Abstract
Background
The objective of this study was to characterize how successfully patients with diabetes are able to distinguish between pens of the same pen type containing long- and short-acting insulins.
Methods
Structured one-on-one interviews were conducted with 400 patients with diabetes in the United States, using either a differentiated (n = 100) or undifferentiated (n = 100) SoloSTAR® (insulin glargine vs insulin glulisine) or (n = 200) FlexPen® (insulin detemir vs insulin aspart). A pair of each pen type was presented simultaneously, and participants were asked to identify the pen that they would use to (1) inject at lunch, (2) inject once daily, and (3) inject at breakfast and how they differentiated between pens. The short-acting insulin pen was then presented, and the interviewer asked whether this was the correct pen to administer insulin once or thrice daily.
Results
More patients successfully identified the correct SoloSTAR pen across the tests vs FlexPen, and the error rate (incorrect selection/inability to select) was significantly lower, respectively [2.7% (n = 8) vs 16.3% (n = 98)]. The most common reason cited for correct responses among all patients was color (of the label/pen, according to pen type).
Conclusions
This study suggests that the full pen body color used on SoloSTAR pens enhances the patient’s ability to differentiate between the pens for long- and short-acting insulin and is a notable improvement compared with the standard approach of differing label color.
Keywords: color differentiation, insulin pen, long-acting insulin, patient preference, short-acting insulin
Background
Insulin injections are essential in type 1 diabetes, which is characterized by the absence of endogenous insulin production. The vast majority of patients with type 2 diabetes will also require insulin therapy at some point in their treatment, with increasing use in the early treatment of type 2 diabetes because of the benefits in terms of hemoglobin A1c reductions.1
The traditional method of injecting insulin with a vial and manual syringe has largely been supplanted by newer methods, particularly insulin pen devices, which offer marked improvements in terms of convenience, flexibility, and social acceptability.2–4 Indeed, studies have shown that patients with diabetes who need to inject insulin tend to prefer pen devices to the vial and syringe option.5,6 Health care professionals also prefer insulin pens for their patients, believing that it is easy to teach patients how to use pens.7 Insulin pens also offer advantages over the vial and syringe in terms of improved dose accuracy.8 However, dosing errors, such as injecting a short-acting insulin when a long-acting insulin should have been administered, can result in serious adverse events.
Patients with type 1 diabetes and some patients with type 2 diabetes inject long- and short-acting insulin as part of a multiple daily injection regimen for the control of basal and prandial glucose fluxes.1 Lantus® SoloSTAR® (sanofi-aventis, Bridgewater, NJ) is a prefilled disposable pen for injection of the basal insulin, insulin glargine (Lantus®, sanofi-aventis), which is administered once daily. Apidra® SoloSTAR® (sanofi-aventis) is used to inject the fast-acting insulin, insulin glulisine (Apidra®, sanofi-aventis), which is administered before or shortly after a meal.
FlexPen® (Novo Nordisk, Princeton, NJ) is another commonly used prefilled pen device used for injection of the basal insulin detemir (Levemir®, Novo Nordisk) and the short-acting insulin aspart (NovoLog®, Novo Nordisk). FlexPen has been shown to be easy to use, with greater patient preference and superior dose accuracy compared with the vial and syringe.9
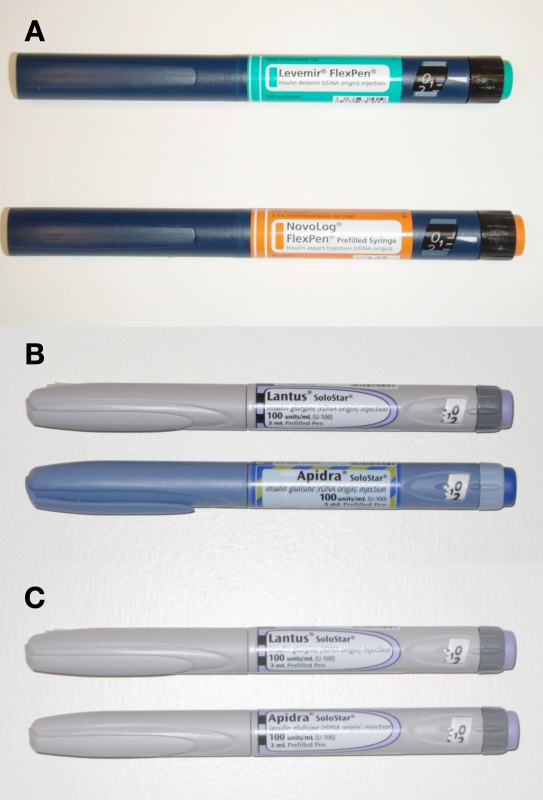
Figure 1 shows photographs of the (A) FlexPen and (B) SoloSTAR prefilled pens. The insulin formulations delivered using the FlexPen set of pens are identified by the different label color/text of the pens as well as tactile and color elements on the injection button and a colored cartridge holder. The colored cartridge holder is not visible when the cap is attached to the pen. In contrast to the FlexPen, SoloSTAR is available in specific body colors for insulin glargine and insulin glulisine in addition to characteristic differences in the label color/text, injection button color, and tactile features on the injection button.10,11 For the purposes of this study, we also used “undifferentiated” SoloSTAR pens, as shown in Figure 1C , in which the same color elements and label shapes were used, and the only differentiating feature was the text on the label. These undifferentiated pens were prepared specifically for this study and are not available for clinical use.
