Abstract
Background
Self-monitoring of blood glucose (SMBG) remains an important component of diabetes management, engendering a need for affordable blood glucose (BG) meters that are accurate, precise, and convenient. The CONTOUR® TS is a BG meter that endeavors to meet this need. It uses glucose dehydrogenase/flavin dinucleotide chemistry, automatic test strip calibration, and autocompensation for hematocrit along with the ease of use that has come to be expected of a modern meter. The objective of this clinical trial was to determine whether the CONTOUR TS system met these criteria.
Methods
The system was evaluated at a single clinical site with 106 subjects with type 1 or type 2 diabetes. Blood glucose values ranged from 60 to 333 mg/dl over all subjects. Both lay users and health care professionals (HCPs) tested the meters, with test strips from three different lots. Results were compared to a reference analyzer of verified precision and accuracy. Forty-nine of the subjects also participated in a home study of the meter. Lay users learned to use the system without assistance and were surveyed on its use at the end of the study.
Results
When used with capillary blood, both subjects and HCPs obtained results that exceeded the International Organization for Standardization 15197:2003 criteria, (i.e., ≥95% of values fell within 20% or 15 mg/dl of the laboratory value for BG levels greater than or less than 75 mg/dl, respectively). Specifically, lay users achieved 97.9% and HCPs 98.6%. When used with venous blood, 99.8% of measurements were within the criteria. All measurements for both capillary and venous blood fell into zones A or B of the Parkes error grid, deemed clinically accurate. Hematocrit was found to have no influence on BG measurements. A large majority of the subjects found the system easy to learn and to use.
Conclusions
The CONTOUR TS BG meter system gave accurate and reproducible results with both capillary and venous blood; subjects learned to use the meter system by following the user guide and quick reference guide.
Keywords: accuracy of blood glucose meters, blood glucose meter, CONTOUR TS, diabetes, hematocrit, no coding, self-monitoring of blood glucose
Introduction
The importance and efficacy of self-monitoring of blood glucose (SMBG) for people with diabetes has been discussed and advocated in a variety of forums.1–4 According to a consensus paper of Hirsch and colleagues,2 SMBG remains the mainstay of diabetes self-management for the majority of patients with type 1 or type 2 diabetes. Both short- and long-term outcomes of self-monitoring are affected by daily actions taken by patients. It is important to consider the performance of blood glucose (BG) meter systems in the context of patients’ information, skills, and knowledge of how to utilize SMBG most effectively to inform their self-management decisions. Optimal utilization of SMBG can lead to improvements in glycemic control by providing patients with immediate feedback on the impact of food, exercise, or medication on their blood sugar.5 Therefore, a simple-to-use, accurate meter can be of benefit.
The CONTOUR® TS meter is one version of the CONTOUR family of products from Bayer. Like CONTOUR, it uses a glucose dehydrogenase (GDH)/flavin dinucleotide (FAD) chemistry, with no interference from maltose or galactose, thus making it suitable for patients on peritoneal dialysis using icodextrin and with patients on maltose-containing immunoglobulins. In addition, it compensates for many other common interfering substances. The GDH/FAD chemistry is insensitive to oxygen, which supports its use in testing blood from various sources (arterial, venous, capillary).
The meter has been cleared for use on alternate anatomical sites (palm and forearm). This may be important to the 35% of diabetes sufferers who report that the discomfort of a finger stick is a barrier to SMBG.6
The CONTOUR TS requires a small blood sample (0.6 μl),and its test strips have a separate electrode that measures hematocrit so that the meter reports a hematocrit-compensated BG. Results are available in 8 seconds. The CONTOUR TS BG monitoring system employs No-CodingTM technology that eliminates miscoding errors that could lead to inaccurate results.7,8 The meter automatically sets to the correct code whenever a new CONTOUR TS strip is inserted. The elimination of this step also contributes to the ease of use of the BG system. Blood glucose levels from 10 to 600 mg/dl can be measured and 250 results can be stored in the meter’s onboard memory. When used with a CONTOUR TS control solution, rather than a blood sample, the system automatically notes, with a checkmark, that it is a control solution test and blocks the use of the measurement from its 14-day averaging calculations.
This article describes a clinical trial that evaluated the performance and ease-of-use of the CONTOUR TS system for BG measurements of capillary and venous blood.
