Abstract
Background
Cardiovascular autonomic neuropathy (CAN) is associated with diabetes and may be related to the development of hypertension, ischemic stroke, and a number of other late complications. The earliest sign of CAN is a reduction of heart rate variability (HRV). Standard HRV tests for CAN include expiration-to-inspiration ratio, response to active standing (30:15), and the Valsalva maneuver. Because of the technical requirements for these tests, they are limited to the point-of-care office or a clinical laboratory setting. It is unknown if a “white-coat“ phenomenon exists in autonomic neuropathy testing and if home testing is feasible. The aims of this study were (1) to evaluate the reproducibility of CAN testing in a clinical setting, (2) to evaluate the feasibility of self-monitoring of cardiovascular autonomic function at home, and (3) report possible differences in measurements taken at the hospital versus those taken at home.
Method
Ten healthy subjects were included. Participants underwent in-hospital testing for CAN before and after home monitoring. For 6 consecutive days, participants measured autonomic function once a day at home. The intra- and interindividual reproducibility was determined by coefficient of variation (CV) and the reproducibility coefficient (RC). Agreement between hospital and home testing was analyzed using Pearson r, mean difference, and Bland–Altman analysis with Pitman’s test of difference in variance.
Results
Pitman’s test showed no significant difference in variance between hospital and home measurements, indicating suitable agreement between the two measurements. Reproducibility was moderate to high in all measures, with RC ranging from 66–94% and CV ranging from 5–10%.
Conclusions
Home testing of CAN is feasible. The evaluations showed no significant systematic error of in-hospital testing compared with self-monitoring at home. In this study, we were not able to demonstrate the presses of “white coat” effect in standard cardiovascular reflex tests.
Keywords: autonomic neuropathy, cardiac, complications, diabetes, neuropathy, risk stratification
Background
Cardiovascular autonomic neuropathy (CAN) is a common and serious complication in both type 1 and type 2 diabetes. It affects heart rhythm and may lead to a significant deterioration of the patient’s quality of life, including increased mortality.1,2 It is estimated that 25% of patients with type 1 and 33% of patients with type 2 diabetes have CAN. The effects on heart rhythm may play a role in the “sudden death in bed syndrome“ described in diabetes patients.3
The diagnosis of CAN is commonly divided into two categories, either clinically by the presence of symptoms and/or signs or subclinically on the basis of cardio-vascular reflex tests.3 The subclinical diagnosis is based on measurements of heart rate variability (HRV) during three cardiovascular reflex tests, all predominantly testing the parasympathetic nervous system, which is affected earlier by CAN than the sympathetic nervous system.4 These three tests comprise (1) expiration-to-inspiration ratio (E:I), (2) the Valsalva maneuver, and (3) the response to active standing ratio (30:15). All tests are validated, reliable, and reproducible; they correlate with each other and test for the somatic nerve function.5 Each test can be described as a normal, border-line, or abnormal test.1,6–9 Using American Diabetes Association criteria, the results are classified as follows: 0, all tests are normal; 1, one test is abnormal, signifying the presence of borderline CAN; and 2–3, two or three tests abnormal, signifying definite CAN. At least two of the three tests should be performed to provide adequate diagnostic information.10
It is recommended that the stage of CAN is determined by the tests described here at the time of diagnosis in type 2 diabetes patients and 5 years after diagnosis in type 1 diabetes patients. Hereafter, annual testing is recommended.
