Abstract
Background
Daily routine for insulin-treated patients with diabetes mellitus requires correct performance of self-monitoring of blood glucose and insulin injections several times a day. Dexterity skills may play an important role in the performance efficacy of these procedures.
Methods
We collected data of insulin-treated (>10 years) patients with different age ranges [healthy controls, 14 female/11 male, age (mean ± standard deviation) 55 ± 7 years; type 1 diabetes mellitus (T1DM) patients, 12/13, 45 ± 9 years, disease duration 23.9 ± 6.5 years; T2DM patients, 8/17, 64 ± 6 years, 16.2 ± 6.9 years; T2DM patients (>70 years of age), 9/16, 75 ± 4 years, 19.7 ± 7.0 years]. After assessment of neuropathy (temperature, pain, and vibration perception), the patients participated in two dexterity test batteries [Jebsen–Taylor hand-function test (JHFT) and motoric performance series (MPS)].
Results
Patients with type 2 diabetes showed disturbed vibration perception as compared to the other groups. The dexterity results were influenced by age to a large extent. Older T2DM patients performed worst in the majority of the subtests (e.g., JHFT, writing nondominant hand: control, 40.8 ± 11.7 s; T1DM, 46.3 ± 50.9 s, not significant versus control; old T2DM, 68.1 ± 29.5 s, p < .05; young T2DM, 52.5 ± 26.2 s, p < .05). Patients with type 1 diabetes showed similar JHFT and MPS results than the 10-year-older control subjects and performed outside of the age-dependent normal reference range.
Conclusions
Manual skills and dexterity differed between the groups, and age-corrected reduced skills were common in both T1DM and T2DM patients in this study. Our findings underline the importance of considering dexterity and manual skills when designing medical devices for patients with diabetes mellitus.
Keywords: dexterity, hand function test, motor function, neuropathy
Introduction
Insulin-treated patients with type 1 and type 2 diabetes mellitus (T1DM and T2DM), are asked to measure their blood glucose several times a day for therapeutic purposes (e.g., in the morning, prior to each major meal, and at bedtime, to be able to correctly calculate the required insulin doses in case of multiple injection therapy).1
Adherence to this requirement is important for achieving the therapeutic targets but is influenced by multiple factors, including, but not limited to, patient education, type of therapy, type and practicability of the used blood glucose meter, age, physical and mental capacity, dexterity, and prevalence of secondary complications.2,3
Psychomotor function is an important domain of mental function that has not been systematically studied with respect to an association with diabetes. Psychomotor performance encompasses motoric strength, hand–eye coordination, balance, dexterity, tracking, and other skills. During episodes of iatrogenic hypoglycemia, people with insulin-treated diabetes have frequently been reported to suffer from a deterioration of fine motor skills.4,5
Manual dexterity may have an enormous impact on occupational performance as well as on activities of daily living, such as frequent daily measurements of blood glucose. This situation has not been thoroughly explored in any larger clinical investigation, and very limited information can be obtained from the current literature.
Using validated clinical tests and standardized controls, Casanova and colleagues6 measured hand function in subjects with diabetes. Fifteen diabetes patients with a median age of 48 years, all having used insulin for a minimum of 5 years, were randomly selected from inpatient and outpatient services. Testing was done on an outpatient basis. The three hand function tests used were the Purdue pegboard, O’Connor tweezer dexterity, and Smith hand-function test. Hand function was significantly decreased in the group with diabetes, and the decrease was out of proportion to patients’ own subjective pretest assessments.