Study Design and Methods
The study included 106 people with diabetes and 4 super-vising health care professionals (HCPs). An institutional review board approved the study protocol, and all subjects completed the informed consent process.
The CONTOUR TS was the only SMBG meter used in the study. Following the viewpoint of most regulatory authorities in the United States and elsewhere, as well as standards committees such as the Clinical and Laboratory Standards Institute, comparisons were restricted to an accepted laboratory instrument (in this case, YSI) that was calibrated using traceable controls, rather than to other SMBG meters. While there are undoubtedly advantages to comparative performance assays among meters, oversight agencies, such as the Food and Drug Administration, discourage such comparisons for a number of reasons; chief among them is the potential for perceived, or real, bias, especially when the work is sponsored by an interested party, such as the manufacturer. Since all SMBG systems must be compared with an accepted laboratory assay in order to gain clearance to be marketed, one can make his/her own assessment of which meter has the best performance, since this data is in the public domain.
Subjects familiarized themselves with the CONTOUR TS system in a clinical setting using only the user guide and quick reference guide, after which a HCP observed and rated each subject’s ability to perform a range of meter tasks. The subjects were rated on a four-point scale with a score of 1, indicating the subject completed the task with no assistance, to a score of 4, indicating the subject needed assistance from the HCP. Each subject then performed two finger sticks pursuant to making four capillary blood measurements (two in duplicate, using strips from two different lots from a total of three lots used in the study). Subjects also carried out control solution measurements on three test solutions of low, normal, and high glucose, marketed with the CONTOUR TS, in order to assure that the meter was operating properly. These are the only control solutions approved for use with the CONTOUR TS meter system. For the reference instrument (YSI), we used control sera that had been assayed by a method traceable to one proposed for use as a national glucose reference method developed through the Centers for Disease Control, the National Bureau of Standards, the American Association for Clinical Chemistry, and the Food and Drug Administration.9
Since there are safety concerns with creating hypoglycemia in subjects in order to obtain blood of low glucose concentration, we also conducted separate studies with “contrived” blood samples in a laboratory setting. That is, blood samples from donors were allowed to undergo glycolysis to remove endogenous glucose, and then various amounts of glucose were added to the samples to create an array of samples covering a large range of glucose concentrations. The contrived samples were then assayed on the CONTOUR TS meter as well as on a laboratory instrument (YSI). This procedure extended the range of BG values beyond what we found in the subject population, which did not contain any samples below 60 mg/dl. Using these contrived samples, we verified that the CONTOUR TS met the International Organization for Standardization (ISO) criteria before embarking on the clinical trial (data not shown).
The HCPs performed four capillary blood measurements (two strip lots in duplicate) on the meter using blood from each subject. They also performed a deep finger puncture on each subject to obtain sufficient capillary blood to assay on a YSI 2300 Stat Plus Analyzer (Yellow Springs, Ohio) for comparison. All YSI measurements were performed using plasma obtained by centrifugation of the capillary finger-stick blood samples.
In addition, a venipuncture to obtain blood for testing on the CONTOUR TS meter and the reference YSI was successfully drawn from 104 subjects. The HCP performed six meter measurements (three strip lots in duplicate) from each subject’s venous blood samples.
Prior to the testing procedures, HCPs measured the ambient temperature and humidity to verify that the local conditions were within the operating range of the CONTOUR TS system (5–45 oC; 10–93% humidity).
In another phase of the study, the first 49 subjects who agreed to participate took the CONTOUR TS meters home and tested fingertip blood three times per day for 7 to 10 days. This was done to evaluate the system in the home setting.
After the subjects and HCPs completed the clinical part of the study, they completed questionnaires designed to gauge their subjective experience with the CONTOUR TS system.
Capillary and venous blood from the subjects was analyzed on a YSI, the accuracy and precision of which was assured with traceable glucose control solutions.
Blood from finger punctures assayed on the CONTOUR TS meter was compared with capillary plasma assayed on the YSI, whereas venous blood assayed on the CONTOUR meter was compared with venous plasma assayed on the YSI.
Hematocrit was determined for each subject using the StatspinTM Microcentrifuge and Critspin Reader (Statspin Technologies, Norwood, MA).