Early diagnosis of CAN may be achieved by annual monitoring for this condition, thereby ensuring timely intervention and treatment. The finding of CAN in a patient suffering symptoms such as sexual dysfunction, dyspepsia, vomiting, diarrhea, and dizziness furthermore supports the clinician in diagnosing diabetic autonomic neuropathy.10 Publications have described an association between autonomic dysfunction and the development of hypertension and ischemic stroke. One paper speculates that testing may be helpful in predicting future stroke events in risk patients.11,12
Testing is scarcely available in hospitals and even less so in private practices despite the described benefits of diagnosing CAN early. The main reasons for this is time consumption with the existing costly stationary technical setups requiring laboratory space and specially trained technicians. Presently, testing is only recommended at the point-of-care office or in a clinical laboratory setting, and this may be due to the stationary complicated technical setups available today, whereas the technology described in this article may allow for home monitoring as well as in-hospital testing.4 We have previously presented and evaluated a handheld, pocket-size device for measuring autonomic reflex test based on electrocardiogram (ECG) recordings.13 Despite recommendations for annual measurements of autonomic function testing, clinical studies for the presence of “white-coat effect“ distinguished from standard reflex tests have never been performed. Blood pressure charges affect autonomic tone and vice versa, and several studies have shown that autonomic dysregulation is present in hypertension.14,15 It is documented that both 24 h and home blood pressure has significantly improved reproducibility and predicts cardiovascular events more accurately than ambulatory blood pressure. It is unknown if a “white-coat phenomenon” exists in autonomic testing and if home testing is feasible. Therefore, the aims of this study were to (1) evaluate the reproducibility of CAN testing in a clinical setting, (2) evaluate the feasibility of self-monitoring of cardio-vascular autonomic function at home, and (3) report possible differences in measurements taken at the hospital versus those taken at home.
Methods
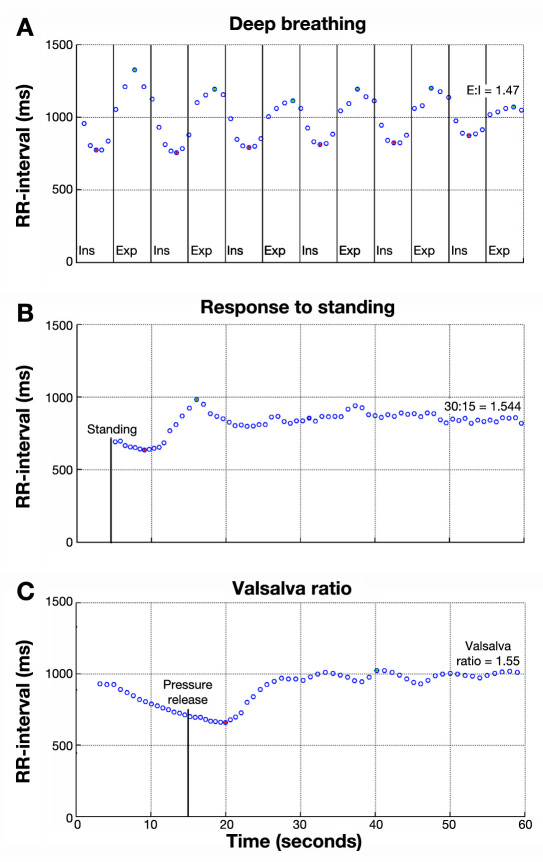
Ten healthy volunteers were included. None of the volunteers were smokers, were on medication, or suffered from chronic diseases. All subjects underwent thorough clinical examinations, including echocardiography (Vivid 5, GE Healthcare). Food and caffeine-containing liquids were prohibited 2 h prior to both home and laboratory testing. All laboratory tests of CAN were performed between 9:00 and 12:00 am in a quiet, isolated examination room. All subjects were, after 5 min supine resting, examined with three cardiovascular function tests, including (1) heart rate response going from lying to standing to examine maximum and minimum R–R interval after 1 min of standing (30:15); (2) deep breathing for 1 min with a respiration frequency of 6 breaths/min to examine the E:I, where E:I was expressed as the mean of the lowest R–R intervals during inhalation divided by the mean of the highest R–R intervals during exhalation; and (3) Valsalva maneuver to determine the ratio of maximum and minimum R–R interval during forced expiration in 15 s against a fixed resistance of 40 mm Hg and 45 s of normal breathing. Each of the three tests was separated by a 2 min resting period. The in-hospital testing for CAN was performed by a technician before and after 6 days of home monitoring. During these 6 consecutive days, participants measured the three cardiac autonomic function tests every morning at home (Figure 1 ).
