Abstract
The continued revolution in multidetector-row CT (MDCT) scanning increases the quality of lung imaging but at the cost of a greater burden of data for review and interpretation. This article discusses our preliminary experience with prototype software for lung nodule detection and characterization using MDCT data sets. We discuss the potential role of computer-assisted detection (CAD) as applied to the automatic detection of lung nodules. We also review the process of CAD, outline its potential results, and explore how it may fit into existing radiology practice. Finally, we discuss MDCT data-acquisition parameters and how they may affect the performance of CAD.
Keywords: Computer-assisted detection, pulmonary nodules, multidetector-row CT
Computed tomography (CT) is the most sensitive test for lung nodule detection. Fundamentally there are two goals for lung nodule detection: (1) to expedite resection of potentially curable cancer, and (2) to minimize the number of benign nodules removed by thoracotomy (and, by inference, to recognize nodules for non-intervention).1 Interpretation of lung nodules involves characterization and assimilation to clinical and other imaging information. Though there are unique, complex qualities of human visualization and image interpretation that defy computer modeling, there are also limitations in operator performance that limit efficiency and accuracy. Multidetector-row helical CT provides very large thoracic data sets (3-400 slices)2 of unprecedented resolution, but its impact will likely be minimal and changes in management and outcome unlikely if interpreted using similar paradigms as sequential or single detector CT (SDCT).
Advances in lung nodule management using MDCT will only be realized through optimal MDCT data acquisition and post-processing (including three-dimensional [3D] tools3 and Computer-assisted detection [CAD]) incorporated into advances in other imaging modalities such as (positron emission tomography-CT) (PET-CT), health programs (e.g., lung cancer screening), and changes in management including medical and surgical therapies. Computer-assisted detection is currently used as a second reader, implying that the physician incorporates the CAD output into his or her decision process but that the final decision is made by the radiologist. It has been explored with some success in the fields of mammography4 and chest radiography,5 although its use in CT carries distinct challenges namely, data acquisition variables, data overload, and the requirements of nodule detection and discrimination. This article discusses the potential role of CAD for lung nodule detection, the variables of CT data acquisition and reconstruction, and the process of CAD segmentation with reference to our preliminary experience of a prototype CAD software system for lung nodule detection with multidetector data sets.
MATERIALS AND METHODS
A prototype R2 ImageChecker CT system (R2 Technology, Inc., Sunnyvale, CA) was installed parallel to an existing system of CT data acquisition and interpretation. The scanner used was a helical system variable array with four active detector units (Siemens Volume Zoom, Siemens Medical Solutions, Malvern, PA). This scanner had previously established connectivity to a soft copy viewing station and three-dimensional work station.
All patients were imaged using our routine non-contrast technique with 0.75-mm detectors and 1-mm slice widths (16 detector system), and interpretation was performed using only the normal image display of our existing systems. The R2 system was not used for any patient management, and it was used only after completion of normal interpretation and report dictation. Patient data were then randomly sent to the R2 work station and included lung cancer screening studies, directed exams, and patients with single or multiple lesions. Cases were reviewed using the various parameters available on the R2 work station to explore its potential for lung nodule detection and characterization in the setting of an active radiology practice.
DISCUSSION
The Potential Role of Computer-Assisted Detection (CAD) for Lung Nodules
Lung nodules may be divided into those discovered incidentally (on chest radiograph or CT) and those found during a dedicated lung nodule search by CT. In the latter group the scan may be performed as a management algorithm for a specific patient with a specific predisposing clinical situation (e.g., metastatic work-up or follow-up) or as part of a program for a population thought to be at risk (e.g., lung cancer screening). Computed tomography must characterize documented isolated nodules as benign, malignant, or unknown according to biologic behavior, and it must assess interval change and help management decisions. Through high spatial and contrast resolution missed nodules are few and of uncertain significance, and detected nodules have good general interobserver and interscan agreement. However, it should be stated that some reader variation remains because of differences in definition of a nodule and the lack of a gold standard to define many small incidental lesions.
