Abstract
Background
This pharmacokinetic (PK) study was designed to investigate the maximum intranasal insulin dose that could be achieved by repeated doses in a single nostril of a nasal spray of recombinant regular human insulin 1% in combination with cyclopentadecalactone (CPE-215) 2%, a compound that enhances absorption of molecules across mucous membranes (Nasulin™, CPEX Pharmaceuticals, Inc.).
Method
A nine-period crossover study of 8 healthy, nonsmoking subjects (ages 18–50, body mass index <33 kg/m2, weight >70 kg) were studied. In a fasted state, subjects were randomly given 25, 50, and 75 U in a single nostril on the first day and randomly given 50, 75, and 100 U doses utilizing both nostrils on two subsequent days. After a 45-minute PK assessment, subjects were given a meal. To determine the mechanism of enhanced absorption in a single nostril, a second study utilizing 24 subjects under similar conditions received 25 U, placebo (P) that included CPE-215 plus 25 U, and 50 U in a single nostril.
Results
Single nostril administration revealed enhanced absorption with maximum concentrations (Cmax) of 13, 65, and 96 µU/ml for the 25, 50, and 75 U doses, respectively. Dual nostril administration in two cohorts resulted in Cmax of 31/42, 65/52, and 88/79 µU/ml for the 50, 75, and 100 U, respectively. In the second cohort, Cmax was 23, 19, 56 µU/ml for the 25, P + 25, and 50 U doses, respectively.
Conclusions
Repeated dosing in a single nostril resulted in enhanced absorption; this was not due to the increased CPE-215 but to the increased insulin administered.
Keywords: CPE-215, cyclopentadecalactone, intranasal insulin, Nasulin, ultrarapid time-action profile
Introduction
The timely delivery of insulin in doses that match the increase in blood glucose after a meal and between meals is a therapeutic challenge. The practice of attempting to use subcutaneous injections of short term, fast-acting insulin before meals in conjunction with less frequent administrations of a longer, slower-acting formulation to mimic pancreatic insulin secretion has been able to produce adequate control in general. To the authors’ best knowledge, there is no insulin on the market with an ultrarapid-acting profile that is able to produce the initial spike of insulin produced by the pancreas in response to a meal.
CPEX Pharmaceuticals, Inc. (Exeter, NH) has developed a formulation of recombinant human insulin for nasal administration under the trade name Nasulin™. The nasal spray is composed of regular short-acting human recombinant insulin dissolved in water in combination with several common excipients [polysorbate 20, sorbitan monolaurate, cottonseed oil, and cyclopentadecalactone (CPE-215)]. The excipient CPE-215 is a compound that occurs naturally in plants (Angelica archangelica)andis a common constituent of many foodstuffs, cosmetics, and personal hygiene products (e.g., deodorants). In preclinical toxicology studies of 3-months’ duration in rats and dogs, no nasal mucosal inflammation was apparent from topical exposure.
The insulin in this formulation is absorbed very rapidly and mimics the initial spike produced by the pancreas.
Initial phase 1 and 2 studies have provided preliminary evidence of the efficacy and absorption of Nasulin at doses up to 50 U, and it appears to be well-tolerated in healthy volunteers and diabetes patients (PK008, PK007, and SC00504, data on file). Smoking1 and the normal physiologic nasal cycle2 did not have a clinically significant effect on absorption or glucodynamic effects, but with nasal route of administration, total nostril blockage decreased the absorption by approximately 50%. There have been transient nasal symptoms of irritation, tickling sensation, and sneezing that are associated with Nasulin administration. However, these symptoms last only a few minutes, are not present with all administrations nor in all patients, and tend to disappear with continued dosing. There are uncommon moderate to severe headaches associated with increased lacrimation most likely due to exposure of the sinuses during spray administration.
Unlike all other marketed insulin products, the PK profile of Nasulin is similar to that of the normal initial pancreatic insulin secretion, peaking in 15–25 minutes.3 Glucodynamic activity begins at 10–20 minutes and peaks at around 30–50 minutes. The advantage of this profile is that it will provide insulin coverage while food is being absorbed and will not be present before subsequent meals. The risk of hypoglycemia may prove to be lessened with this route of administration compared to that of injectable insulins. It may also allow a more normal anabolic-catabolic cycle, which may lead to weight stability unlike the usual weight gain experienced by patients when injectable insulins are initiated. It could lead to enhanced compliance and earlier insulin use in patients with type 2 diabetes, as well as aid those who do not wish to utilize injectable insulins in public.