Cederlund and associates7 examined hand disorders, symptoms, overall hand function, activities of daily living, and life satisfaction in elderly men with T2DM, with impaired glucose tolerance, and with normal glucose tolerance. The subjects were interviewed and evaluated with a battery of clinical and laboratory tests, including hand assessment, and a questionnaire. As expected, hemoglobin A1c was highest in the diabetes patients. A limited joint mobility (e.g., prayer sign and Dupuytren’s contracture) was most common in individuals with diabetes, followed by individuals with impaired glucose tolerance. Vibrotactile sense was impaired symmetrically in the index and little fingers in diabetes patients. The authors, however, found no differences for sensibility, dexterity, grip strength, and cold intolerance between the study cohorts when applying their methods of investigation. In an earlier investigation, the same group described increased vibration thresholds in the fingertips in subjects with T2DM, which may lead to decreased dexterity.8
A general evaluation of these few study reports on dexterity in diabetes is basically impossible, as no standardized methods of dexterity assessment have been applied in the different investigations. A systematic exploratory approach requires, therefore, a cross-sectional pilot examination to assess the prevalence of potential dexterity impairment by means of scalable and reproducible methods.
One standardized way to assess hand function is the modified Jebsen test of hand function, which has been validated in patients with stroke, multiple sclerosis, and other head injury and which has been suggested as a measure for gross functional dexterity.9,10 Another standardized and scalable method is the Schoppe test, which has been predominately reported to be used with patients suffering from Parkinson’s disease.11,12
The goal of this pilot investigation was to assess the prevalence of potential dexterity problems in different patient populations (25 healthy control subjects 40–70 years of age, 25 T1DM patients <60 years of age, 25 T2DM patients 40–70 years of age, and 24 T2DM patients >70 years of age) in the context of potential confounding prevalence of neuropathy.
Patients and Methods
This cross-sectional single-center study was approved by an institutional review board and was performed in compliance with the Declaration of Helsinki and local legal and ethical regulations. Type 1 and type 2 diabetes patients were selected based on the following inclusion criteria: insulin treatment >10 years, experience with self-monitoring of blood glucose (SMBG), and specific age ranges (25 T1DM <60 years of age, 25 T2DM at 40–70 years, and 24 T2DM >70 years). In addition, 25 nondiabetic healthy control subjects were enrolled (40–70 years). Exclusion criteria were disease duration <10 years, any neurological disorder known to impair dexterity (e.g., Parkinson’s disease), known neuropathy of other origin than diabetes, arthritis/arthrosis, other impairments of manual dexterity (e.g., accident or handicap), central neurologic disorders (e.g., Alzheimer’s disease, dementia, stroke, chronic dihydration syndrome), any neurodegenerative or musculodegenerative disease resulting in impairment of manual dexterity, cancer, or any other life-threatening condition. Patients with visual impairment (visus <0.5) were excluded from study participation. After signing written informed consent, the patients participated in a series of neuropathy assessment and dexterity tests during a single visit.
Jebsen–Taylor Hand-Function Test
The Jebsen–Taylor hand-function test (JHFT) is a widely used assessment of common everyday motor skills.13 It has a good validity and reliability, and normative data are available for different ages and both genders.14 The test consists of seven subtests: writing a sentence, turning over cards, picking up small objects and placing them in a can, picking up small objects with a teaspoon and placing them in a can (simulated feeding), stacking checkers, moving large light cans, and moving heavy cans. Patients were instructed to perform the tasks as rapidly and accurately as possible according to standardized instructions.13 Subtest JHFT times were recorded with a stopwatch for analysis.
Motoric Performance Series (Motorischer Leistungstest)
The motoric performance series (MPS) is a comprehensive fine motor abilities test battery. The battery measures fine motor abilities through static and dynamic tasks for finger, hand, and arm movement and is applicable from 7 years onward. It has been developed by Schoppe11 based on Fleishman’s factor-analytic examinations of fine motor abilities.15 The MPS is administered via a work panel with boreholes, grooves, and contact surfaces and consists of four subtests (steadiness, line tracing, aiming, and tapping) for each hand. Results are expressed as speed or accuracy measurements. After short introduction to the work panel, patients had to perform one sequence (left and right hand) within 10 minutes.