Figure 1.
Protocol for the study process.
The previous device we evaluated was able to measure and analyze HRV during two standard tests (deep breathing and lying to standing).13 The present device has a built-in pressure sensor that optimizes the device for the measuring and analyzing of HRV during three standard tests (deep breathing, lying to standing, and Valsalva). A specially designed mouthpiece should be connected to the device with silicone tubing when performing the Valsalva maneuver. The mouthpiece has a small air leakage to prevent closure of the glottis, thus ensuring that the expiratory pressure was transmitted to the chest. Both devices are based on two hand electrodes for recording the ECG with a sampling frequency of 1000 Hz as recommended.16 The volunteer places one hand each on two separate metal electrodes, and the device guides the user during the test and automatically records an ECG signal from which heart rate and HRV are deduced and shown in the display.
All data during the tests, including ECG measurements and the actual exhale pressure during the Valsalva maneuver, were recorded and automatically stored on a memory card in the device. To ensure correct use, the device has a built-in user interface, which actively guides the subject through all tests, giving both audio and visual commands, and finally shows the results in the display.
Statistical Analysis
The correlations between repeated measurements in hospital and between hospital and home testing were calculated using Pearson r and the mean difference between laboratory and home measurements. The Bland–Altman analysis with Pitman’s test of difference in variance was used to test the agreement between hospital and home measurements. Natural logarithm of all measurements, e.g., E:I, Valsalva, and 30:15, were performed to obtain a normal distribution. Subsequently, for the presentation of intra- and interindividual reproducibility, the ln values were back-transformed. The intra- and interindividual reproducibility was determined, both from laboratory and home measurements, by coefficient of variation [CV; CV = standard deviation (SD)within/mean × 100] and the reproducibility coefficient [RC; RC = SD2between/(SD2 between + SD2within) × 100] were calculated by analysis of variance to obtain test–retest reliability. Paired t-tests were applied and a p-value of less than 0.05 was considered statistically significant.
Results
The 10 healthy volunteers consisted of 3 females and 7 males, ages 35 ± 4 years. Subject characteristics are shown in Table 1 .
Table 1.
Subject Characteristicsa
| n | 10 |
| sex ratio M:F | 7:3 |
| age (years) | 35 ± 4 |
| Heart rate | 58 ± 10 |
| Body mass index | 26 ± 4 |
| Ejection fraction (%) | 70.5 ± 4 |
| Blood pressure (mm Hg) | 122/77 ± 8/10 |
| Q–T interval (ms) | 422.8 ± 20.2 |
| Q–T interval corrected for heart rate (ms) | 409.2 ± 17.5 |
Results are mean ± SD.
No signs of cardiac disease were found, and ejection fraction was 70.4 ± 0.5 as showed in Table 1 .
Printable test results from the prototype device are shown in Figure 2 .
Figure 2.
Printable test results from the prototype device. Green dots indicate automated detected maximum, and red dots indicate detected minimum.
A high degree of correlation between repeated measurements in hospital and between hospital and home testing was evident, as shown in Tables 2 and 3 . There were no significant differences between repeated measurements within the same session in the hospital for any test result (Table 2 ). The evaluations showed no significant difference between laboratory testing and self-monitoring at home when using standard cardiovascular reflex tests (30:15, Valsalva, and E:I; Table 3 ). As shown in Table 3 , all tests, but the response going from lying to standing (30:15), showed a tendency toward higher values when testing at home as compared to hospital testing, e.g., mean differences of hospital versus home measurements were negative, indicating a higher home measurement. The Bland–Altman analysis with Pitman’s test of difference in variance showed no significant difference in variance between hospital and home measurements, indicating suitable agreement between the two measurements. Reproducibility was moderate to high in all measures, with RC ranging from 66–94% and CV ranging from 5–10% (Table 4 ); RC less than 40% representing poor reproducibility and RC more than 75% representing good reproducibility and low values of CV representing good reproducibility.17–19
Table 2.