The initial studies utilized doses up to 50 U, one spray of 100 µl containing 25 U in each nostril. This dose level did not produce adequate glucodynamic activity in all individuals. This study was designed to determine the maximal dose that could be administered easily in order to optimize glucodynamic activity.
Methods
This article reports on two open-label studies in healthy subjects that were conducted to determine the maximum feasible clinical dose of Nasulin and the enhanced mechanism of absorption when a single nostril was used in the initial study. The study was conducted at the Orlando Clinical Research Center, Orlando, Florida. Prior to initiation of the studies, the protocols for the studies, the written informed consent forms, as well as all written information provided to subjects were reviewed by the Independent Investigational Review Board of Plantation, Florida. All subjects provided written, informed consent.
Maximum Dose Determination Study
Eight healthy, nonsmoking subjects [ages 18–50, body mass index (BMI) <33 kg/m2, weight >70 kg] participated in the initial nine-way crossover study. Subjects were admitted the day prior to the first treatment day and remained in the clinic until the end of the last administration day. All subjects self-administered all Nasulin doses, with proper training practice on Day -1 using a placebo sprayer (normal saline). The latest meals the subjects had were at least 5 hours before dose administration. Prior to dosing, patency of the nostrils was tested and assured. After baseline blood sampling, subjects received doses of Nasulin in the fasted state followed by blood sampling at 10, 15, 20, 25, 30, and 45 minutes after dosing. Earlier studies indicated insulin levels would be close to baseline at 45 minutes and that hypoglycemia risk would go up substantially after that time. Standardized meals of 600 kcal (50% carbohydrate, 30% fat, 20% protein) were provided at 45 minutes post-dosing or after the last timed blood samples were collected to ameliorate or prevent symptoms of hypoglycemia.
Nasulin intranasal 1% insulin spray, Cardinal Health Lot No. XT0603 (Research Triangle Park, NC) was used in this trial. The dosing schedule was as follows:
Day 1
Using the same nostril, each subject was randomly dosed three times over the day with 25, 50, and 75 U.
Day 3
Using both nostrils, each subject randomly received doses of 50, 75, and 100 U.
Day 5
Same as day 3.
Each spray actuation contained 100 µl. Each spray of the 1% insulin emulsion contained 25 U. On day 1, the subjects received one, two, or three sprays in the same nostril in a randomized fashion. On days 3 and 5, they randomly received 50 U (one spray in each nostril), 75 U (two sprays in one nostril and one spray in the other nostril), or 100 U (two sprays in each nostril). The 1% has 27.5 U per spray and for convenience purposes was rounded to 25 U for dose calculation.
Mechanism of Absorption Determination
This is a portion of a larger study, the other portion having been reported elsewhere.4 Twenty-four healthy, nonsmoking subjects (ages 18–50, BMI <33 kg/m2, weight >70 kg) participated in this three-way crossover study under conditions as outlined in the Maximum Dose Determination Study. As it was not possible to dose all 24 subjects at once, two cohorts of 12 were dosed on consecutive weeks. Blood samples were taken at -7, -3, 5, 10, 15, 20, 25, 30, 40, and 45 minutes (ten time points).
Nasulin intranasal 1% insulin spray administered intra-nasally in 25 and 50 U doses [batch number: Catalent Lot No. CT0728 (Catalent Pharma Solutions, Research Triangle Park, NC)] and placebo containing all ingredients of Nasulin minus insulin administered intranasally [batch number: DPT Lot No. 809925 (DPT Laboratories, LTD, Lakewood, NJ)] were used in this trial.