Neuropathy Assessment
Temperature, pain, and vibration perception thresholds were assessed by means of the Medoc TSA 2001 device (Medoc Advanced Medical Systems, Eilat, Israel). For thermal testing, a thermode (30 × 30 mm) was secured at the tested side (right-hand palmar thenar) with an elastic band. To avoid tactile or pressure stimulation, the probe was kept in contact with the skin for the entire duration of the test. Cold and warm detection thresholds were measured first. Then thermal pain thresholds (hot and cold) were determined. The mean threshold temperature from five consecutive trials per site and test was calculated. To detect the vibration threshold, the middle fingertip was placed on the simulating probe with a diameter of 1.00 cm. The vibratory stimulus was delivered at 100 Hz, and the method of limits was used for analysis. The machine delivered the stimulus with increasing intensity in the steps of 0.3 µm. The participant was instructed to click the mouse as soon as she/he perceived the vibration. The test was repeated five times. The mean vibration detection threshold was computed from the five consecutive experiments.
Statistical Methods
All statistical analyses were performed with SPSS (Version 15.0). Hypotheses were tested with a significance level of α = 0.05. Adjustment of the α-level for multiple comparisons was done according to the Sidak procedure.16
Continuous data were analyzed using analysis of variance procedures with experimental group as a fixed factor. Possible interfering variables were controlled as covariate using analysis of covariance procedures. In case of violations of the assumption of normal distributed test scores (testing for kurtosis and skewness), Kruskal–Wallis tests were performed for these variables. Relations between variables were analyzed by means of correlation techniques (Pearson, Spearman). A p value < .05 was considered to be statistically significant.
Results
A total of 100 patients were initially enrolled into the study. One patient (T2DM, >70 years) did not participate in all of the applied tests. The demographic characteristics of the patients are provided in Table 1 .
Table 1.
Demographic Characteristics of the Study Participants
| Characteristics | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| Healthy control (40–70 years) | T1DM (<60 years) | T2DM (40–70 years) | T2DM (>70 years) | |
| N | 25 | 25 | 25 | 25 |
| Gender | ||||
| female | 14 | 12 | 8 | 9 |
| male | 11 | 13 | 17 | 16 |
| age (years) | 55 ± 7a | 45 ± 9a | 64 ± 6a | 75 ± 4a |
| Body mass index (kg/m²) | 300 ± 5.1 | 27.0 ± 4.1b | 34.5 ± 6.7b | 31.4 ± 4.7 |
| Diabetes duration (years) | — | 23.9 ± 6.5 | 16.2 ± 6.9 | 19.7 ± 7.0 |
| Hemoglobin A1c (%) | — | 7.5 ± 1.2 | 6.4 ± 0.5 | 7.4 ± 1.3 |
| Neuropathy | 0% | 28% | 44% | 52% |
| Retinopathy | 0% | 36% | 16% | 12% |
| Nephropathy | 0% | 12% | 8% | 8% |
p < .05 versus all other groups. bp < .05 versus control.