Repeated Measurements Separated by a 2-Minute Resting Period in the Laboratory Setting
| Test | Pearson r | Mean difference | 95% confidence interval | p |
|---|---|---|---|---|
| 30:15 | 0.85 | -0.03 | -0.08–0.01 | Not significant |
| Valsalva ratio | 0.86 | 0.01 | -0.03–0.05 | Not significant |
| E:I | 0.91 | 0.02 | -0.07–0.05 | Not significant |
Table 3.
Difference between Laboratory Testing and Self-Monitoring at Home
| Test | Pearson r | Mean difference | 95% confidence interval | p | Pitman’s test |
|---|---|---|---|---|---|
| 30:15 | 0.64 | 0.022 | -0.08–0.03 | Not significant | Not significant |
| Valsalva ratio | 0.75 | -0.071 | -0.15–0.01 | Not significant | Not significant |
| E:I | 0.90 | -0.017 | -0.06–0.02 | Not significant | Not significant |
Table 4.
Coefficient of Variation and Reliability Coefficient of Tests for Cardiovascular Autonomic Neuropathy within 1, 2, and 6 Days
| Test | n | Hospital: two measurements within one day | Hospital: two measurements within two days | Home: six measurements within six days | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mean | CV (%) | RC (%) | mean | CV (%) | RC (%) | mean | CV (%) | RC (%) | ||
| 30:15 | 10 | 1.63 | 9 | 94 | 1.55 | 9 | 77 | 1.52 | 8 | 66 |
| Valsalva ratio | 10 | 1.90 | 7 | 80 | 1.89 | 7 | 83 | 1.98 | 10 | 82 |
| E:I | 10 | 1.42 | 6 | 90 | 1.42 | 6 | 84 | 1.44 | 6 | 86 |
Discussion
The results show that it is indeed possible to test cardiac autonomic function at nonclinical venues. We have described that the prototype device is suitable for ambulatory testing and is interchangeable with the hitherto-used research-based setup.13 We now suggest that patients may even be able to perform testing of the cardiac autonomic function themselves after a short introduction to the device and the built-in user interface.
The sampling size included 10 young, healthy, well-educated volunteers naïve to this test method. The design of the methodology study was to demonstrate whether this small number of patients would demonstrate a difference between home self-monitoring and hospital testing results. A short introduction (15 min) was given to each study subject by a trained technician on how to use the device. Special emphasis was placed on the graphical and textual information on the display, assuring that the patients were able to perform the tests correctly. None of the subjects accessed the “telephone hotline“ set up for this project. In the laboratory settings, the repeatability or test–retest reliability, e.g., the variation in measurements, was taken by the same technician using the same instrument and under the same conditions. However, when participants measured at home, there was no control over the environment, and the only controlling element was the text and audio guides from the device. Despite this, the reproducibility of all three reflex tests was high; all measures showed low CV values and high RC values. Reproducibility of the standard autonomic tests has been reported previously, i.e., CV of 4–14% for Valsalva, CV of 4–8% for E:I, and CV of 6–7% for 30:15.18,20–23 Our results appear to be in agreement with these results. There was a tendency toward higher reproducibility in the laboratory setting in the response to standing and Valsalva maneuver but not the deep breathing test. Ewing and colleagues24 showed, in the case of the response to standing test, that it is not the speed of the response to standing that determines rebound bradycardia but the muscular activity involved in standing up. Lawrence and associates25 showed that spontaneous breathing during tests, e.g., active standing and Valsalva, is associated with poor repeatability. Therefore, controlled breathing after active standing and probably after pressure release in the Valsalva maneuver may improve the reproducibility and should be further investigated. Adding to this complexity, there is evidence that the reproducibility of the cardiovascular reflex tests are higher in diabetes patients than in healthy controls due to a well-described reduction in HRV in the diabetes population.26–28 Further studies in the diabetes population are therefore necessary to investigate whether or not it is of clinical relevance to perform self-monitoring of CAN.