Computer-assisted detection serving as a second reader, may provide better sensitivity for small nodules, easier enumeration and documentation, improved interobserver and interscan consistency on longitudinal follow-up, and a more objective assessment of significant temporal change in lesion size and number.6 Automated volume measurements remove the subjective measurement variations and limitations of two-dimensional (2D) measures of irregular structures and can therefore lay the foundation for a systematic approach to management and monitoring of small nodules of low specificity on CT. Differentiation of biologic behavior could be improved using automated volume measurements as an indirect measure of doubling times. It may address user oversight error, increasingly an issue with MDCT data information overload (e.g., 16 detector 1-mm slices of the entire chest) and with lesions of low conspicuity such as ground-glass opacities and lesions near the chest wall or mediastinum. For patients with lesions “too numerous to count” the CAD tool can serve as an efficient means of rapidly assessing stability with printout reports to aid clinician communication and management.
For the evaluation or practice of such a screening program where many patients will have abnormalities but few will have cancer, CAD can be integrated to improve work flow and aid patient throughput. An immediate review of the CAD result could facilitate identifying those patients that should await counseling. Agreed-upon screening parameters may have a role in quality assurance and may provide a systematic approach to the management and monitoring of small nodules of low specificity on CT. We would also hope that a standardized CAD system may offer the potential to refine imaging parameters for improved accuracy with reductions in radiation exposure. In addition, CAD has the potential to compensate for the signal loss with lower dose scanning techniques. A well-defined case database will help determine CAD parameter refinement for greater specificity.
The Process of CAD, Its Potential Results, and How It May Fit into Future Imaging Algorithms
We reviewed a series of cases on a prototype R2 ImageChecker CT CAD system (R2 Technology, Inc.). The software is designed for automatic detection and analysis of lung nodules. It is equipped with a number of work flow enhancing tools such as automatic volume measurement of the detected nodule (Fig 1), the ability for the user to read at varying, lower-resolution, axial collimations (CAD reads at the highest acquired resolution), the ability to add to and subtract from CAD detected findings (Figs 2, 3), a reporting tool for nodule characterization including tracking of nodule location, number, volume, and level of suspicion (Figs 4, 5, 6, 7). The next-generation system will have the ability to perform temporal comparison between current and prior scans. A volume-centric design takes advantage of MDCT data sets by using volumetric review, visualization, and assisted detection.
Figure 1.
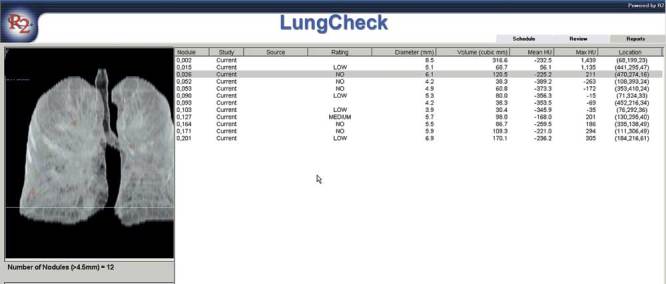
The patient report that is generated giving a comprehensive review of each nodule and its volumetric size, location, HU (Hounsfield) measurements, and the level of suspicion.
Figure 2.
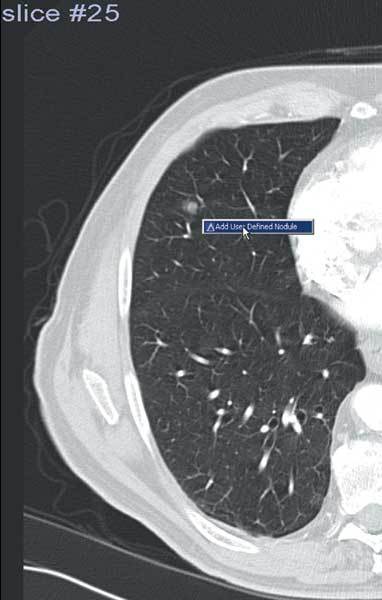
A missed nodule may be marked for reporting.