Figure 1.
FlexPen and SoloSTAR sets of pens. (A) FlexPens. (B) Differentiated SoloSTAR pens. (C) Undifferentiated SoloSTAR. The label shown in (B) for Lantus SoloSTAR is not the currently used one, as modest modifications of the Lantus SoloSTAR label were tested in this study that had not yet been implemented into production.
Because of the similarities between the long- and short-acting pens, it is a concern that mix-ups could occur, particularly among people who regularly inject a combination of basal and/or prandial and/or premixed insulins.12 Poor visual acuity and impaired color vision are relatively common in people with diabetes, partly as a result of diabetic retinopathy. In 2005, 5.5 million people with diabetes in the United States were estimated to have diabetic retinopathy, a figure predicted to increase to 16 million by 2050.13 Being able to correctly identify the insulin pen is important in order to reduce the likelihood of injecting the wrong type of insulin and thus avoid potential risk of adverse events, such as hypoglycemia, which may ensue, particularly if a short-acting insulin is administered instead of a long-acting insulin.
The objective of this study was to characterize how successfully patients with diabetes are able to distinguish between pens of the same pen type containing long- and short-acting insulins using SoloSTAR and FlexPen devices.
Methods
This study involved two independent but identically designed surveys of people with diabetes to compare the ability to differentiate between long- and short-acting insulin-containing prefilled pens using either SoloSTAR or FlexPen sets of pens. The independent nature of the surveys was used to avoid learning and positive (or negative) reinforcement on subsequent differentiation tests.
Each study involved face-to-face one-on-one interviews conducted with patients with diabetes in 10 different locations across the United States. All interviews were delivered by staff from Lieberman Research Group (Great Neck, NY) who were trained in advance to ensure standardized delivery. The screening procedure is summarized in Appendix 1 and the main questionnaires in Appendices 2 and 3 . Identical procedures were used in both surveys. The surveys were conducted in a well-lit office setting.
Participants were provided with one of three pairs of pens in order to compare a pen for long-acting insulin versus a pen containing a short-acting insulin from the same manufacturer [i.e., differentiated/undifferentiated Lantus SoloSTAR vs Apidra SoloSTAR and Levemir FlexPen (insulin detemir) vs NovoRapid FlexPen (insulin aspart)]. The participants were given a verbal description of how to use the pens. Those given the SoloSTAR pens were randomly provided with either a differentiated pair or with an undifferentiated pair.
The participants were shown the pens side by side and were asked to identify which pen would be used for Scenario 1: injecting insulin at lunch (short-acting insulin); Scenario 2: injecting once daily (long-acting insulin); and Scenario 3: injecting at breakfast (short-acting insulin). The participants were then asked to state whether they believed that they would have trouble remembering that the pens were different. In order to understand which feature(s) participants used to distinguish between pens, they were asked at the end of each question how they differentiated between the pens.
At the end of the interview, the participants were given the pen containing the short-acting insulin. According to the randomization schedule, the participants were then asked one of two questions: (1) “It is time to give yourself your three times a day insulin. Is this the right insulin pen?” (correct response, yes), or (2) “It is time to give yourself your one time a day insulin. Is this the right insulin pen?” (correct response, no).
Study Outcomes
The following parameters were assessed: (1) error percentage rate: the proportion of patients failing to select the appropriate pen to use in each of the three different scenarios; (2) the features used by patients to distinguish between the pens; (3) the proportion of patients who thought they would have trouble remembering that the pens were different; and (4) the proportion of patients able to identify the short-acting insulin pen in isolation.
Participant Eligibility
Potential participants were approached at shopping malls in 10 cities in the United States, and eligibility was assessed using a screening questionnaire. Participants with type 1 or type 2 diabetes who were taking oral antidiabetic medication or insulin (pen device or vial and syringe) were eligible for the study.