Conclusions
This study is the first to demonstrate that self-monitoring of the cardiac autonomic function at home is feasible.
The correlation between repeated measurements in hospital and between self-monitoring and hospital testing were high in all cardiovascular reflex tests.
In this study, we were not able to demonstrate the presses of “white coat” effect in standard cardiovascular reflex tests.
Acknowledgments
The authors thank all volunteers for their time and cooperation.
Abbreviations
- CAN
cardiovascular autonomic neuropathy
- CV
coefficient of variation
- E:I
expiration-to-inspiration ratio
- ECG
electrocardiogram
- HRV
heart rate variability
- RC
reproducibility coefficient
- SD
standard deviation
References:
- 1.Vinik AI, Erbas T. Recognizing and treating diabetic autonomic neuropathy. Cleve Clin J Med. 2001;68(11):928–930. 932, 934–944. doi: 10.3949/ccjm.68.11.928. [DOI] [PubMed] [Google Scholar]
- 2.Pop-Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care. 2010;33(2):434–441. doi: 10.2337/dc09-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziegler D. Diabetic cardiovascular autonomic neuropathy: prognosis, diagnosis and treatment. Diabetes Metab Rev. 1994;10(4):339–383. doi: 10.1002/dmr.5610100403. [DOI] [PubMed] [Google Scholar]
- 4.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26(5):1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 5.Kempler P. Autonomic neuropathy: a marker of cardiovascular risk. Br J Diabetes Vasc Dis. 2003;3:84–90. [Google Scholar]
- 6.Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care. 1985;8(5):491–498. doi: 10.2337/diacare.8.5.491. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler D, Laux G, Dannehl K, Spüler M, Mühlen H, Mayer P, Gries FA. Assessment of cardiovascular autonomic function: age-related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabet Med. 1992;9(2):166–175. doi: 10.1111/j.1464-5491.1992.tb01754.x. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien IA, O’Hare P, Corrall RJ. Heart rate variability in healthy subjects: effect of age and the derivation of normal ranges for tests of autonomic function. Br Heart J. 1986;55(4):348–354. doi: 10.1136/hrt.55.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assessment: Clinical autonomic testing report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1996;46(3):873–880. [PubMed] [Google Scholar]
- 10.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D, American Diabetes Association Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 11.Ko SH, Song KH, Park SA, Kim SR, Cha BY, Son HY, Moon KW, Yoo KD, Park YM, Cho JH, Yoon KH, Ahn YB. Cardiovascular autonomic dysfunction predicts acute ischaemic stroke in patients with type 2 diabetes mellitus: a 7-year follow-up study. Diabet Med. 2008;25(10):1171–1177. doi: 10.1111/j.1464-5491.2008.02567.x. [DOI] [PubMed] [Google Scholar]
- 12.Istenes I, Keresztes K, Hermányi Z, Putz Z, Vargha P, Gandhi R, Tesfaye S, Kempler P. Relationship between autonomic neuropathy and hypertension–are we underestimating the problem? Diabet Med. 2008;25(7):863–866. doi: 10.1111/j.1464-5491.2008.02458.x. [DOI] [PubMed] [Google Scholar]
- 13.Ejskjaer N, Fleischer J, Fleischer J, Jacobsen PE, Poulsen PL, Nygaard H. A pocket-size device to detect autonomic neuropathy. J Diabetes Sci Technol. 2008;2(4):692–696. doi: 10.1177/193229680800200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh JP, Larson MG, Tsuji H, Evans JC, O’Donnell CJ, Levy D. Reduced heart rate variability and new-onset hypertension: insights into pathogenesis of hypertension: the Framingham Heart Study. Hypertension. 1998;32(2):293–297. doi: 10.1161/01.hyp.32.2.293. [DOI] [PubMed] [Google Scholar]