Figure 3.
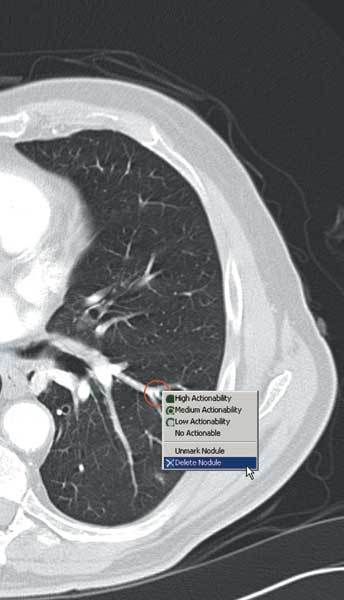
A false positive nodule may be removed from the final report.
Figure 4.
An example of a single right lung nodule on two- and three-dimensional display.
Figure 5.
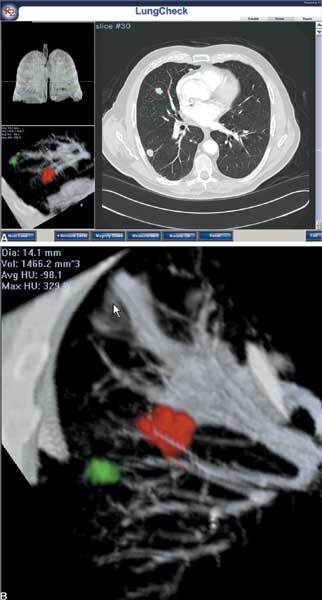
a. An example of multiple right lung nodules on two and three-dimensional display. b. Alternate imaging perspectives of two right lung nodules aids their discrimination from vessels.
Figure 6.
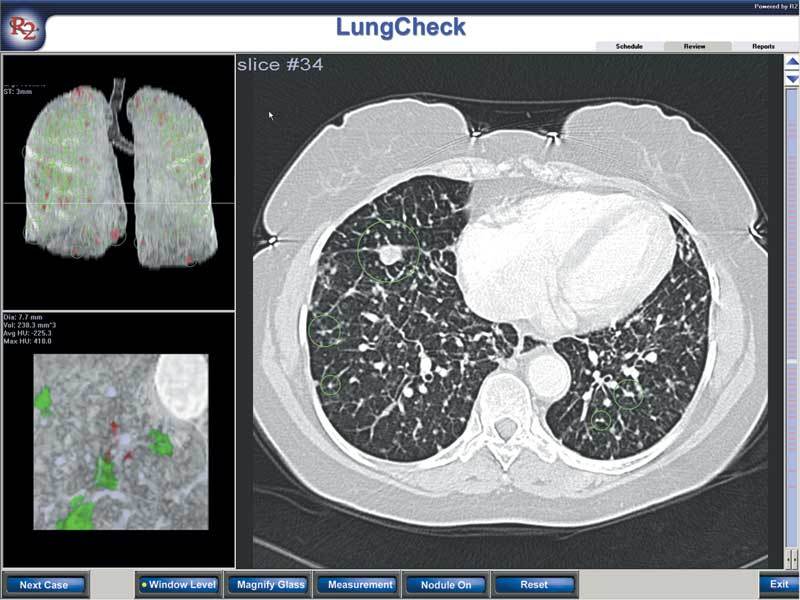
Large numbers of nodules are documented in this case.
Figure 7.