Serum insulin levels were determined using the Immulite® 2000 Analyzer (Siemens Healthcare Diagnostics, Deerfield, IL), which is a solid-phase, two-site chemiluminescent immunometric assay for the first study and the Unicell DX1 800 immunoassay by Beckman-Coulter, Inc. (Brea, CA) for the second study. The change of laboratories was for logistical reason only; a subset of sample was analyzed in both laboratories, yielding similar results. All samples were labeled, handled, processed, and shipped according to Orlando Regional Medical Center (ORMC) Standard Operating Procedures and Clinical Laboratory Procedures using the tubes provided by ORMC Clinical Laboratory. Variables considered for insulin were the maximum measured concentration (Cmax), the area under the plasma concentration time curve (AUC) estimated using PK modeling, and time to maximum concentration (Tmax). Each PK parameter was analyzed using an analysis of variance model that included the fixed effects of sequence, treatment, and period, and the random effects of subjects within sequences and within-subject errors.
Each subject was individually monitored by trained medical personnel. Bedside glucometer readings (One Touch®, LifeScan, Inc., Milpitas, CA) were performed at each blood draw. If symptoms of hypoglycemia occurred, a last blood sample was immediately taken, glucose was administered, and then a meal was given to the subject. Safety data was compiled by regular nondirectional questioning of the subjects and by direct reporting of adverse symptoms.
The studies were conducted in accordance with the International Conference on Harmonisation’s Guidelines for Good Clinical Practice with ethical principles that have their origins in the Declaration of Helsinki, and in compliance with approved protocols and applicable regulatory requirements. CPEX Pharmaceuticals, Inc. affirmed and upheld the principle of the subjects’ right to protection against the invasion of privacy. Throughout the studies, all data were identified only by subject number and subject initials.
Results
Maximum Dose Determination Study
Eight subjects who met the inclusion/exclusion criteria were enrolled in and completed the trial. Samples of all subjects were used in the analysis of the data.
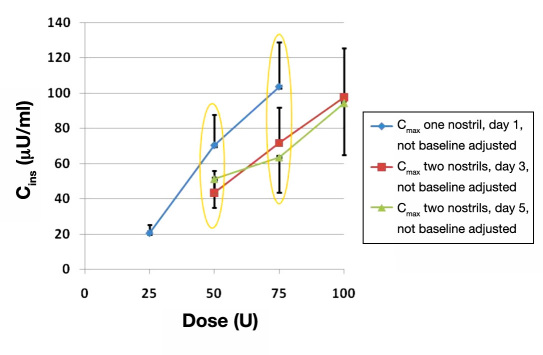
Repeated intranasal administration of 25 U insulin in immediate succession led to a steep rise in exposure, with one-nostril repeated administration achieving relatively higher exposures than the two-nostril repeated administration with the 50 and 75 U doses (Figure 1 ). The ellipses in Figure 1 demonstrate the direct comparison of doses utilizing one or two nostrils and illustrate the PK advantage of delivering the same dose in a single nostril. The maximum dosing using a single nostril was 75 U (three sprays of 25 U each).
Figure 1.
Comparison of Cmax values for single and dual nostril administration. Maximal peripheral blood insulin concentration (mean ± standard error of the mean) after one, two, or three 25 U puffs in a single nostril (blue), or two, three or four 25 U puffs in two nostrils (red and green). Note that the same dose at either 50 or 75 U leads to higher exposures when delivered in one rather than two nostrils.
Overall, good concordances in exposure between Cmax and AUC values were seen throughout the study. Table 1 contains the data for days 1 and 3. Day 5 revealed similar results as day 3. There were high correlations between the dose exposure ratios calculated with Cmax and AUC values of different doses. Regardless of which exposure estimations were used, the dose exposure relationships were essentially the same. There was no statistically significant difference in the Tmax values between different doses on all three test days as the levels peaked between 18 and 20 minutes for all regimens.
Table 1.