Potential participants were excluded if they or an immediate family member were currently an employee, a paid consultant, or a clinical researcher for any pharmaceutical manufacturer or their agents; a paid consultant for a government health-related agency (e.g., The Food and Drug Administration) or an advertising agency; or a physician, nurse, or an employee of a public relations agency or marketing research agency; had participated in any market research studies in the past 3 months; or used an insulin pump for insulin administration. All enrolled patients provided written informed consent, and subjects who were deemed by the instructors to be uncooperative, not paying attention, or unable to focus on the questions were excluded from the survey.
The target sample size was 100 participants for each SoloSTAR group and 200 for the FlexPen group.
Statistical Analysis
All data were analyzed descriptively, with means ± standard deviation for continuous variables, and n and percentage for categorical variables. Statistical t-testing was performed at the 95% and 90% levels.
Results
The characteristics of the participants in each group are summarized in Table 1 .
Table 1.
Participant Characteristicsa
| Characteristics | Group A Undifferentiated SoloSTARb | Group B Differentiated SoloSTARc | Group C FlexPen |
|---|---|---|---|
| N | 100 | 100 | 200 |
| Males/females (%) | 44/56 | 51/49 | 50/50 |
| Age (average) | 50.3 | 49.0 | 48.8 |
| Age (%) | |||
| 18–49 years | 45 | 52 | 50 |
| 50 years or older | 55 | 48 | 50 |
| Diabetes (%) | |||
| Type 1 | 20 C | 16 | 12 |
| Type 2 | 80 | 84 | 88 A |
| Treatment type (%) | |||
| Insulin (± OADsd) | 34 | 37 | 36 |
| OADs (no insulin) | 66 | 63 | 64 |
Statistical testing: Upper case letter indicates a statistic is significantly higher at the 90% confidence level between groups.
Two participant(s) were color blind.
One participant was color blind.
(OAD) oral antidiabetic drug
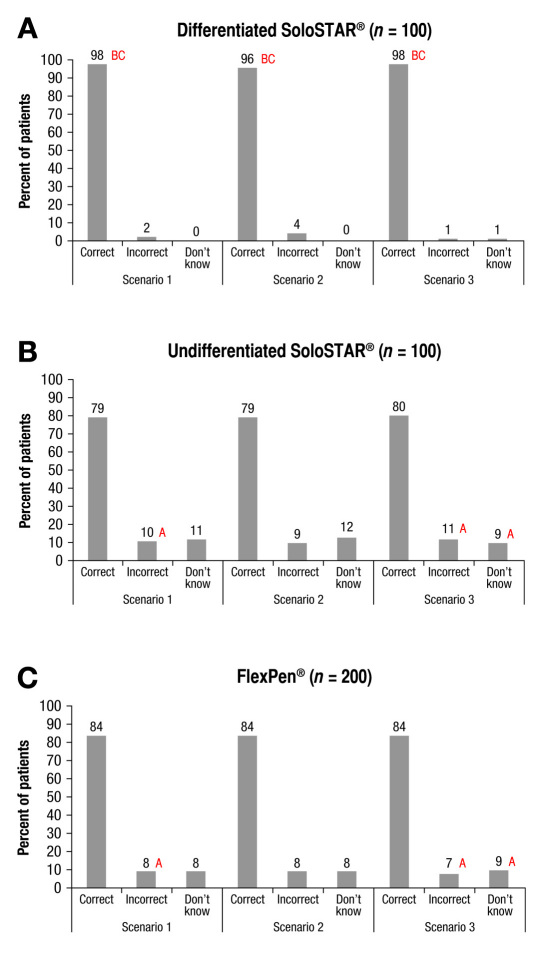
The majority of the participants correctly identified the insulin/pen to be used in a specific scenario (Figure 2 ). In each of the three scenarios, the differentiated SoloSTAR was consistently selected correctly more frequently than the undifferentiated SoloSTAR or the FlexPen (Figure 2 ).As shown in Table 2 , 97.3% of the responses for the differentiated SoloSTAR were correct, compared with 83.7% for FlexPen and 79.3% for undifferentiated SoloSTAR, which translates to error percentage rates of 2.7, 16.3, and 20.7%, respectively (p < 0.05 for differentiated SoloSTAR vs FlexPen and undifferentiated SoloSTAR).
Figure 2.
Proportion of participants who correctly identified the short-acting or long-acting insulin. (A) Differentiated SoloSTAR. (B) Undifferentiated SoloSTAR. (C) FlexPen in three individual scenarios. Scenario 1: inject insulin at lunch (short-acting insulin), Scenario 2: inject once a day, and Scenario 3: inject at breakfast (short-acting insulin). Statistical testing: upper case letter indicates a statistic is significantly higher at the 95% confidence level between groups.
