Abstract
Anesthetics produce immobility and depress spinal nociceptive processing, but the exact sites and mechanisms of anesthetic action are unknown. The gamma-aminobutyric acid type-A (GABAA) receptor is thought to be important to anesthetic action. We studied knockin mice that had mutations in the alpha1 subunit of the GABAA receptor that imparts resistance to isoflurane in in vitro systems. We determined the isoflurane minimum alveolar concentration (MAC) that produces immobility in 50% of subjects and responses of lumbar neurons (single-unit recordings) to noxious stimulation (5 s pinch) of the hindpaw. Isoflurane MAC did not differ between wild-type (1.1 ± 0.1%) and knock-in (1.1 ± 0.1%) mice. Isoflurane depressed neuronal responses to noxious stimulation (60 sec period during and after pinch) similarly in both wild-type and knock-in mice (555 ± 133 and 636 ±106 impulses/min, respectively, at 0.8 MAC and 374 ± 81 and 409 ±85 impulses/min at 1.2 MAC). We conclude that isoflurane enhancement of alpha1 -containing GABAA receptors is not required to produce immobility or depress spinal nociceptive processing.
Keywords: Spinal cord, GABA receptor, isoflurane, immobility, anesthesia
Anesthetics are widely used but we still do not fully understand how these powerful and potentially unsafe drugs produce general anesthesia, which includes amnesia, unconsciousness and immobility. Analgesia and control of autonomic responses to noxious stimuli are also desirable components of general anesthesia [1]. Immobility is determined using the alveolar concentration of an inhaled anesthetic that produces immobility in 50% of subjects: the minimum alveolar concentration, or MAC. Immobility likely arises by anesthetic action in the spinal cord [2,16]. Because physiologically relevant concentrations of many anesthetics robustly enhance the effect of gamma-aminobutyric acid (GABA) at GABA type A (GABAA) receptors, the GABAergic system is thought to be an important target of general anesthetics. GABAA receptors are pentamers encoded by 19 different subunits [19]. The alpha1 subunit is the predominant alpha subunit in may regions of brain and spinal cord of adult animals and is a component of over half of all GABAA receptors [13,19].
Recent advances in genetic engineering have allowed the construction of mouse models harboring point mutations in specific gene targets. These models, termed gene knock-ins, have been invaluable in elucidating drug action. Typically, the only effect of the mutation is to abolish enhancement of receptor function by the drug of interest; normal, endogenous receptor function is unaltered. Recently, GABAA receptor alpha1 subunit gene knock-in mice with mutations that render the receptor resistant to isoflurane have been produced [3]. A serine to histidine change at position 270 produces isoflurane resistance and a leucine to alanine change at position 277 restores normal responses to GABA [3]. Mice possessing both mutations are viable, overtly normal, and have reduced cellular responses to isoflurane [3,22]. We investigated the effects of isoflurane in these mice, hypothesizing that this mutation would increase anesthetic requirements (MAC) and would attenuate isoflurane depression of nociceptive responses in lumbar spinal neurons.
Methods
The University of California, Davis and the University of Pittsburgh Animal Care and Use Committees approved this study which conformed to NIH Guidelines for the use and care of vertebrate animals. Male and female wild-type (WT) or homozygous knock-in (KI) littermates aged 26-32 weeks were used. These mice were generated from heterozygous breeders and genotyped as described [3]. All mice were a mixture of C57BL/6J and 129SvJ background from the F8-F11 generations. The investigators were blinded with regard to genotype.
The mice were placed into a small plastic chamber and anesthetized with isoflurane 3-5%. The mice were removed and allowed to spontaneously breathe isoflurane 1-2% in oxygen that was delivered via a custom-made, tight fitting mask. Isoflurane concentration was measured from the inspired gas flowing into the mask using a calibrated agent analyzer (Puritan-Bennett, Model 254). Rectal temperature was measured with a small thermistor and body temperature was maintained at 36-38°C using a warming blanket and heat lamp. A small incision was made over the back and the muscle and tissue were scraped off the lower thoracic and lumbar spine, as previously described [4]. The lower thoracic spinous processes were removed to expose the lumbar cord. MAC was then determined using a tail clamp / withdrawal assay. After equilibration of the mice with isoflurane (1% atm) for at least 20 min, the tail clamp was applied for 30 sec to evoke gross and purposeful movement, which included turning of the head towards the stimulus or shaking motions of a hindlimb or forelimb. The isoflurane was either decreased or increased 0.2%, depending on the response, and after 15-20 min the clamp was applied again. This process was continued until concentrations were found that just prevented and just permitted movement. The MAC was the mean of these two concentrations. MAC was determined in a total of 38 mice (18 WT and 20 KI). We did not determine MAC nor perform electrophysiological studies in heterozygous mice.