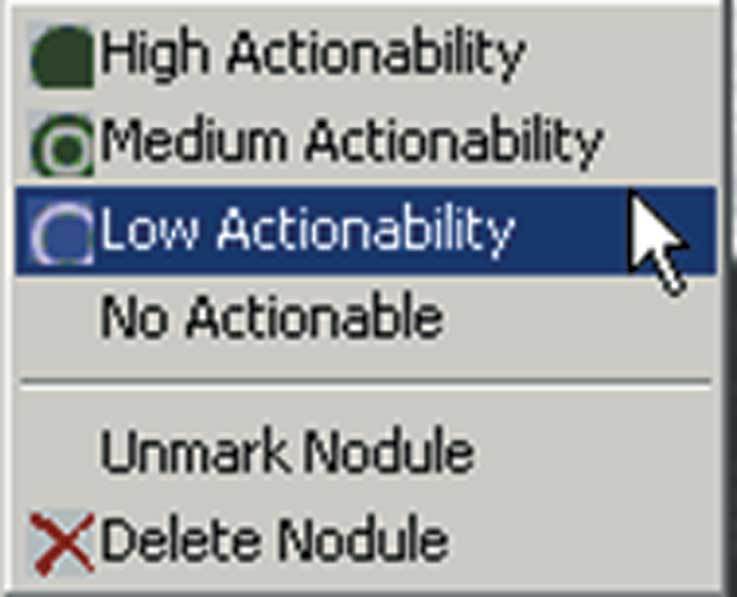
A level of suspicion for longitudinal follow-up maybe assigned.
The CAD system works via a series of volume-centric segmentation techniques that delineate normal from abnormal lung tissue.1 Multiple geometric parameters are calculated for each nodule, including shape, elongation, size, spiculation, density, and other features. Based on these parameters and a series of rules, the candidate nodule is given a likelihood rating. If that likelihood rating falls above a defined threshold for features indicative of a lung nodule, CAD will mark the lesion as an area requiring a second review. If the candidate lesion falls below the threshold, CAD will not mark the lesion as a suspicious area. Through neural networks a learning system may be established for lung nodule detection and discrimination as new reference data are added to the database.
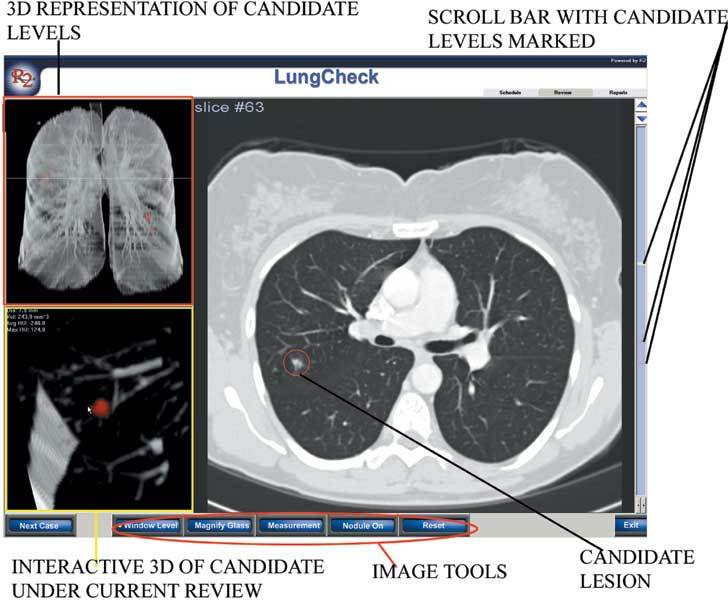
A user interface allows navigation between 2D and 3D images and is linked to a server and archive (Figs 8, 9). Lesions under active review are color-coded. Image slices with nodules for review are marked for ease of scrolling, and measurements are automatically calculated, although the user may define other 2D dimensions. A 3D image of the nodule under review is generated to demonstrate its relationship to adjacent vascularity. The user may assign a level of suspicion to the nodule (Fig 7) or completely discard it from the review. After a completed review, a report is automatically generated (Fig 1). The entire system allows seamless Ethernet integration to the scanner, a volume work station, or a picture archiving and communication system (PACS) and has full digital imaging and communication in medicine (DICOM) compatability.
Figure 8.
The user interface with patient lists for review.
Figure 9.