Comparison of the investigational meter and laboratory values were performed by regression analysis using the method of Passing and Bablok,10 by Parkes error grid analysis,11 and by following system accuracy presentation and assessments described in ISO 15197.12
We used the Parkes (or Consensus) error grid11 rather than the older Clarke error grid,13 in order to take advantage of the collective expertise of the 100 endocrinologists who were surveyed in order to create it. The older error grid has been criticized on the basis of the placement of its risk boundaries,14 some of which skip risk categories. The Parkes (Consensus) error grid retained the five-risk-level format of the Clarke grid, but the definitions of the risk levels were slightly altered to align with the expert opinions, and none of the boundaries skip risk levels. It has become widely referenced and employed in many analyses, publications, and regulatory and standards documents, including ISO 19157:2003, which is the main Standard recognized by the BG monitoring community.
Results
Demographics
There were 63 women and 43 men in the overall study population, ranging in age from 20 to 74 (median age 54). Twelve had type 1 diabetes; the remaining 94 had type 2 diabetes. All reported that they monitor their BG at home. The home-study subgroup of 49 subjects had a median age of 51. Demographic data are summarized in Table 1 .
Table 1.
Subject Demographics (n = 106)
| In clinic | Home | |
|---|---|---|
| Number of subjects | 106 | 49 |
| Median age (range in years) | 54 (20 to 79) | 51 (20 to 74) |
| Number of females | 63 | 31 |
| Number of males | 43 | 18 |
| Race/ethnic origin | ||
| African American | 7 | 2 |
| Latino | 3 | 1 |
| Caucasian | 92 | 44 |
| Other | 4 | 2 |
| Educational level | ||
| Less than high school | 12 | 3 |
| High school | 44 | 25 |
| Some college or technical school | 26 | 11 |
| College degrees | 20 | 6 |
| Graduate degree | 4 | 4 |
| Type of diabetes | ||
| Type 1 | 12 | 7 |
| Type 2 | 94 | 42 |
| Diabetes therapy | ||
| Insulin | 25 | 12 |
| Oral agents | 64 | 29 |
| Combination therapy | 12 | 4 |
| Meal plan and exercise only | 5 | 4 |
CONTOUR TS System Performance with Control Solutions
The subjects made a total of 212 measurements with each of the control solutions (low, normal, and high). Ninety-nine percent (low) and 100% (normal and high) were within the recommended range, and all of the measurements were correctly identified as control solutions and flagged with a check mark by the meter.
Performance with Capillary Blood
In the clinic, a total of 420 finger-stick SMBG measurements were made by the subjects, and 418 measurements were made by the HCPs using finger-stick blood from the subjects. The BG values ranged from 70.5 to 333.3 mg/dl. All measurements were performed in duplicate and the average within-run coefficient of variation (CV) was determined as a measure of precision. The combined average CV across the three test strip lots was 6% for the subjects and 5.3% for the HCPs.
The CONTOUR TS results were compared to the YSI results and found to be in good agreement. The correlation coefficient was 0.97 for subjects’ self-measurements and 0.98 for the HCPs, with y intercepts of -1.73 mg/dl or less. The results were well within the ISO criteria of 20% or 15 mg/dl 95% of the time, meeting them, in fact, 97.9% and 98.6% of the time, respectively, as seen in Table 2 .
Table 2.
CONTOUR TS Results within ISO 15197 Performance Criteria
| Blood sample | Meter operator | Percentage of results within | |
|---|---|---|---|
| ±20% or ±15 mg/dl of YSI result | ±15% or ±10 mg/dl of YSI result | ||
| Capillary | Subject | 97.9% (411/420) | 91.7% (385/420) |
| HCP | 98.6% (412/418) | 93.3% (390/418) | |
| All results | 98.2% (823/838) | 92.5% (775/838) | |
| Venous | HCP | 99.8% (623/624) | 97.9% (611/624) |
We carried out a more detailed comparison of the meter and YSI values, as recommended in the ISO document, and found that 91.7% and 93.3 % of the results (respectively) were within 15% or 10 mg/dl of the YSI values (Table 2 ).