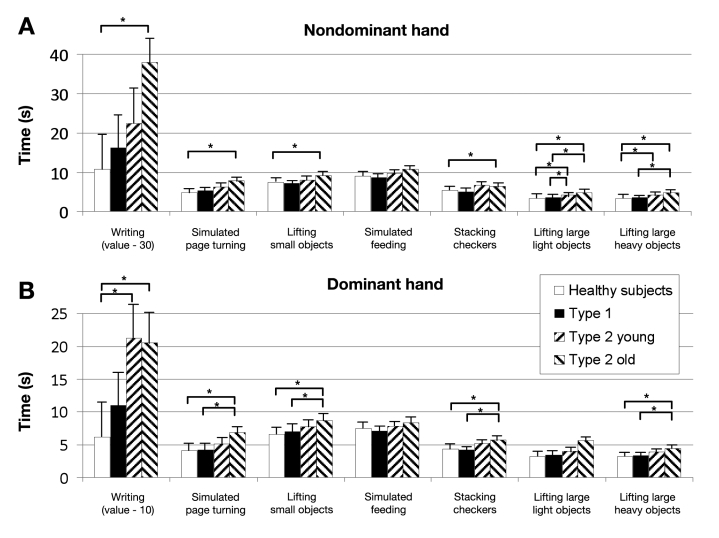
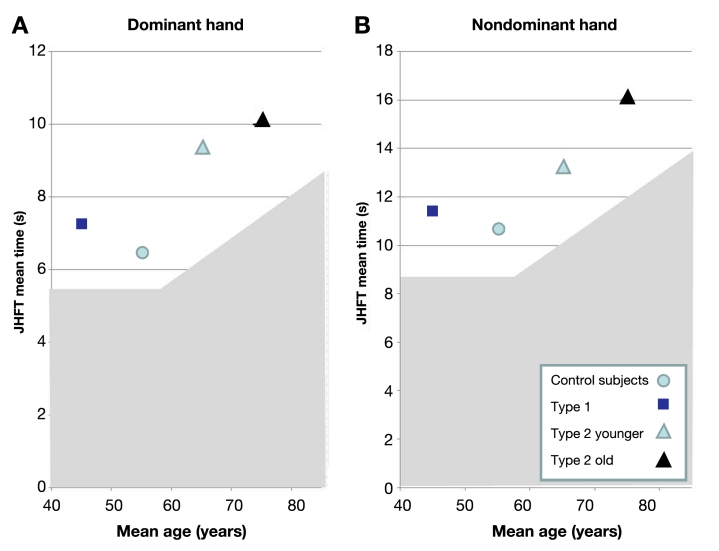
As shown in Figure 1 , the results of the JHFT were consistent if the test was performed with the dominant hand or the nondominant hand. For both hands, T2DM patients older than 70 years show consistently worse results and needed more time for the subtests as compared to the healthy controls. Post hoc group comparisons revealed significant differences for several of the individual subtests, e.g., for the nondominant hand, simulated page turning, healthy controls < T2DM (old); lifting large, light objects, healthy controls = T1DM < T2DM (young) = T2DM (old); and lifting large, heavy objects, healthy controls < T2DM (young and old); T1DM < T2DM (old). As shown in Figure 2 , all three groups with diabetes showed impaired results when compared to the age-correlated normal reference values.14
Figure 1.
Results of the individual JHFT items. Values are given as mean values ± standard deviation. The asterisk represents p < .01.
Figure 2.
Mean JHFT times for the dominant and nondominant hand for all four groups in relation to their age. The gray shadowed areas present the normal references.14
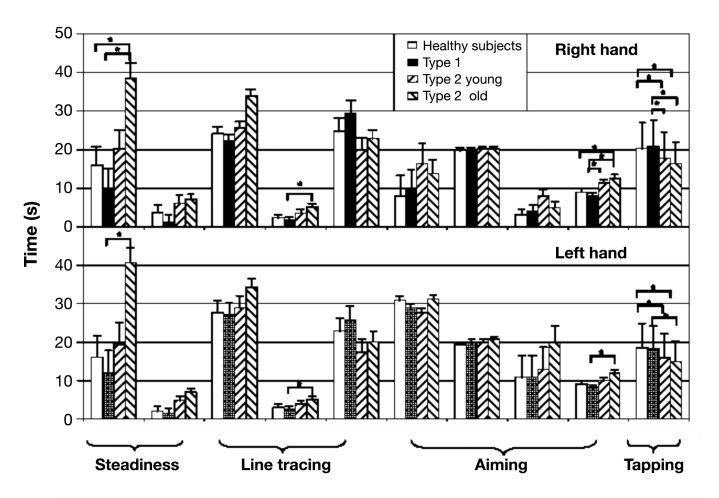
For the analysis of the MPS dexterity test, single subtests were summarized to four factors of fine motor abilities (hand flurry/tremor, precision of arm–hand movements, arm–hand speed, and wrist–finger speed). The scores (means ± standard deviation) for the individual subtests are provided in Figure 3 . Group differences on the MPS factors tremor and wrist–finger speed reached significance with T2DM (old) patients showing significantly lower values than T1DM patients (p < .001). For wrist–finger speed, T2DM patients older than 70 years additionally show higher values than healthy controls (p < .001).