- 15.Ayad F, Belhadj M, Pariés J, Attali JR, Valensi P. Association between cardiac autonomic neuropathy and hypertension and its potential influence on diabetic complications. Diabet Med. 2010;27(7):804–811. doi: 10.1111/j.1464-5491.2010.03027.x. [DOI] [PubMed] [Google Scholar]
- 16.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17(3):354–381. [PubMed] [Google Scholar]
- 17.Gerritsen J, TenVoorde BJ, Dekker JM, Kingma R, Kostense PJ, Bouter LM, Heethaar RM. Measures of cardiovascular autonomic nervous function: agreement, reproducibility, and reference values in middle age and elderly subjects. Diabetologia. 2003;46(3):330–338. doi: 10.1007/s00125-003-1032-9. [DOI] [PubMed] [Google Scholar]
- 18.Koskinen T, Kähönen M, Jula A, Laitinen T, Keltikangas-Järvinen L, Viikari J, Välimäki I, Raitakari OT. Short-term heart rate variability in healthy young adults: the Cardiovascular Risk in Young Finns Study. Auton Neurosci. 2009;145(1-2):81–88. doi: 10.1016/j.autneu.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Bolger C, Sheahan N, Coakley D, Malone J. High frequency eye tremor: reliability of measurement. Clin Phys Physiol Meas. 1992;13(2):151–159. doi: 10.1088/0143-0815/13/2/007. [DOI] [PubMed] [Google Scholar]
- 20.Risk M, Bril V, Broadbridge C, Cohen A. Heart rate variability measurement in diabetic neuropathy: review of methods. Diabetes Technol Ther. 2001;3(1):63–76. doi: 10.1089/152091501750220028. [DOI] [PubMed] [Google Scholar]
- 21.Piha SJ, Puukka P, Seppänen A. Short- and long-term reproducibility of cardiovascular tests of autonomic function in normal subjects. Clin Auton Res. 1991;1(2):115–118. doi: 10.1007/BF01826206. [DOI] [PubMed] [Google Scholar]
- 22.Maser RE, Lenhard MJ. Obesity is not a confounding factor for performing autonomic function tests in individuals with diabetes mellitus. Diabetes Obes Metab. 2002;4(2):113–117. doi: 10.1046/j.1463-1326.2002.00188.x. [DOI] [PubMed] [Google Scholar]
- 23.Maser RE, Pfeifer MA, Dorman JS, Kuller LH, Becker DJ, Orchard TJ. Diabetic autonomic neuropathy and cardiovascular risk. Pittsburgh Epidemiology of Diabetes Complications Study III. Arch Intern Med. 1990;150(6):1218–1222. [PubMed] [Google Scholar]
- 24.Ewing DJ, Hume L, Campbell IW, Murray A, Neilson JM, Clarke BF. Autonomic mechanisms in the initial heart rate response to standing. J Appl Physiol. 1980;49(5):809–814. doi: 10.1152/jappl.1980.49.5.809. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence GP, Home PD, Murray A. Repeatability of measurements and sources of variability in tests of cardiovascular autonomic function. Br Heart J. 1992;68(2):205–211. doi: 10.1136/hrt.68.8.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Javorka M, Javorková J, Tonhajzerová I, Calkovska A, Javorka K. Heart rate variability in young patients with diabetes mellitus and healthy subjects explored by Poincaré and sequence plots. Clin Physiol Funct Imaging. 2005;25(2):119–127. doi: 10.1111/j.1475-097X.2004.00601.x. [DOI] [PubMed] [Google Scholar]
- 27.Krönert K, Luft D, Baumann B, Müller PH, Eggstein M. Reduced intraindividual variability of repeated cardiovascular reflex tests: an additional marker of autonomic neuropathy in insulin-dependent (type I) diabetes mellitus? Acta Diabetol Lat. 1986;23(4):279–289. doi: 10.1007/BF02582061. [DOI] [PubMed] [Google Scholar]
- 28.Burger AJ, Charlamb M, Sherman HB. Circadian patterns of heart rate variability in normals, chronic stable angina and diabetes mellitus. Int J Cardiol. 1999;71(1):41–48. doi: 10.1016/s0167-5273(99)00110-2. [DOI] [PubMed] [Google Scholar]