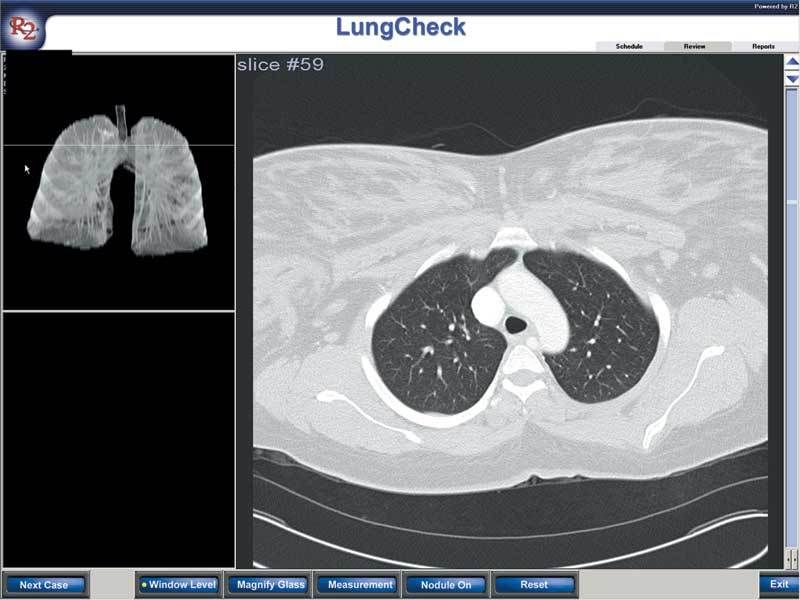
The image interface with a two-dimensional view (on the right) and three-dimensional interactive representation (on the left).
There are few studies to date on the effectiveness of CAD for lung nodules using CT. Using 10-mm slices and eight patients, Giger et al. found 94% sensitivity for nodule detection with 1.25 false positives per case with a range of 0 to 4 per case.7 Armato III et al. documented sensitivity of 70% with 3 false positives per case for a total of 17 cases,1 whereas Ko et al.8 found 86% sensitivity for lung nodule detection in eight oncology patients with a total of 370 nodules. At follow-up, automated measurement of change in nodule size matched that calculated by a radiologist. One study suggested improved radiologist performance for micronodule detection using thin-section CT.9 In our preliminary experience, though, we found high sensitivity (up to 80%) for a range of lesions including those less than 4 mm. Most false positives were normal bronchovascular structures (up to 50%) which were easily discriminated. Interestingly, a significant number of false positives (up to 30%) were not lung nodules but were abnormal findings (including parenchymal scar and pleural thickening). There are no CAD studies using MDCT with high-resolution data sets to date. We would anticipate greater sensitivity and more accurate volume measurements with MDCT. Thinner sections and greater temporal resolution may improve specificity. We would also anticipate improved separation of nodules and vascular structures through CAD and 3D reformations. However, it is likely there will be an increase in the detection of smaller nodules lacking morphologic detail for characterization. It is important to state that differences in definition make radiologist interpretation an imperfect gold standard against which to measure CAD systems. This lack of clarity may affect the performance of the CAD system. Nevertheless the adverse impact of this lack of clarity on nodule detection should be less than its effect on chest radiograph.10,11
In our early experience we anticipate the R2 Lung Checker as fitting in seamlessly to our network of data transfer for lung nodule assessment, which includes other post-processing tools (e.g., volume measurements, contrast enhancement, and fluorodeoxyglucose activity). For single and multiple lung nodules, it will fit into a system for comprehensive assessment and reporting with archiving for longitudinal follow-up. In addition, CAD may have a critical role in any lung cancer screening program. At present CT is under review as a potential screening tool, and it must be shown to improve disease-specific mortality as well as survival. To detect more true positives than false positives with prevalence <5%, the test must have sensitivity greater than 95% if specificity is less than or equal to 95%, and vice versa. Most screening tests are not that good, and false positives must be absorbed.12 Any screening population will have a large number of normals and will likely produce a large number of true small nodules of uncertain significance.13,14,15 Computer-assisted detection is one method to deal with this data burden in a time-efficient and consistent manner.