Table 2.
Participant Responses to the Differentiation Exercisesa
| A. Undifferentiated SoloSTAR (n =100) | B. Differentiated SoloSTAR (n =100) | C. FlexPen (n =200) | |
|---|---|---|---|
| Total number of responses | 300 | 300 | 600 |
| Correct, n (%) | 238 (79.3) | 292 (97.3) ACD | 502 (83.7) D |
| Incorrect/inability, n(%) | 62 (20.7) B | 8 (2.7) | 98 (16.3) B |
| Participants with at least one scenario incorrect (n) | 30 | 7 | 39 A |
| All three | 11 | – | 28 |
| Any two | 10 CD | 1 | 3 |
| Any one | 9 | 6 ACD | 8 |
| Trouble remembering (%) | |||
| No | 49 | 90 AD | 86 AD |
| Yes | 48 BCD | 9 | 11 |
| Do not know | 3 | 1 | 3 D |
Statistical testing: upper case letter indicates a statistic is significantly higher at the 95% confidence level between groups.
The most common reason cited for correct responses among all patients was color of the label or the pen, according to pen type. For the differentiated SoloSTAR, 65% (scenario 1), 62% (scenario 2), and 38% (scenario 3) of the participants reported that they remembered the color difference. In addition to the 38% in scenario 3, another 31% correctly associated the blue pen body color with the short-acting insulin.
The color of the label was the most frequently cited reason for the correct responses in terms of insulin type for FlexPen (scenario 1, 61%; scenario 2, 56%; and scenario 3, 60%).
By contrast, for the undifferentiated SoloSTAR lacking any differing color elements, the most common reason was that the participant remembered the name/brand when responding to scenarios 1, 2, and 3 (41, 40, and 35%, respectively).
For the differentiated SoloSTAR, 68, 62, and 34% of participants in scenarios 1, 2, and 3, respectively, reported that they remembered the number of times per day for each pen (multiple answers were allowed; p < 0.05 vs undifferentiated SoloSTAR and FlexPen in scenarios 1 and 2). In Scenarios 1, 2, and 3, the rates for the undifferentiated SoloSTAR were 43, 38, and 38%, respectively. By contrast, the proportion of participants who remembered the number of times each pen/insulin type should be used per day was lower for FlexPen (scenario 1, 19%; scenario 2, 17%; and scenario 3, 12%).
Overall, 90% of the participants given the differentiated SoloSTAR believed they would have no trouble remembering that the pens are different, compared with 86 and 49% of participants given FlexPen and the undifferentiated SoloSTAR, respectively (p < 0.05 for differentiated SoloSTAR and FlexPen vs undifferentiated SoloSTAR).
The correct identification of the insulin pen in isolation was highest for the differentiated SoloSTAR. When the participants were presented with the pen containing the short-acting insulin and asked whether the pen was for once daily injection or thrice daily injection, more patients correctly identified the differentiated Apidra SoloSTAR pen. All participants (100%) correctly reported that the Apidra SoloSTAR pen was for thrice daily insulin, while 96% of the participants correctly stated that this pen was not for once daily insulin (both: p < 0.05 vs undifferentiated SoloSTAR and FlexPen). In contrast, the proportion of participants with correct responses was 77 and 75%, respectively, for the undifferentiated Apidra SoloSTAR pen, and 85 and 88%, respectively, for NovoLog FlexPen.
Discussion
In the present study, participants with diabetes were presented with two pens of the same model (i.e., differentiated or undifferentiated SoloSTAR, or FlexPen); one pen contained short-acting insulin and the other contained long-acting insulin. The participants were then presented with three scenarios commonly encountered in everyday life, namely: (1) you are about to eat lunch, and you need to give yourself the insulin that is used three times a day; (2) it is 9 o’clock, and you are getting ready for bed; you need to give yourself the insulin that is used once a day; and (3) you are getting ready for breakfast, and you need to give yourself the insulin that is used three times a day. Overall, the majority of participants presented with either model identified the correct insulin for each scenario. Notably, the proportion of participants with correct responses was highest for the differentiated SoloSTAR (97.3%), followed by FlexPen (83.7%) and undifferentiated SoloSTAR (76.7%) (p < 0.05 for differentiated SoloSTAR vs FlexPen and undifferentiated SoloSTAR). For differentiated SoloSTAR, the most common reason given for the correct response was that they remembered whether the pen was for once or thrice daily in each scenario, followed by the color difference (scenario 1, 56%; scenario 2, 62%; and scenario 3, 38% plus 31% for blue is for three times a day). For FlexPen, the color of the label was the most commonly cited reason for the correct response. By contrast, for undifferentiated SoloSTAR, the most commonly cited reason was that they remembered whether the insulin was for once or thrice daily administration. These findings suggest that color features, such as body color and label color, are important factors that helped the participants select the correct pen.