We then performed electrophysiological studies in a subset of these mice (10 WT and 12 KI). The mice were placed into a stereotaxic frame and the spinal column rigidly secured to the frame using two vertebral clamps caudal and rostral to the laminectomy. The dura was incised and warm agar poured onto the cord. The mice spontaneously breathed isoflurane via mask. A microelectrode (tungsten, 8-10 MOhm resistance; FHC, Bowdoinham, ME) was advanced into the lumbar cord using a hydraulic microdrive (Kopf Instruments, Tujunga, CA). Action potential signals were amplified, filtered (300-3000 Hz), displayed on an oscilloscope and sent to computer (Chart5, ADInstruments, Colorado Springs, CO). We sought neurons that responded to innocuous and noxious stimuli applied to the ipsilateral hindpaw. Neurons were classified as wide-dynamic range (WDR) if they increased their response to increasing stimulus strength (from light touch to pinching with a forceps). If the neurons responded only to noxious pinch stimuli they were classified as nociceptive-specific (NS). In many mice, pinching the hindpaw at 0.8 MAC resulted in movement, indicating that the pinch was nearly supramaximal. We did not further study light threshold neurons. Recording depth was estimated from the micrometer reading on the microdrive.
Once a neuron was found, we applied a pinch stimulus to the center of the receptive field using forceps that had been modified by placing a force transducer (SensoTec, Columbus, OH) between the arms of the forceps. The force transducer output was amplified, digitized and displayed on computer. The pinch was applied for 5 s. For any one neuron the force used was constant but the force varied from neuron to neuron, depending on the response characteristics of the neuron. Overall, the force ranged from ≈30-45 N. We applied 4 ± 1 pinches to obtain neuronal responses with 4-5 min between pinches. We recorded responses at 0.8 MAC and at 1.2 MAC, starting with the lower concentration in about half the experiments and the higher concentration in the other half. In two-thirds of the mice we returned to the first isoflurane concentration and re-determined neuronal responses. We waited 15-20 min after changing isoflurane concentrations before determining neuronal responses. In 14 animals, after finishing studying a neuron, we found another neuron and studied it using the same protocol. In addition, in two animals we were able to discriminate two simultaneously recorded action potentials based on differences in amplitude and waveform.
Neuronal responses were counted for the 30 s period before stimulation, during stimulation and for the 55 s period after stimulation (afterdischarge). The responses during the pinch and afterdischarge were combined to make the 60 s response. Responses for each of these periods were averaged for each anesthetic concentration for the KI and WT groups. We also evaluated the neuronal response during the period of noxious stimulation. The neuronal response data were not normally distributed (based on the Kolmogorov-Smirnov test) and therefore we log transformed the data [25] and compared responses at 0.8 MAC and 1.2 MAC using the paired t-test, and responses between the WT and KI groups were compared using the unpaired t-test. MACs of the two groups were compared using the unpaired t-test. A P<0.05 was considered significant. Data are presented as mean ± SEM.