Figure 3.
Results of the individual items of the MPS. Values are given as mean values ± standard deviation. The asterisk represents p < .001.
To check whether both JHFT and MPS are measuring related constructs, bivariate correlations were computed as well as a principal component analysis for all MPS and JHFT measures. There were mixed results with the whole range from low to medium-high correlations present. However, these results have to be seen as preliminary and can be interpreted only with caution for this analysis since the number of patients was rather small.
In order to investigate a potential effect of neuropathy on the obtained results, sensory perception thresholds were determined by means of the MEDOC TSA 2001 device. The results of these tests are summarized in Table 2 . Since assumption of normality seemed to be violated in some groups, for consistency of results Kruskal–Wallis tests were performed for all five variables. In five related tests, the hypothesis was tested that groups do not differ in neuropathy. According to Holland and DiPonzio Copenhaver,16 the α level was corrected to 0.0102. With this approach, highly significant differences (p < .001) between the healthy controls and the T2DM patients were identified for both sensitivity tests and the vibration perception test.
Table 2.
Summary of the Neuropathy Test Scores for All Four Study Groups
| Variable | Group A | Group B | Group C | Group D |
|---|---|---|---|---|
| Healthy control (40–70 years) | T1DM (<60 years) | T2DM (40–70 years) | T2DM (>70 years) | |
| Sensitivity heat right hand | 34.2 ± 1.1 | 34.1 ± 2.1 | 35.1 ± 1.6 | 36.2 ± 3.2 |
| Sensitivity cold left hand | 30.5 ± 0.8 | 29.9 ± 3.6 | 30.0 ± 1.0 | 29.2 ± 1.5a |
| Pain heat right hand | 46.1 ± 3.8 | 45.4 ± 4.5 | 47.5 ± 2.5a | 45.5 ± 4.3 |
| Pain cold left hand | 11.6 ± 8.2 | 11.7 ± 9.2 | 9.2 ± 8.7 | 12.3 ± 9.3 |
| Vibration right finger ball | 1.6 ± 1.3 | 2.1 ± 3.9 | 3.8 ± 3.4a | 3.1 ± 1.7a |
p < .05 versus healthy control.
Discussion
In our cross-sectional study with different groups of diabetes patients, we investigated motoric skills that may have an influence on the performance of complex routine diagnostic and therapeutic procedures, such as blood glucose measurements or insulin injections. In any case, the results of this pilot investigation need to be interpreted with caution. Mean age differed significantly in the four study groups. In this respect, an interesting result of this study is that T1DM patients and nondiabetic controls performed similar in the dexterity tests, although the T1DM cohort was about 10 years younger. In turn, psychomotor function seems to be less affected by hypoglycemia in patients with T1DM as compared to healthy subjects.5
It has been shown previously that patients with neuropathy and visual impairment have tactual deficits.17 Highly significant differences in neuropathy were identified for both sensitivity tests and for the vibration perception test versus controls. Sensitivity measures show similar results for healthy subjects and T1DM patients group, with mean diabetes duration of 23 years. Interestingly, heat applied to the right hand showed opposite results than cold applied to the left hand. Patients with type 2 diabetes—especially the older population—had a higher prevalence of neuropathy. The differences were most pronounced for vibration perception at the finger ball. Cederlund and associates7 have investigated hand disorders, symptoms, overall hand function, activities of daily living, and life satisfaction in elderly men with T2DM, impaired glucose tolerance, and normal glucose tolerance. They showed that hand disorders and impaired vibrotactile sense in fingertips occurred in patients with diabetes but not in those with impaired glucose tolerance, although limited joint mobility was observed in both groups. A longer duration of diabetes was associated with more severe neuropathy. Life satisfaction was high, and hand disorders did not have a significant impact on activities of daily living.