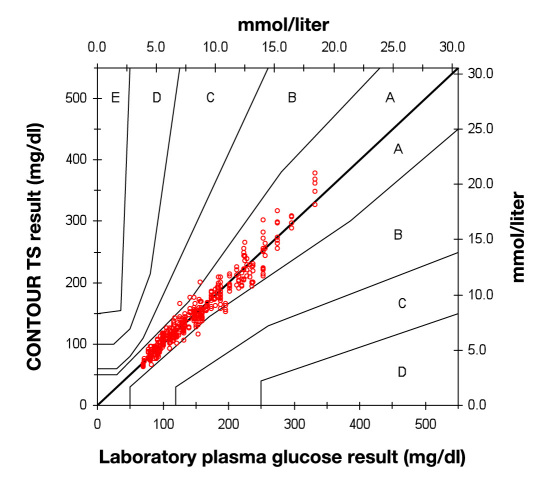
Capillary blood results were subjected to error grid analysis.11 A total of 97.1% of subjects’ measurements were in zone A (no effect on clinical action). The remainder were in zone B (altered clinical action with little or no effect on clinical outcome). The results are shown in Figure 1 . A total of 98.7% of HCPs measurements were in zone A; the remainder were in zone B (data not shown).
Figure 1.
Error grid analysis of CONTOUR TS subject capillary results (n = 420).
Performance with Venous Blood
The HCPs made a total of 624 measurement of venous blood (312 duplicates from three strip lots from 104 subjects) using the CONTOUR TS system. The BG values ranged from 60.8 to 330.5 mg/dl. The within-run CV average over the three test strip lots was 4.2 %. The meter results were in good agreement with the YSI results. We calculated a correlation coefficient of 0.99, with a y intercept of -3.79 mg/dl. A total of 99.8% of the venous blood measurements were within the ISO criteria, and 97.9% were within the 15% or 10 mg/dl cutoff (Table 2 ).
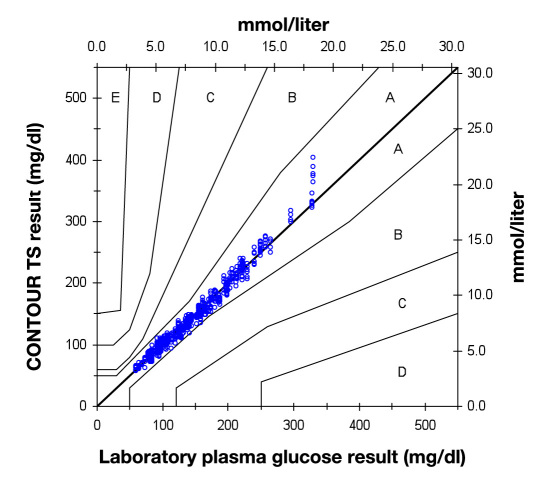
Error grid analysis of the venous blood data is shown in Figure 2 . In total, 99.8% of the measurements are in zone A, with the remainder in zone B.
Figure 2.
Error grid analysis of CONTOUR TS: venous results (n = 624).
Effect of Hematocrit
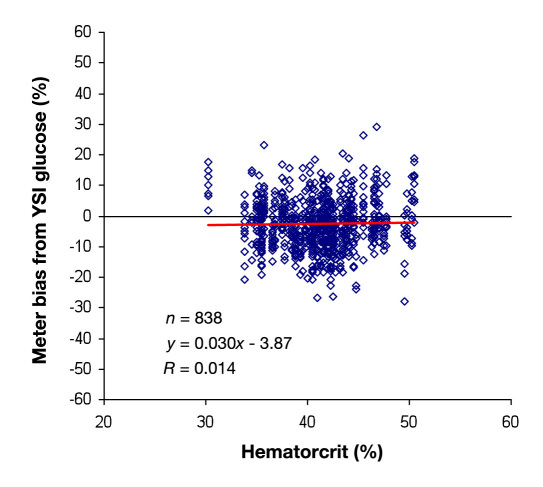
The deviations of the meter measurements from the YSI measurements are plotted against the hematocrit of the blood sample for all the data collected in the clinic (n = 838) in Figure 3 . The meter was found to be essentially unbiased by the hematocrit (correlation coefficient of 0.014).
Figure 3.
CONTOUR TS meter bias compared with hematocrit.
Ease of Use of the CONTOUR TS System in the Clinic and at Home
According to observations of the HCPs, over 99% of subjects performed the CONTOUR TS BG tests correctly with no instructions beyond the user guide and quick reference guide.
The majority of subjects were also able to complete a battery of basic tasks associated with using the meter. The most troublesome task was setting the time and date, which was mastered by 71% of the subjects without assistance.