Variables Including Collimation, Interscan Spacing, and a Reconstruction Algorithm That May Affect the Success of CAD Nodule Detection
The quality of any CT post-processing is only as good as the original data acquisition. There is a greater choice of user-defined variables with MDCT. Initial scan set-up involves selection of a series of detectors either of the same size (fixed array) or of variable size (variable array). This discussion focuses on a 4-detector variable array, although the principles are applicable to other systems and newer detectors.
For the best 2D and 3D data sets, narrow collimation and near-isotropic or isotropic resolution is preferred. This combination provides voxels with spatial resolution independent of the acquisition plane, which affords a better chance of detecting small nodules and differentiating vascular structures. Smaller pixels should provide a more accurate assignment for measurement. Furthermore, MDCT allows for arbitrary slice position and width reconstruction, although slice widths cannot be made smaller than the smallest detector chosen. Larger slice widths may be created for images with less “noise.” For our initial evaluation of the R2 system, the x-ray fan beam was collimated to 0.75-mm detectors producing 1.0-mm slice widths. A maximum slice thickness of 3 mm must be used with a spacing of 3 or less to obtain the highest sensitivity. Because of the interpolation method, the slice sensitivity profile is not broadened with higher values of pitch so that the entire lung may be scanned using a table speed that permits a breath-hold study. The kernel chosen for reconstruction is important. High-frequency kernels are noisy and may give the false impression of calcifications. Generally, soft-tissue lower-frequency spatial frequency algorithms are used, although we have found that the optimal kernel may vary with detector arrays. The dose exposure is automatically modulated to the patient geometry, and the naturally high contrast between nodules and lung parenchyma permits lower doses to be employed without loss of sensitivity (20-60 mA). Although the standard dose of a conventional chest CT is 5.8 mSv (effective radiation dose) low-dose scans for screening may be as low as 0.6 mSv (effective radiation dose). Wasted irradiation is limited through focal spot tracking and larger detector arrays with decreased contribution from penumbra. Cardiac gating is not routinely used, but it can limit movement misregistration in the medial lower lung regions. Respiratory misregistration is limited through the fast acquisition of MDCT, which has a gantry rotation of 0.5 s and simultaneous 4-detector activation allowing temporal resolution in the order of 125 ms. With the ongoing developments of MDCT, it is not yet known what the uniform agreement on chest scanning protocol will be for routine interpretation or CAD. However, we can say it will likely be a low-dose protocol (80 mA or less) and collimation that creates isotropic data sets (0.75-1 mm). Users will then decide whether to apply CAD to a particular slice width or a range of slice widths, all of which will be available from a single acquisition. For example, readers may prefer to use conventional reading for 3-5 mm slice widths and apply CAD to the large number of smaller slice widths available.
CONCLUSIONS
From our early experience of prototype, CAD has clear potential to improve the diagnostic accuracy and consistency of CT examinations for pulmonary nodule assessment and follow-up. It can be used to enhance the existing role of CT for single and multiple pulmonary nodule assessment and may be critical for lung cancer population screening. The key to successful CAD implementation will be a high true detection rate with a low rate of false positives integrated into the established work flow and clinical assessment. Ultimately CAD must be part of a work station or PACS system rather than a freestanding unit, and it must contribute to efficient management of the larger data sets currently being produced. Future studies will include prospective scientific evaluation of this effectiveness together with a radiologist consensus development on the definition of nodules and their management. It is likely that CAD will fulfill potential roles such as detection, characterization, and follow-up, although how it is employed will depend on individual practice needs.
Acknowledgement
R2 Lung Checker is an investigational tool. R2 Technology (Sunnyvale, CA) is supporting our research through an agreement with Johns Hopkins Medical Institutions.
Footnotes
An erratum to this article is available at http://dx.doi.org/10.1007/s10278-003-9000-y.