Insulin vials and pens have commonly had a significant limitation, in that the different products have been differentiated only through the text printed on the label, which may raise the risk for dosing errors caused by picking the wrong vial.13 This problem is further compounded by the increasing use of insulin pens, where the pen cap may obscure any text on the pen cartridge. Recent developments have included the introduction of color features on the label of prefilled pens. However, regarding FlexPen, it seems that label color features provide only small improvements in error rates compared with the undifferentiated SoloSTAR, which was evident in each of the scenarios and in the final identification of the short-acting insulin pen. We found that with the extension of color features to the pen body, as in the differentiated SoloSTAR, there was a notable improvement in the error rate in all three scenarios and in the final identification of the short-acting insulin pen.
Overall, more participants correctly identified the differentiated SoloSTAR sets of pens in each of the three scenarios compared with the FlexPen sets. Furthermore, many of the participants reported that they memorized the body color appropriate for the specific pen/insulin for SoloSTAR, whereas for FlexPen, a smaller proportion of patients reported that they considered the label color when selecting the pen/insulin.
Overall, the results of the study should be considered in conjunction with its limitations. First, only 100 participants were surveyed for each of the undifferentiated and differentiated SoloSTAR sets of pens, whereas 200 participants were surveyed for the FlexPen set of pens. Accordingly, the results in the SoloSTAR groups may be underestimated compared with the other groups. The time taken to explain to patients the difference between the different pens was not recorded as part of the survey. Although all interviews were performed with a standardized approach across all surveys, some patients may have inadvertently spent longer learning the differences between the pens. Moreover, only current treatments for diabetes were recorded as part of the survey; it is possible that some patients may have had prior, but not current, exposure to insulin pens and could have been more familiar with the differences between pens than patients with no prior exposure. Although it would be interesting to analyze the differences between these groups of patients, the numbers of patients enrolled were too low to allow further subgroup analyses. The limit on patient numbers also meant that analysis according to current pen use or other demographic features was not possible.
In this study, we only used devices bearing the U.S. label. Therefore, studies performed in other countries using the locally approved labels may show differing results, although the essential features of the labels are broadly comparable across countries.
The results reported in this analysis provide a good indication of how patients can use the features of the pens to differentiate between insulin formulations; however, it is important to recognize that within a clinical setting, patients will receive complete guidance and information on how to remember the differences between pens. This study was purposefully designed to avoid learning and positive (or negative) reinforcement, and the results presented, therefore, may not reflect those obtained in the clinic, where patients may be shown additional features that can be used to distinguish pens and minimize injection errors.
Ultimately, enhancing the ability to differentiate between pens and insulin formulations is an important consideration for improving insulin therapy and reducing the likelihood of potentially serious medication errors. The introduction of specific pen body colors could help to reduce such errors, as was originally achieved by teaching people with diabetes the difference between cloudy and clear insulin in the neutral protamine Hagedorn era.13
Conclusions
This study suggests that the full pen body color used on SoloSTAR pens enhances the patients’ ability to differentiate between the long- and short-acting insulin compared with the standard approach of differing colors for label and injection button. The results of this study should offer patients and health care professionals reassurance that using insulin pens with a different body color according to the type of insulin used may reduce the risk of erroneously selecting (and injecting) the incorrect insulin, as compared to pens that differ only in their label and injection button color.
Acknowledgments
The author thanks Lieberman Research Group (Great Neck, NY) for conducting the interviews.
Appendix 1. Screening Questionnaire
Market: Check One
| [ ]1 Atlanta |
| [ ]2 Chicago |
| [ ]3 Connecticut |
| [ ]4 Dallas |
| [ ]5 Ft. Lauderdale |
| [ ]6 Los Angeles |
| [ ]7 Minneapolis |
| [ ]8 New York |
| [ ]9 Phoenix |
| [ ]10 San Diego |
Hello my name is ______________ from *******, a marketing research company. We are conducting a survey on health issues and would like to include your opinions. This is strictly a research project. You will not be asked to buy anything during or as a result of this survey. We are interested only in learning your opinions on this subject. This will take only a few minutes of your time.