Results
The MAC for isoflurane did not differ between WT (1.1± 0.1%) and KI (1.1 ± 0.1%) mice. For electrophysiological studies, we recorded from 20 neurons in 10 WT animals and 21 neurons in 12 KI animals. Most neurons were of the WDR type: 18 WDR neurons in the WT mice and 17 WDR neurons in the KI mice; 2 NS neurons were in the WT group and 4 NS neurons in the KI group. The recording depth was 450 ± 19 microns in the WT mice and 442 ± 18 microns in the KI mice (no significant difference). Spontaneous activity was generally low, with mean activity around 1 Hz. Figure 1 shows individual examples of action potentials at 0.8 and 1.2 MAC from a WT mouse and a KI mouse. Isoflurane depressed the responses in both animals. Summary data are shown in Figure 2. Increasing isoflurane from 0.8 MAC to 1.2 MAC significantly depressed 60 sec neuronal responses to noxious stimulation by 33% and 36% in the WT and KI mice, respectively (no significant difference between the groups). The neuronal responses during the 5 sec period of noxious pinching showed a similar pattern of reduction from 0.8 to 1.2 MAC, with no difference between the WT and KI mice (29% and 30% reduction, respectively).
Figure 1.
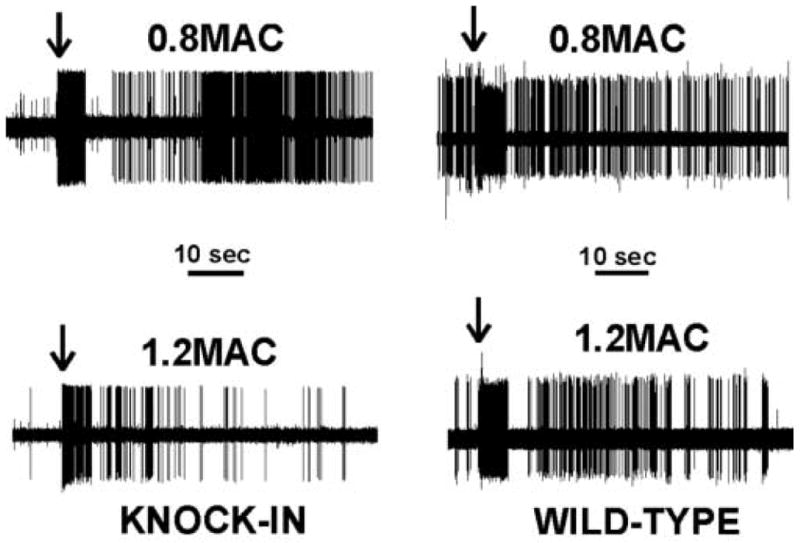
Shown are action potential recordings from knock-in and wild-type mice at 0.8 and 1.2 minimum alveolar concentration (MAC) of isoflurane. Arrows indicate the time at which the pinch was applied to the receptive field on the hindpaw. Isoflurane depressed the responses from 0.8 to 1.2 MAC in both wild-type and knock-in animals.
Figure 2.
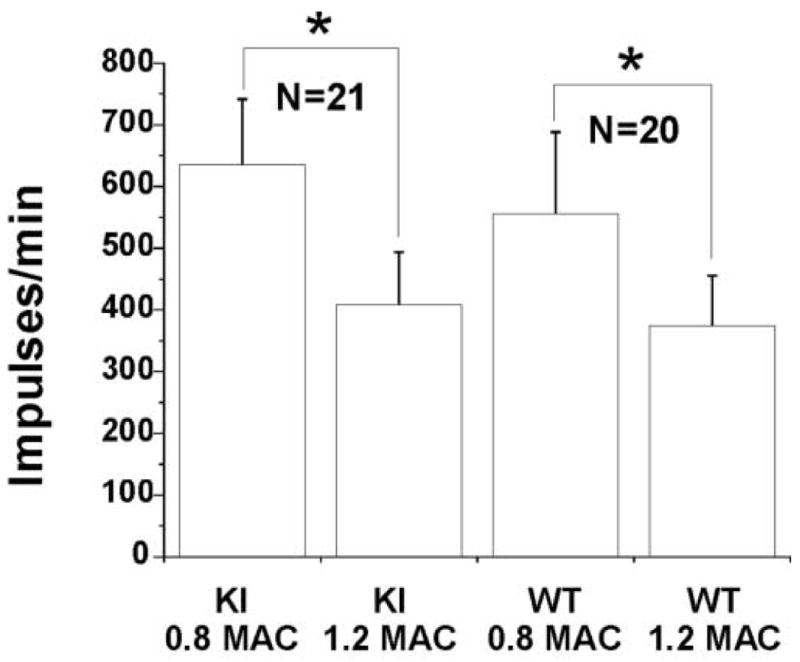
Shown are summary data (mean±SEM) of neuronal responses to noxious stimulation in knock-in (KI) and wild-type (WT) mice at 0.8 and 1.2 minimum alveolar concentration (MAC) of isoflurane. Increasing isoflurane from 0.8 MAC to 1.2 MAC depressed neuronal responses (* p <0.05). No differences were observed between genotypes.