Cmax, AUC(0–inf), and Tmax. Days 1 and 3, Eight Subjects
| Day 1: one-nostril repeated administrations | |||||||||
| Parameter | Unit | Dose (U) | Estimate (SEa) | 50/25 (SE) | pvalue | 75/25 (SE) | pvalue | 75/50 (SE) | pvalue |
| Cmax (adjusted) | µU/ml | 25 | 13.2 (3.4) | 4.905 | .0018 | 7.293 | .0003 | 1.487 | .3599 |
| 50 | 64.9 (19.0) | (0.079) | (0.053) | (0.279) | |||||
| 75 | 96.4 (28.2) | ||||||||
| AUC(0–inf)a | µU·min/ml | 25 | 414.7 (110.2) | 4.976 | .0022 | 7.011 | .0005 | 1.409 | .4419 |
| 50 | 2063.2 (625.5) | (0.081) | (0.057) | (0.305) | |||||
| 75 | 2907.3 (881.4) | ||||||||
| Tmax | min | 25 | 18.33 (1.92) | -1.29 | .6655 | 2.89 | .3434 | 4.18 | .2057 |
| 50 | 17.04 (2.19) | (2.91) | (2.91) | (3.11) | |||||
| 75 | 21.22 (2.19) | ||||||||
| Day 3: two-nostril repeated administrations | |||||||||
| Parameter | Unit | Dose (U) | Estimate (SE) | 75/50 (SE) | pvalue | 100/50 (SE) | pvalue | 100/75 (SE) | pvalue |
| Cmax (adjusted) | µU/ml | 50 | 30.9 (10.3) | 2.112 | .1382 | 2.845 | .0464 | 1.347 | .5289 |
| 75 | 65.2 (21.3) | (0.223) | (0.165) | (0.341) | |||||
| 100 | 87.9 (28.7) | ||||||||
| AUC(0–inf) | µU·min/ml | 50 | 839.6 (351.5) | 2.300 | .1859 | 3.406 | .0613 | 1.481 | .5107 |
| 75 | 1931.0 (795.3) | (0.258) | (0.174) | (0.391) | |||||
| 100 | 2859.7 (1177.8) | ||||||||
| Tmax | min | 50 | 17.31 (2.20) | 2.09 | .5161 | 5.17 | .1235 | 3.08 | .3316 |
| 75 | 19.40 (2.16) | (3.12) | (3.12) | (3.04) | |||||
| 100 | 22.48 (2.16) | ||||||||
SE, standard error of the mean; AUC(0–inf),area under the curve from zero time (dose administration) to infinity.
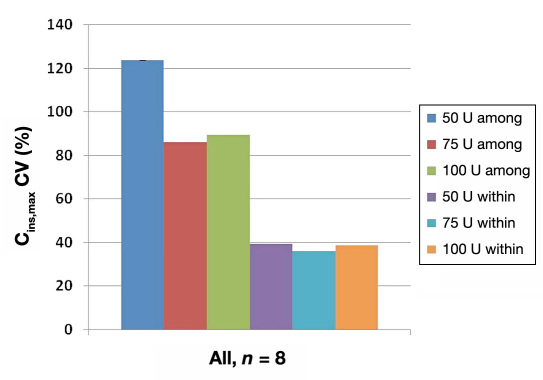
There was high intersubject (among) variation and small intrasubject (within) variation in exposures with repeated intranasal Nasulin administrations of doses ranging from 50 to 100 U (Figure 2 ). However, the intrasubject variability was at ~40%, which is close to what has been reported with subcutaneous insulin administration (Binder, 19845 and Heinemann, 20046).
Figure 2.
Cmax. Large intersubject (among) but small intrasubject (within) variability. Cmax inter- and intrasubject variability following repeat administration of two, three, or four 25 U puffs in two nostrils. The high total variability is primarily derived from intersubject variability.
Mechanism of Absorption Determination
To investigate the mechanism of enhanced absorption with repeated dosing in the same nostril, the treatment of placebo (P), which contained all ingredients minus insulin, followed by 25 U (administered with one spray of P followed by one spray of Nasulin) in a single nostril was evaluated against 25 U administered with a single spray and 50 U administered with two sprays in a single nostril in a crossover design. The adjusted insulin AUC and Cmax values of the three treatments were compared using a mixed model analysis of variance (individual and combined cohorts). As shown in Table 2 , the 50 U dose of Nasulin generated a statistically significant (higher) difference that was more than double the exposure than that of both the 25 U and the P + 25 U doses, in terms of both geometric least squares mean Cmax and AUC(0–45 min) measures in each cohort and both cohorts combined (p < .05). The P + 25 U dose of Nasulin, however, generated similar but less exposure than that of the 25 U dose (p > .05).