S1 Are you or anyone in your immediate family currently an employee, a paid consultant, or a clinical researcher for any pharmaceutical manufacturer or their agents; a paid consultant for a government health-related agency (e.g., The Food and Drug Administration) or an advertising agency; a physician, nurse, public relations agency, or marketing research agency?
[ ] Yes THANK AND TERMINATE
[ ] No CONTINUE
[ ] Don’t know THANK AND TERMINATE
[ ] Refused THANK AND TERMINATE
S2 Have you participated in any market research studies in the past 3 months?
[ ] Yes THANK AND TERMINATE
[ ] No CONTINUE
[ ] Don’t know THANK AND TERMINATE
[ ] Refused THANK AND TERMINATE
S3a To be sure we represent the opinions of all ages, please tell me your age:
Age (in years) _____________
[ ] Refused THANK AND TERMINATE
INTERVIEWER: IF UNDER 18 – THANK AND TERMINATE
S3b INTERVIEWER : Record gender by sight
[ ] 1 Male
[ ] 2 Female
SHOW CARD S4
S4 Which, if any, of these health conditions do you currently have that has been diagnosed by a doctor? INTERVIEWER : Check all that apply. NOTE : Multiple answers are possible except for type 1 or type 2 diabetes, where only one answer is possible.
[ ] 1 Heart condition or coronary heart disease
[ ] 2 High blood pressure or hypertension
[ ] 3 High cholesterol
[ ] 4 Juvenile or type 1 diabetes
[ ] 5 Type 2 adult diabetes
[ ] 6 None of the above
MUST HAVE TYPE ONE OR TYPE TWO DIABETES TO CONTINUE, ELSE THANK AND TERMINATE
SHOW CARD S5
S5 And which of these, if any, do you currently do to treat your diabetes? INTERVIEWER : Check all that apply
[ ] 1 Take insulin (with or without oral diabetes medication)
[ ] 2 Take oral diabetes medication(s), meaning you swallow a pill(s) that was prescribed by your doctor (do NOT take insulin)
[ ] 3 Do NOT take oral diabetes medications or insulin – TERMINATE
NOTE: IF TAKE INSULIN (WITH OR WITHOUT ORAL DIABETES MEDICATION), CONTINUE WITH Q.S6. IF TAKE ORAL DIABETES MEDICATION(S) BUT NOT INSULIN, GO TO INSTRUCTION FOR ISHIHARA COLOR BLIND TEST AFTER Q.S8.
S6 Do you currently use a vial and syringe, an insulin pen, or a pump?
[ ] 1 Vial and syringe
[ ] 2 Insulin pen
[ ] 3 Pump – THANK AND TERMINATE
S7 And do you typically give yourself the injection, or does someone else give it to you?
[ ] 1 Give self
[ ] 2 Someone else
SHOW CARD S8
S8 Which type of insulin(s) do you currently take? INTERVIEWER : Check all that apply
[ ] 1 Apidra or glulisine (glue-ly-seen)
[ ] 2 Humalog or lispro (hume-ah-log or liss-pro)
[ ] 3 Lantus or glargine (lann-tuss or glarr-geen)
[ ] 4 Levemir or detemir (lev-a-meer or det-a-meer)
[ ] 5 NovoLog or Aspart (novo-log or as-part)
[ ] 6 NPH (Humulin N or Novolin N)
[ ] 7 Pre-mixed insulin (Humulin 70/30, Humulin 50/50, Novolin 70/30, Humalog 75/25 or Novolog 70/30, etc.)
[ ] 8 Regular human insulin (Humulin R, Novolin R)
[ ] 9 Other (Please write in:__________________________)
[ ] 10 Don’t know
HAND ISHIHARA COLOR BLIND TEST CARD TO RESPONDENT
Starting with the top row, please call off the numbers you see from left to right. INTERVIEWER : Check those that the respondent correctly identified. If the respondent says an incorrect number, write it in the space provided below it.
| Top row (left to right) | → | [ ] 25 | ||
| If incorrect, # given was: | → | _____ | _____ | _____ |
Now the bottom row, please call off the numbers you see from left to right. INTERVIEWER : Check those that the respondent correctly identified. If the respondent says an incorrect number, write it in the space provided below it.
| Bottom row (left to right) | → | [ ] 56 | ||
| If incorrect, # given was: | → | _____ | _____ | _____ |
S9 INTERVIEWER : CHECK BOXED NUMBERS ABOVE CAREFULLY, THEN CHECK ONE BOX BELOW.
[ ] RESPONDENT IS COLOR BLIND -- each of the four boxed numbers 45, 6, 29, and 8 MUST NOT be checked (that is not correctly identified).