References
- 1.Armato 3rd SG, Giger ML, Moran CJ, et al. Computerized detection of pulmonary nodules on CT scans. Radiographics. 1999;19:1303–1311. doi: 10.1148/radiographics.19.5.g99se181303. [DOI] [PubMed] [Google Scholar]
- 2.Schoepf UJ, Bruening RD, Hong C, et al. Multislice helical CT of focal and diffuse lung disease: comprehensive diagnosis with reconstruction of contiguous and high-resolution CT sections from a single thin-collimation scan. AJR Am J Roentgenol. 2001;177:179–184. doi: 10.2214/ajr.177.1.1770179. [DOI] [PubMed] [Google Scholar]
- 3.Ravenel JG, McAdams HP, Remy-Jardin M, et al. Multidimensional imaging of the thorax: practical applications. J Thorac Imaging. 2001;16:269–281. doi: 10.1097/00005382-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Freer TW, Ulissey MJ. Screening mammography with computer-aided detection: prospective study of 12,860 patients in a community breast center. Radiology. 2001;220:781–786. doi: 10.1148/radiol.2203001282. [DOI] [PubMed] [Google Scholar]
- 5.Abe H, MacMahon H, Engelmann R, et al. Computer-aided diagnosis in chest radiography: results of large-scale observer tests at the 1996-2001 RSNA Scientific Assemblies. Radiographics. 2003;23:255–265. doi: 10.1148/rg.231025129. [DOI] [PubMed] [Google Scholar]
- 6.Yankelevitz DF, Reeves AP, Kostis WJ, et al. Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology. 2000;217:251–256. doi: 10.1148/radiology.217.1.r00oc33251. [DOI] [PubMed] [Google Scholar]
- 7.Giger ML, Bae KT, MacMahon H. Computerized detection of pulmonary nodules in computed tomography images. Invest Radiol. 1994;29:459–465. doi: 10.1097/00004424-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Ko JP, Betke M. Chest CT: automated nodule detection and assessment of change over time—preliminary experience. Radiology. 2001;218:267–273. doi: 10.1148/radiology.218.1.r01ja39267. [DOI] [PubMed] [Google Scholar]
- 9.Brown MS, Goldin JG, Suh RD, et al. Lung micronodules: automated method for detection at thin-section CT—initial experience. Radiology. 2003;226:256–262. doi: 10.1148/radiol.2261011708. [DOI] [PubMed] [Google Scholar]
- 10.MacMahon H, Engelmann R, Behlen FM, et al. Computer-aided diagnosis of pulmonary nodules: results of a large-scale observer test. Radiology. 1999;213:723–726. doi: 10.1148/radiology.213.3.r99dc27723. [DOI] [PubMed] [Google Scholar]
- 11.MacMahon H. Improvement in detection of pulmonary nodules: digital image processing and computer-aided diagnosis. Radiographics. 2000;20:1169–1177. doi: 10.1148/radiographics.20.4.g00jl211169. [DOI] [PubMed] [Google Scholar]
- 12.Obuchowski NA, Graham RJ, Baker ME, et al. Ten criteria for effective screening: their application to multislice CT screening for pulmonary and colorectal cancers. AJR Am J Roentgenol. 2001;176:1357–1362. doi: 10.2214/ajr.176.6.1761357. [DOI] [PubMed] [Google Scholar]
- 13.Aberle DR, Gamsu G, Henschke CI, et al. A consensus statement of the Society of Thoracic Radiology: screening for lung cancer with helical computed tomography. J Thorac Imaging. 2001;16:65–68. doi: 10.1097/00005382-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Ellis SM, Husband JE, Armstrong P, et al. Computed tomography screening for lung cancer: back to basics. Clin Radiol. 2001;56:691–699. doi: 10.1053/crad.2001.0850. [DOI] [PubMed] [Google Scholar]
- 15.Ellis JR, Gleeson FV. Lung cancer screening. Br J Radiol. 2001;74:478–485. doi: 10.1259/bjr.74.882.740478. [DOI] [PubMed] [Google Scholar]