Our dexterity tests showed consistent results (some reaching significance) for the JHFT; the dominant hand assessment shows that patients with T2DM older than 70 years of age perform worse in most subtests than nondiabetic controls. Emphasizing very fine motor skills, the MPS seems to add a different piece of information: it was observed that, in two out of four subscales (“tremor” and “wrist–finger speed”), again the T2DM patients older than 70 years of age performed worse than controls. It is to be expected that the higher prevalence of neuropathy is interfering with these results. Studies with nondiabetic patients with hereditary motoric and sensoric neuropathy also applying the JHFT have shown major limitations in activities related to upper extremity function in 25% of this population.18,19
This was a pilot study with a limited number of patients, and the study was conducted to collect first data on dexterity and confounding variables in patients with insulin-treated diabetes mellitus. Levels of significance should not be overestimated and significant results could not be expected.
However, we believe that the following conclusions can be drawn from our results:
-
1.
The JHFT and the MPS are simple, established methods and may be suitable for dexterity assessment in patients with diabetes mellitus. It has to be investigated in future studies whether it is possible to generate a link between these performance measures and safety or performance constraints for diabetes patients. Also, patients with visual impairment should be included in these evaluations because of the major impact of this condition on dexterity skills.20
-
2.
This pilot data set provides first results on dexterity measures for insulin-treated T1DM and T2DM patients compared to nondiabetic controls. The results show that reduced dexterity skills were common in T1DM and in both groups of T2DM patients. In further studies, these results should be confirmed and study protocols should be adapted in line with results of this pilot study.
In conclusion, insulin-treated T1DM and T2DM patients showed different degrees of impaired results regarding dexterity and nerve function as compared to a healthy control group. These factors may influence the outcome of complex diagnostic and treatment procedures routinely performed by these patients in daily life, such as SMBG and insulin injections. The tests used in this investigation should be more intensively investigated in future clinical trials, because impaired dexterity and sensory nerve function may be more prevalent, even in younger patients with T1DM. It may be worthwhile to take this into consideration when new devices are being developed for blood glucose measurement and insulin delivery.
Acknowledgments
The authors thank all participating patients, physicians, and study nurses from IKFE clinic and research laboratory who participated in the study.
Abbreviations
- JHFT
Jebsen–Taylor hand-function test
- MPS
motoric performance series
- SMBG
self-monitoring of blood glucose
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
References:
- 1.American Diabetes Association. Clinical treatment recommendations 2009. Diabetes Care. 2009;32:S1–95. [Google Scholar]
- 2.Snoek F. Barriers to good glycaemic control: the patient’s perspective. Int J Obes Relat Metab Disord. 2000;24(Suppl 3):S12–S20. doi: 10.1038/sj.ijo.0801421. [DOI] [PubMed] [Google Scholar]
- 3.Adili F, Larijani B, Haghighatpanah M. Diabetic patients: psychological aspects. Ann N Y Acad Sci. 2006;1084:329–349. doi: 10.1196/annals.1372.016. [DOI] [PubMed] [Google Scholar]
- 4.Geddes J, Deary IJ, Frier BM. Effects of acute insulin-induced hypoglycaemia on psychomotor function: people with type 1 diabetes are less affected than non-diabetic adults. Diabetologia. 2008;51(10):1814–1821. doi: 10.1007/s00125-008-1112-y. [DOI] [PubMed] [Google Scholar]