Discussion
The main findings of the present study were that the KI mutation in the alpha1 subunit of the GABAA receptor did not affect anesthetic requirements to prevent movement in response to a noxious stimulus nor did it alter nociceptive responses in the spinal cord. We discuss these findings in relation to the role of GABAA receptors in mediating isoflurane's anesthetic properties.
GABA is the major inhibitory neurotransmitter in the central nervous system and many anesthetics, including isoflurane, robustly enhance the effect of GABA at the GABAA receptor [11]. Much of this work, however, has been done in reduced preparations, such as recombinant receptors expressed in vitro [11]. The fact that an anesthetic alters a receptor in vitro may not necessarily translate into a similar effect in the intact animal. Thus, studies of GABAA-R involvement in anesthetic action in intact animals are vital.
Pharmacological studies employing GABAA receptor antagonists, such as picrotoxin or bicuculline, have suggested at most a minor role for the GABAA receptor in MAC [26-28]. These studies imply that other targets are the primary mediators of MAC for inhaled anesthetics; these targets include receptors for N-methyl-D-aspartate and glycine, and possibly voltage gated sodium channels [5,6,21]. However, pharmacologic studies are limited by the specificity of the drugs used.
Gene knockout studies have also suggested a minor role of specific GABAA receptor subunits in MAC [7,10,14,15]. However, knockout studies are often confounded by compensatory changes that may mask the true function of the inactivated gene [24]. Interpretation of gene KI studies is theoretically more straightforward as the mutation that is introduced typically has no effect on endogenous receptor function. The gene KI approach has been used with great success to clearly define the role of the beta 2 and beta 3 subunits of the GABAA receptor with respect to propofol and etomidate anesthesia [8,17]. The beta 3 KI mice also suggest a minor role for this subunit in inhaled anesthetic action [8,12]. Our gene KI results with anesthetic insensitive alpha1 GABAA receptor gene KI also suggests that these receptors play little role in isoflurane MAC. However, the KI mutations that have been made in the alpha1 subunit are not completely innocuous [3]. Although unlikely, it is conceivable that compensatory changes may be masking the contribution of alpha1 containing GABAA-Rs to isoflurane MAC. Tobler et al similarly found that the alpha1 subunit of the GABAA-R was not important to the effect of diazepam on sleep patterns [23], although the same group has reported that the alpha1 subunit does play a role in the sedative and amnesic actions of benzodiazepines [18].
It is also possible that isoflurane might act additionally at supraspinal GABAA receptors to mediate some aspects of anesthetic immobility. For example, although anesthetics, including isoflurane, act primarily in spinal cord to suppress movement in response to noxious stimulation [2,16], isoflurane has supraspinal effects on nociception. Kingery et al reported that isoflurane had a supraspinal pro-nociceptive effect that dominated a supraspinal antinociceptive effect; this action occurred, in part, at pontine noradrenergic neurons [9]. While the most parsimonious explanation of the present findings is that the GABAA receptor does not play a major role in anesthetic immobility, we cannot rule out a scenario wherein the KI mutation makes the spinal cord more resistant to isoflurane, but simultaneously ablates a supraspinal pro-nociceptive effect. Thus, the overall anesthetic requirement for immobility is not different between the KI and WT mice. Tissue-specific gene KI mice [20] should help to illuminate the contribution of spinal and supraspinal anesthetic targets.
In summary, isoflurane-insensitive GABAA receptor alpha1 subunit gene KI mice did not display increased isoflurane requirements for immobility, nor was spinal nociceptive processing altered. These data suggest that isoflurane produces immobility at sites other than the alpha1 subunit of the GABAA receptor.