[ ] RESPONDENT IS NOT COLOR BLIND -- if one or more of these #’s are checked (correctly identified).
Appendix 2. Main Questionnaire for SoloSTAR
Differentiation Exercise:
We are testing the appearance of two pens that people with diabetes might use to take insulin. One pen is taken ONE time each day. The other pen is taken THREE times a day with your meals.
Please look at these two pens.
This insulin (show pen labeled Lantus SoloStar) is used ONE time each day.
This insulin (show pen labeled Apidra SoloStar) is used THREE times a day with your meals.
I am now going to present three scenarios to you, and I would like you to tell me which pen you would use for each. If you are not sure, just tell me.
NOTE: Before each scenario, the interviewer mixed up the pens under the table.
Scenario #1:
-
1a.
First, you are about to eat lunch, and you need to give yourself the insulin that is used THREE times a day. Please pick up the pen that you need to use.
-
1b.
Please tell me why you picked this pen. (PROBE: What is it specifically about the appearance of the pen itself that made you select this pen?)
-
1c.
You seem unsure. Please tell me why. (PROBE: What is it specifically about the appearance of the pen itself that makes you unsure?)
Scenario #2:
-
2a.
Second, it is 9 o’clock, and you are getting ready for bed; you need to give yourself the insulin that is used ONE time a day. Please pick up the pen that you need to use.
-
2b.
Please tell me why you picked this pen. (PROBE: What is it specifically about the appearance of the pen itself that made you select this pen?)
-
2c.
You seem unsure. Please tell me why. (PROBE: What is it specifically about the appearance of the pen itself that makes you unsure?)
Scenario #3 :
-
3a.
Third, you are getting ready for your breakfast, and you need to give yourself the insulin that is used THREE times a day. Please pick up the pen that you need to use.
-
3b.
Please tell me why you picked this pen. (PROBE: What is it specifically about the appearance of the pen itself that made you select this pen?)
-
3c.
You seem unsure. Please tell me why. (PROBE: What is it specifically about the appearance of the pen itself that makes you unsure?)
Trouble Remembering Pens Are Different:
-
4.
And, looking at these two pens, do you think you would have trouble remembering that they are different?
-
5.
Please tell me why.
Demographics:
-
6.
Marital status
-
7.
Employment status
-
8.
Level of education completed or highest degree received
-
9.
Household income before taxes for the past 12 months
-
10.
Ethnic background
-
11a.
Is English the language you primarily speak on a day to day basis?
-
11b.
Do you speak any other languages?
Apidra Correct Identification in Isolation:
(Rotated)
-
12a.
It is time to give yourself your ONE time a day insulin. Is this the right insulin pen?
-
12b.
Why did you say (yes/no)?
-
12a.
It is time to give yourself your THREE times a day insulin. Is this the right insulin pen?
-
12b.
Why did you say (yes/no)?
Additional Demographic:
-
13.
People with diabetes were asked to read a sentence aloud to test for literacy.
Appendix 3. Main Questionnaire for FlexPen
Differentiation Exercise:
We are testing the appearance of two pens that people with diabetes might use to take insulin. One pen is taken ONE time each day. The other pen is taken THREE times a day with your meals.
Please look at these two pens.
This insulin (show pen labeled Levemir FlexPen) is used ONE time each day.
This insulin (show pen labeled NovoLog FlexPen) is used THREE times a day with your meals.
I am now going to present three scenarios to you, and I would like you to tell me which pen you would use for each. If you are not sure, just tell me.
NOTE: Before each scenario, the interviewer mixed up the pens under the table.
Scenario #1:
-
1a.
First, you are about to eat lunch, and you need to give yourself the insulin that is used THREE times a day. Please pick up the pen that you need to use.
-
1b.
Please tell me why you picked this pen. (PROBE: What is it specifically about the appearance of the pen itself that made you select this pen?)
-
1c.
You seem unsure. Please tell me why. (PROBE: What is it specifically about the appearance of the pen itself that makes you unsure?)
Scenario #2 :
-
2a.
Second, it is 9 o’clock, and you are getting ready for bed; you need to give yourself the insulin that is used ONE time a day. Please pick up the pen that you need to use.
-
2b.
Please tell me why you picked this pen. (PROBE: What is it specifically about the appearance of the pen itself that made you select this pen?)
-
2c.
You seem unsure. Please tell me why. (PROBE: What is it specifically about the appearance of the pen itself that makes you unsure?)
Scenario #3 :
-
3a.
Third, you are getting ready for your breakfast, and you need to give yourself the insulin that is used THREE times a day. Please pick up the pen that you need to use.
-
3b.