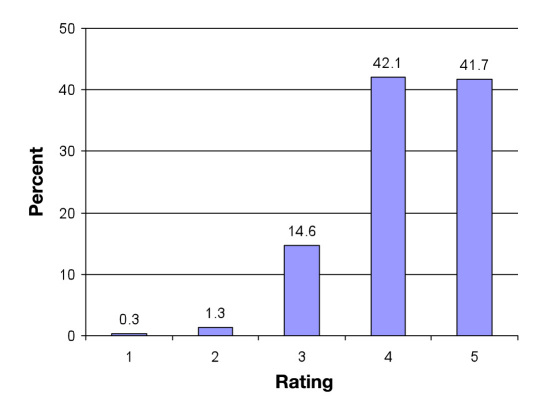
The results of subjects’ own evaluations of using the CONTOUR TS system in the clinic are summarized in Figure 4 . The subjects rated 22 features on scale of 1 to 5. In total, 83.5% of the ratings were in the “very good” or “excellent” category (i.e., scores 4 and 5). Particularly well rated were “ease of applying blood” (92%), “performing a blood test” (94%), and “no-coding technology” (89%). Ninety-six percent of the subjects reported that the CONTOUR TS system would meet their needs for self-testing.
Figure 4.
CONTOUR TS meter user satisfaction ratings. Results for 22 system features (combined) rated by 106 subjects. Rating scale: 1, unacceptable; 2, poor; 3, average; 4, very good; and 5, excellent.
The subset of subjects (49) who took CONTOUR TS meters home (to use in parallel with their personal meters) for 7–10 days were asked afterward to report on their experiences with it. In summary, 89.8% (i.e., 44/49) of the subjects reported no problems with using the CONTOUR TS meter.
Conclusions
The CONTOUR TS system is an easy-to-learn and easy-to-use basic meter in the CONTOUR family of BG monitoring systems. We report here that CONTOUR TS displays excellent accuracy and precision with our subject population of people with type 1 and type 2 diabetes. The ISO 15197:2003 performance guidelines call for 95% of measurements to be within 20% or 15 mg/dl of the reference results, and in this study, 97.9% and 98.6% of capillary measurements in the hands of lay users and HCPs, respectively, were within those limits.
When HCPs tested subjects’ venous blood, 99.8% of results were within the ISO limits. Furthermore, 92.5% of capillary and 97.9% of venous blood results were within 15% or 10 mg/dl of YSI results.
These results are reflected in the error grid analysis, which showed that 100% of the measurements were within the clinically acceptable zones A and B, with most of the data in the A zone.
The subjects, albeit regular practitioners of SMBG, were readily able to learn to use the system from the user guide and quick reference guide alone.
The accurate measurement of BG can be confounded by a number of factors, such as oxygen, extreme values of hematocrit, and reducing agents in the blood.15–17 For example, glucose-oxidase-based systems can be sensitive to blood oxygen tension, and thus, under conditions where oxygen saturation may vary, such as high altitude or certain pulmonary diseases, the accuracy of BG results may be affected. While GDH-based meters are not affected by oxygen, certain substrates of GDH may pose problems. For example, systems using GDH/pyrroloquinoline quinone chemistry will respond to sugars other than glucose, such as maltose and galactose, and thus can produce falsely elevated glucose readings in the presence of these sugars (a serious concern for patients undergoing certain therapies).
The CONTOUR TS system is based on GDH/FAD chemistry, which is insensitive to oxygen and is highly selective for glucose, thus minimizing the potential for interference.
Hematocrit is known to affect BG measurement and is generally accounted for by applying a conversion factor to whole blood measurements based on an assumed hematocrit of 45%. This can lead to significant errors when the hematocrit is far from this value. The CONTOUR TS strip has a third electrode that is used to estimate the hematocrit of each sample so that the meter can compensate for it. Our results show that, in using this approach, the meter exhibited essentially no bias due to hematocrit levels across our entire subject sample.
In addition to SMBG errors associated with the hardware, there are potentially significant errors due to human fallibility. Operator error has long been impugned as the largest source of overall error in point-of-care and home BG measurements,18,19 including errors such as improper storage of test strips, failure to clean the puncture site, and improper meter calibration. The latter, in particular, can lead to substantial errors.8 The CONTOUR TS system addresses this concern by the use of no-coding test strips, which make such miscalibration effectively impossible.