Table 2.
Geometric Least Squares Mean Adjusted Insulin Cmax and AUC(0–45min) by Dose Comparison of P + 25 U versus 25 and 50 U
| Parameter | Unit | Cohort | Dose (U) | Estimate (SEa) | 50/25 (SE) | pvalue | (P* + 25)/25 (SE) | pvalue | 50/(P* + 25) (SE) | pvalue |
|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (adjusted) | µU/ml | Total | 25 | 22.44 (4.694) | 2.478 | .0004 | 0.708 | .1527 | 3.502 | <.0001 |
| 50 | 55.61 (11.63) | (.5878) | (.1679) | (.8305) | ||||||
| P* + 25 | 15.88 (3.322) | |||||||||
| AUC45 (adjusted) | µU·min/ml | Total | 25 | 490.8 (107.4) | 2.507 | .0008 | 0.712 | .1885 | 3.520 | <.0001 |
| 50 | 1230 (269.3) | (.6361) | (.1807) | (.8931) | ||||||
| P* + 25 | 349.5 (76.51) |
SE, standard error of the mean; P*, placebo containing all ingredients of the formulation except insulin.
Compared to the single spray of the 25 U dose, the second spray of the P + 25 U dose increased only the volume of excipient, whereas the second spray of the 50 U dose increased both the dose of insulin and the spray volume of excipient. Therefore, the excipient CPE-215 did not show any effect in increasing the dose response of Nasulin for an immediate second administration. In contrast, the increased dose response from repeated dosing of the 50 U Nasulin was apparently due to the increasing dose of insulin indicating that the maximal effect of CPE-215 is attained after the first spray.
Summary of Adverse Events
Maximum Dose Determination Study
Overall, Nasulin was well-tolerated with a generally good safety profile. The most noticeable adverse events were administration site reactions and hypoglycemia, both of which seemed to be dose dependent with occurrences increasing with increasing doses. The most common administration site reactions were increased lacrimation, nasal irritation, headache, cough, nasal congestion, sneezing, and throat irritation. The percentage of subjects with hypoglycemic events (characteristic symptoms of hypo-glycemia or blood glucose levels ≤59 mg/dl) increased with increasing exposures of insulin on all three days. On day 1, with single-nostril administrations of 25, 50, and 75 U, there were dose-dependent increases in hypo-glycemia (0, 37.5, and 42.9%, respectively). On days 3 and 5, with dual nostril administration of 50, 75, and 100 U, there were dose-dependent increases in hypoglycemia (12.5, 31.3, and 43.8%, respectively).
Mechanism of Absorption Determination
All subjects experienced adverse events and administration site reactions during the course of the study. The most frequent adverse events were administration site reactions, and in order of decreasing frequency were nasal irritation, sneezing, throat irritation, increased lacrimation, dysgeusia, headache, cough, and nasal congestion. The percentages of subjects experiencing hypoglycemia at doses of 25, P + 25, and 50 U were 12.5, 12.5, and 29.2%, respectively.
Discussion
In this study, the maximum dose for Nasulin was determined. In addition, an unexpected finding was the enhanced absorption when a single nostril is used for administration as compared to dual nostril administration. At the 25 and 50 U doses in a single nostril, the Cmax values were 13.2 (3.4) and 64.9 (19.0) µU/ml, respectively, an almost five-fold increase. At the 75 U dose, the Cmax was 96.4 (28.2) µU/ml, an expected 50% increase over the 50 U dose. In further comparisons, when the 50 U dose in a single nostril was compared to the dual nostril (one spray in each nostril), the Cmax value was about double. Comparison of the 75 U single dose to dual nostril administration (two sprays in one nostril and one spray in the other nostril), the values were ~1.5–1.9 times higher for the single nostril (see Figure 2 ). AUC values paralleled the Cmax values (see Table 1 ). For both single and dual nostril administration over all doses, the Tmax values were similar, ranging from 17 to 23 minutes.