Please tell me why you picked this pen. (PROBE: What is it specifically about the appearance of the pen itself that made you select this pen?)
-
3c.
You seem unsure. Please tell me why. (PROBE: What is it specifically about the appearance of the pen itself that makes you unsure?)
Trouble Remembering Pens Are Different:
-
4.
And, looking at these two pens, do you think you would have trouble remembering that they are different?
-
5.
Please tell me why.
Demographics:
-
6.
Marital status
-
7.
Employment status
-
8.
Level of education completed or highest degree received
-
9.
Household income before taxes for the past 12 months
-
10.
Ethnic background
-
11a.
Is English the language you primarily speak on a day to day basis?
-
11b.
Do you speak any other languages?
NovoLog Correct Identification in Isolation:
(Rotated)
-
12a.
It is time to give yourself your ONE time a day insulin. Is this the right insulin pen?
-
12b.
Why did you say (yes/no)?
-
12a.
It is time to give yourself your THREE times a day insulin. Is this the right insulin pen?
-
12b.
Why did you say (yes/no)?
Additional Demographic:
-
13.
People with diabetes were asked to read a sentence aloud to test for literacy.
References:
- 1.American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care. 2009;32(Suppl 1):S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coscelli C, Lostia S, Lunetta M, Nosari I, Coronel GA. Safety, efficacy, acceptability of a pre-filled insulin pen in diabetic patients over 60 years old. Diabetes Res Clin Pract. 1995;28(3):173–177. doi: 10.1016/0168-8227(95)01092-r. [DOI] [PubMed] [Google Scholar]
- 3.D’Eliseo P, Blaauw J, Milicević Z, Wyatt J, Ignaut DA, Malone JK. Patient acceptability of a new 3.0 ml pre-filled insulin pen. Curr Med Res Opin. 2000;16(2):125–133. [PubMed] [Google Scholar]
- 4.Haak T, Edelman S, Walter C, Lecointre B, Spollett G. Comparison of usability and patient preference for the new disposable insulin device Solostar versus Flexpen, Lilly disposable pen, and a prototype pen: an open-label study. Clin Ther. 2007;29(4):650–660. doi: 10.1016/j.clinthera.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Korytkowski M, Bell D, Jacobsen C, Suwannasari R, FlexPen Study Team A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25(11):2836–2848. doi: 10.1016/s0149-2918(03)80337-5. [DOI] [PubMed] [Google Scholar]
- 6.Summers KH, Szeinbach SL, Lenox SM. Preference for insulin delivery systems among current insulin users and nonusers. Clin Ther. 2004;26(9):1498–1505. doi: 10.1016/j.clinthera.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Carter J, Roberts A. Usability of a pre-filled insulin injection device in a 3-month observational survey of everyday clinical practice in Australia. Curr Med Res Opin. 2008;24(10):2741–2749. doi: 10.1185/03007990802367579. [DOI] [PubMed] [Google Scholar]
- 8.Asakura T, Seino H, Nakano R, Muto T, Toraishi K, Sako Y, Kageyama M, Yohkoh N. A comparison of the handling and accuracy of syringe and vial versus prefilled insulin pen (FlexPen) Diabetes Technol Ther. 2009;11(10):657–661. doi: 10.1089/dia.2009.0006. [DOI] [PubMed] [Google Scholar]
- 9.Ignaut DA, Schwartz SL, Sarwat S, Murphy HL. Comparative device assessments: Humalog KwikPen compared with vial and syringe and FlexPen. Diabetes Educ. 2009;35(5):789–798. doi: 10.1177/0145721709340056. [DOI] [PubMed] [Google Scholar]
- 10.Bode A. Development of the SoloSTAR insulin pen device: design verification and validation. Expert Opin Drug Deliv. 2009;6(1):103–112. doi: 10.1517/17425240802636187. [DOI] [PubMed] [Google Scholar]
- 11.Clarke A, Spollett G. Dose accuracy and injection force dynamics of a novel disposable insulin pen. Expert Opin Drug Deliv. 2007;4(2):165–174. doi: 10.1517/17425247.4.2.165. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch IB. Insulin packaging: a medical error waiting to happen. American Diabetes Society. DOCNEWS; 2006. Available from: http://docnews.diabetesjournals.org/content/3/5/7.full. Accessed May 2010. [Google Scholar]
- 13.Saaddine JB, Honeycutt AA, Narayan KM, Zhang X, Klein R, Boyle JP. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005-2050. Arch Ophthalmol. 2008;126(12):1740–1747. doi: 10.1001/archopht.126.12.1740. [DOI] [PubMed] [Google Scholar]