Accuracy and precision are essential criteria in choosing a BG meter. Caution should be exercised in comparing reports of meter performance, as they may not use identical study design and methodology. For example, some may compare blood from different sources (e.g., capillary versus venous) for the hand-held meter and laboratory assay, or some may use trained health professionals only, rather than lay users, to perform the meter BG measurement.
Handling of blood samples destined for laboratory analysis (e.g., time delay, temperature) is another potential source of error. While various standards for such clinical research are extensive, compliance by investigators varies, making comparisons between different studies problematic.20 Clearly, a universally accepted set of protocols is desired,21 but a consensus has yet to emerge.22 A few studies have appeared that directly compare the performance of various meters.23–25 Such studies, while valuable, are themselves potentially subject to miscues. For example, possible bias in the reference measurement may favor one meter system over another.26 In addition, if analysis is limited to a single lot of test strips for each meter, the desired statistical validity and representative performance of the system as a whole may be compromised.
With the above caveats noted, the evaluations here show that the CONTOUR TS BG monitoring system exceeds current standards for accuracy and precision. It offers no-coding technology, automatic hematocrit compensation, and a control solution detection feature. Testers found it easy to master and convenient for both home use by lay people and for point-of-care use by HCPs. The CONTOUR TS can be a useful tool to manage glycemic control for people with diabetes.
Acknowledgments
The authors thank Dr. Stephen Slatin for help in preparing the manuscript.
Abbreviations
- BG
blood glucose
- CV
coefficient of variation
- FAD
flavin dinucleotide
- GDH
glucose dehydrogenase
- HCP
health care professional
- ISO
International Organization for Standardization
- SMBG
self-monitoring of blood glucose
References:
- 1.Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002;48(3):436–472. [PubMed] [Google Scholar]
- 2.Hirsch IB, Bode BW, Childs BP, Close KL, Fisher WA, Gavin JR, Ginsberg BH, Raine CH, Verderese CA. Self-monitoring of blood glucose (SMBG) in insulin- and non-insulin-using adults with diabetes: consensus recommendations for improving SMBG accuracy, utilization, and research. Diabetes Technol Ther. 2008;10(6):419–439. doi: 10.1089/dia.2008.0104. [DOI] [PubMed] [Google Scholar]
- 3.Montagnana M, Caputo M, Giavarina D, Lippi G. Overview on self-monitoring of blood glucose. Clin Chim Acta. 2009;402(1-2):7–13. doi: 10.1016/j.cca.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Klonoff DC. Benefits and limitations of self-monitoring of blood glucose. J Diabetes Sci Technol. 2007;1(1):130–132. doi: 10.1177/193229680700100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klonoff DC, Bergenstal R, Blonde L, Boren SA, Church TS, Gaffaney J, Jovanovic L, Kendall DM, Kollman C, Kovatchev BP, Leippert C, Owens DR, Polonsky WH, Reach G, Renard E, Riddell MC, Rubin RR, Schnell O, Siminiero LM, Vigersky RA, Wilson DM, Wollitzer AO. Consensus report of the coalition for clinical research-self-monitoring of blood glucose. J Diabetes Sci Technol. 2008;2(6):1030–1053. doi: 10.1177/193229680800200612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher WA, Schachner H, Stenger P. Understanding self-monitoring of blood glucose: an information-motivation-behavioral skills analysis [abstract] Diabetes. 2010;59(suppl 1):A518. doi: 10.1177/0145721710391479. [DOI] [PubMed] [Google Scholar]
- 7.Raine CH 3rd, Pardo S, Parkes JL. Predicted blood glucose from insulin administration based on values from miscoded glucose meters. J Diabetes Sci Technol. 2008;2(4):557–562. doi: 10.1177/193229680800200404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raine CH 3rd, Schrock LE, Edelman SV, Mudaliar SR, Zhong W, Proud LJ, Parkes JL. Significant insulin dose errors may occur if blood glucose results are obtained from miscoded meters. J Diabetes Sci Technol. 2007;1(2):205–210. doi: 10.1177/193229680700100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neese JW, Duncan P, Bayse D, Robinson M, Cooper T, Stewart C. Development and evaluation of a hexokinase/glucose-6-phosphate dehydrogenase procedure for use as a national glucose reference method. Atlanta: Centers for Disease Control; 1976. [Google Scholar]