To determine the mechanism of the enhanced absorption, a second study investigated the finding comparing 25, P + 25, and 50 U utilizing a single nostril. The 50 U dose of Nasulin generated a statistically significant (higher) difference that was more than double the exposure than that of both the 25 and the P + 25 U dose, in terms of both geometric least squares mean Cmax and AUC(0–45 min) measures in each cohort and both cohorts combined (p < .05). The P + 25 U dose of Nasulin, however, generated similar but less exposure than that of the 25 U dose (p > .05). Compared to the single spray of the 25 U dose, the second spray of the P + 25 U dose increased only the volume of excipient, whereas the second spray of the 50 U dose increased both the dose of insulin and the spray volume of excipient. Therefore, the excipient CPE-215 did not show any effect in increasing the dose response of Nasulin for an immediate second administration. In contrast, the increased dose response from repeated dosing of the 50 U Nasulin was apparently due to the increasing dose of insulin, not due to the excipient CPE-215.
Adverse events included mainly application site reactions as reported earlier and hypoglycemia in a dose-related fashion as expected in fasting individuals. To date, the longest human exposure to Nasulin has been 3 months. Longer-term studies will be needed to see if chronic administration to the nasal mucosa would lead to chronic inflammation or other adverse local events. Effects on the CNS will also need to be carefully monitored. Because the Nasulin particle size is >10 µm, little, if any lung exposure is expected, as inhalation requires the particle size to be <5 µm.
Conclusions
This study demonstrated that with repeated intranasal administrations of Nasulin in one or two nostrils, substantial exposures of insulin were achieved with rapid onset of actions. The maximum dosing via a single nostril was three repeated administrations in immediate succession for a total dose of 75 U. This study demonstrated enhanced absorption with single nostril administration compared to double nostril administration. Tmax was unaffected by increased doses. There was relatively higher intersubject (among) variation and smaller intra-subject (within) variation in the study. The intrasubject variability of intranasal Nasulin administration was at ~40%, which is close to what has been seen in subcutaneous insulin administration. It was relatively well-tolerated. The mechanism of enhanced absorption with repeated dosing in the same nostril was found to be due to an increasing dose of insulin, not due to the excipient CPE-215 increase.
Acknowledgments
The authors wish to thank Lance Berman, M.D., M.S. for his contribution to the review of this document and for his overall leadership in the Nasulin Development Program.
Abbreviations
- AUC
area under the curve
- BMI
body mass index
- Cmax
maximum concentration
- CPE-215
cyclopentadecalactone
- ORMC
Orlando Regional Medical Center
- P
placebo
- PK
pharmacokinetic
- SE
standard error of the mean
- Tmax
time to maximum concentration
- U
unit
References:
- 1.Schwartz S, Ryan T, Stote R. Results of a randomized, single-dose, 2/3-way crossover comparison study of intranasal insulin spray (NasulinTM) injectable fast-acting insulin (Humalog®) in normal nonsmoking and smoking subjects. Diabetes. 2007;56(Suppl 1A):02-LB. [Google Scholar]
- 2.Leary AC, Dowling M, Cussen K, O’Brien J, Stote RM. Pharma-cokinetics and pharmacodynamics of intranasal insulin spray (Nasulin) administered to healthy male volunteers: influence of the nasal cycle. J Diabetes Sci Technol. 2008;2(6):1054–1060. doi: 10.1177/193229680800200613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stote R, Schwartz S, Dehn C, Strange P. Two randomized crossover glucose clamp studies of NasulinTM and lispro SC. Poster, Diabetes Technology Meeting; November, 2009. [Google Scholar]
- 4.Stote R, Marbury T, Shi L, Miller M, Strange P. Comparison pharmacokinetics of two concentrations (0.7% and 1.0%) of Nasulin, an ultra-rapid-acting intranasal insulin formulation. J Diabetes Sci Technol. 2010;4(3):603–609. doi: 10.1177/193229681000400314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binder C, Lauritzen T, Faber O, Pramming S. Insulin pharmaco-kinetics. Diabetes Care. 1984;7(2):188–199. doi: 10.2337/diacare.7.2.188. [DOI] [PubMed] [Google Scholar]
- 6.Heinemann L. Time-action profiles of insulin preparations. Mainz, Germany: Kirchheim & Co GmbH; 2004. [Google Scholar]