- 5.Schächinger H, Cox D, Linder L, Brody S, Keller U. Cognitive and psychomotor function in hypoglycemia: response error patterns and retest reliability. Pharmacol Biochem Behav. 2003;75(4):915–920. doi: 10.1016/s0091-3057(03)00167-9. [DOI] [PubMed] [Google Scholar]
- 6.Casanova JE, Casanova JS, Young MJ. Hand function in patients with diabetes mellitus. South Med J. 1991;84(9):1111–1113. doi: 10.1097/00007611-199109000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Cederlund RI, Thomsen N, Thrainsdottir S, Eriksson KF, Sundkvist G, Dahlin LB. Hand disorders, hand function, and activities of daily living in elderly men with type 2 diabetes. J Diabetes Complications. 200;23(1):32–39. doi: 10.1016/j.jdiacomp.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Dahlin LB, Thrainsdottir S, Cederlund R, Thomsen NO, Eriksson KF, Rosén I, Speidel T, Sundqvist G. Vibrotactile sense in median and ulnar nerve innervated fingers of men with type 2 diabetes, normal or impaired glucose tolerance. Diabet Med. 2008;25(5):543–549. doi: 10.1111/j.1464-5491.2008.02433.x. [DOI] [PubMed] [Google Scholar]
- 9.Bovend’Eerdt TJ, Dawes H, Johansen-Berg H, Wade DT. Evaluation of the modified Jebsen test of hand function and the University of Maryland Arm Questionnaire for Stroke. Clin Rehabil. 2004;18(2):195–202. doi: 10.1191/0269215504cr722oa. (2004) [DOI] [PubMed] [Google Scholar]
- 10.Ringendahl H. Factor structure, normative data and retest-reliability of a test of fine motor functions in patients with idiopathic Parkinson‘s disease. J Clin Exp Neuropsychol. 2002;24(4):491–502. doi: 10.1076/jcen.24.4.491.1031. [DOI] [PubMed] [Google Scholar]
- 11.Schoppe KJ. Das MLS-Gerät: ein neuer Testapparat zur Messung feinmotorischer Leistungen. Diagnostica. 1974;20:43–47. [Google Scholar]
- 12.Sturm W, Büssing A. Ergänzende Normierungsdaten und Retest-Reliabilitätskoeffizienten zur Motorischen Leistungsserie (MLS) nach Schoppe. Diagnostica. 1985;31:234–245. [Google Scholar]
- 13.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardised test of hand function. Arch Phys Med Rehab. 1969;50:311–319. [PubMed] [Google Scholar]
- 14.Hackel ME, Wolfe GA, Bang SM, Canfield JS. Changes in hand function in the aging adult as determined by the Jebsen test of hand function. Phys Ther. 1992;72(5):373–377. doi: 10.1093/ptj/72.5.373. [DOI] [PubMed] [Google Scholar]
- 15.Fleishman EA. Structure and measurement of psychomotor abilities. In: Singer RN, editor. The psychomotor domain. Philadelphia: Lea and Febiger; 1972. pp. 78–196. [Google Scholar]
- 16.Holland B, DiPonzio Copenhaver M. An improved sequentially rejective Beonferroni test procedure. Biometrics. 1987;43(2):417–423. [Google Scholar]
- 17.Travieso D, Lederman SJ. Assessing subclinical tactual deficits in the hand function of diabetic blind persons at risk for peripheral neuropathy. Arch Phys Med Rehabil. 2007;88(12):1662–1672. doi: 10.1016/j.apmr.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Videler AJ, Beelen A, van Schaik IN, de Visser M, Nollet F. Manual dexterity in hereditary motor and sensory neuropathy type 1a: severity of limitations and feasibility and reliability of two assessment instruments. J Rehabil Med. 2008;40(2):132–136. doi: 10.2340/16501977-0143. [DOI] [PubMed] [Google Scholar]
- 19.Videler AJ, Beelen A, Nollet F. Manual dexterity and related functional limitations in hereditary motor and sensory neuropathy. An explorative study. Disabil Rehabil. 2008;30(8):634–638. doi: 10.1080/09638280701400755. [DOI] [PubMed] [Google Scholar]
- 20.Uslan M, Blubaugh M. Analysis: including visual impaired participants in validation design studies of diabetes technology. J Diabetes Sci Technol. 2010;4(5):1236–1237. doi: 10.1177/193229681000400524. [DOI] [PMC free article] [PubMed] [Google Scholar]