- 10.Passing H, Bablok A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem. 1983;21(11):709–720. doi: 10.1515/cclm.1983.21.11.709. [DOI] [PubMed] [Google Scholar]
- 11.Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23(8):1143–1148. doi: 10.2337/diacare.23.8.1143. [DOI] [PubMed] [Google Scholar]
- 12.ISO 15197. In vitro diagnostic test system requirements for blood glucose monitoring systems for self-testing in managing diabetes mellitus. Geneva: International Organization for Standardization; 2003. [Google Scholar]
- 13.Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10(5):622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 14.Gough DA, Botvinick EL. Reservations on the use of error grid analysis for the validation of blood glucose assays. Diabetes Care. 1997;20(6):1034–1036. doi: 10.2337/diacare.20.6.1034. [DOI] [PubMed] [Google Scholar]
- 15.Dungan K, Chapman J, Braithwaite SS, Buse J. Glucose measurement: confounding issues in setting targets for inpatient management. Diabetes Care. 2007;30(2):403–409. doi: 10.2337/dc06-1679. [DOI] [PubMed] [Google Scholar]
- 16.Tonyushkina K, Nichols JH. Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol. 2009;3(4):971–980. doi: 10.1177/193229680900300446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginsberg BH. Factors affecting blood glucose monitoring: sources of errors in measurement. J Diabetes Sci Technol. 2009;3(4):903–913. doi: 10.1177/193229680900300438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewandrowski K, Cheek R, Nathan DM, Godine JE, Hurxthal K, Eschenbach K, Laposata M. Implementation of capillary blood glucose monitoring in a teaching hospital and determination of program requirements to maintain quality testing. Am J Med. 1992;93(4):419–426. doi: 10.1016/0002-9343(92)90172-8. [DOI] [PubMed] [Google Scholar]
- 19.Bergenstal R, Pearson J, Cembrowski GS, Bina D, Davidson J, List S. Identifying variables associated with inaccurate self-monitoring of blood glucose: proposed guidelines to improve accuracy. Diabetes Educ. 2000;26(6):981–989. doi: 10.1177/014572170002600610. [DOI] [PubMed] [Google Scholar]
- 20.Mahoney J, Ellison J. Assessing the quality of glucose monitor studies: a critical evaluation of published reports. Clin Chem. 2007;53(6):1122–1128. doi: 10.1373/clinchem.2006.083493. [DOI] [PubMed] [Google Scholar]
- 21.Mahoney JJ, Ellison JM. Assessing glucose monitor performance–a standardized approach. Diabetes Technol Ther. 2007;9(6):545–552. doi: 10.1089/dia.2007.0245. [DOI] [PubMed] [Google Scholar]
- 22.Baum JM, Pardo SA, Schachner HC, Parkes JL, Simmons DA. Re-evaluating a standard approach to assessing glucose monitor performance. Diabetes Technol Ther. 2009;11(5):323–325. doi: 10.1089/dia.2008.0084. [DOI] [PubMed] [Google Scholar]
- 23.Kristensen GB, Monsen G, Skeie S, Sandberg S. Standardized evaluation of nine instruments for self-monitoring of blood glucose. Diabetes Technol Ther. 2008;10(6):467–477. doi: 10.1089/dia.2008.0034. [DOI] [PubMed] [Google Scholar]
- 24.Thomas LE, Kane MP, Bakst G, Busch RS, Hamilton RA, Abelseth JM. A glucose meter accuracy and precision comparison: the FreeStyle Flash versus the Accu-Chek Advantage, Accu-Chek Compact Plus, Ascensia Contour, and the BD Logic. Diabetes Technol Ther. 2008;10(2):102–110. doi: 10.1089/dia.2007.0244. [DOI] [PubMed] [Google Scholar]
- 25.Freckmann G, Baumstark A, Jendrike N, Zschornack E, Kocher S, Tshiananga J, Heister F, Haug C. System accuracy evaluation of 27 blood glucose monitoring systems according to DIN EN ISO 15197. Diabetes Technol Ther. 2010;12(3):221–231. doi: 10.1089/dia.2009.0128. [DOI] [PubMed] [Google Scholar]
- 26.Krouwer JS, Cembrowski GS. A review of standards and statistics used to describe blood glucose monitor performance. J Diabetes Sci Technol. 2010;4(1):75–83. doi: 10.1177/193229681000400110. [DOI] [PMC free article] [PubMed] [Google Scholar]