Acknowledgments
Supported in part by NIH grants 1P01-47818 and GM61283
NIH grants 1PO1GM47818 (to JFA and GEH) and R01-GM61283 (to JFA) supported this work
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Antognini JF, Carstens E. In vivo characterization of clinical anaesthesia and its components. Br J Anaesth. 2002;89:156–166. doi: 10.1093/bja/aef156. [DOI] [PubMed] [Google Scholar]
- 2.Antognini JF, Schwartz K. Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology. 1993;79:1244–1249. doi: 10.1097/00000542-199312000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Borghese CM, Werner DF, Topf N, Baron NV, Henderson LA, Boehm SL, Blednov YA, Saad A, Dai S, Pearce RA, Harris RA, Homanics GE, Harrison NL. An isoflurane- and alcohol-insensitive mutant GABA(A) receptor alpha(1) subunit with near-normal apparent affinity for GABA: characterization in heterologous systems and production of knockin mice. J Pharmacol Exp Ther. 2006;319:208–218. doi: 10.1124/jpet.106.104406. [DOI] [PubMed] [Google Scholar]
- 4.Cuellar JM, Antognini JF, Carstens E. An in vivo method for recording single unit activity in lumbar spinal cord in mice anesthetized with a volatile anesthetic. Brain Res Brain Res Protoc. 2004;13:126–134. doi: 10.1016/j.brainresprot.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Dutton RC, Laster MJ, Xing Y, Sonner JM, Raines DE, Solt K, Eger EI. Do N-methyl-d-aspartate receptors mediate the capacity of inhaled anesthetics to suppress the temporal summation that contributes to minimum alveolar concentration? Anesth Analg. 2006;102:1412–1418. doi: 10.1213/01.ane.0000205759.67123.76. [DOI] [PubMed] [Google Scholar]
- 6.Eger EI, Liao M, Laster MJ, Won A, Popovich J, Raines DE, Solt K, Dutton RC, Cobos FV, Sonner JM. Contrasting roles of the N-methyl-d-aspartate receptor in the production of immobilization by conventional and aromatic anesthetics. Anesth Analg. 2006;102:1397–1406. doi: 10.1213/01.ANE.0000219019.91281.51. [DOI] [PubMed] [Google Scholar]
- 7.Homanics GE, Ferguson C, Quinlan JJ, Daggett J, Snyder K, Lagenaur C, Mi ZP, Wang XH, Grayson DR, Firestone LL. Gene knockout of the alpha6 subunit of the gamma-aminobutyric acid type A receptor: lack of effect on responses to ethanol, pentobarbital, and general anesthetics. Mol Pharmacol. 1997;51:588–596. doi: 10.1124/mol.51.4.588. [DOI] [PubMed] [Google Scholar]
- 8.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 9.Kingery WS, Agashe GS, Guo TZ, Sawamura S, Frances DM, David CJ, Kobilka BK, Maze M. Isoflurane and Nociception: Spinal alpha2A Adrenoceptors Mediate Antinociception while Supraspinal alpha1 Adrenoceptors Mediate Pronociception. Anesthesiology. 2002;96:367–374. doi: 10.1097/00000542-200202000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Kralic JE, Wheeler M, Renzi K, Ferguson C, O'Buckley TK, Grobin AC, Morrow AL, Homanics GE. Deletion of GABAA receptor alpha 1 subunit-containing receptors alters responses to ethanol and other anesthetics. J Pharmacol Exp Ther. 2003;305:600–607. doi: 10.1124/jpet.102.048124. [DOI] [PubMed] [Google Scholar]
- 11.Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cell Mol Life Sci. 1999;55:1278–1303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao M, Sonner JM, Jurd R, Rudolph U, Borghese CM, Harris RA, Laster MJ, Eger EI. Beta3-containing gamma-aminobutyric acidA receptors are not major targets for the amnesic and immobilizing actions of isoflurane. Anesth Analg. 2005;101:412–8. doi: 10.1213/01.ANE.0000154196.86587.35. table. [DOI] [PubMed] [Google Scholar]
- 13.McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 14.Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci U S A. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinlan JJ, Homanics GE, Firestone LL. Anesthesia sensitivity in mice that lack the beta3 subunit of the gamma-aminobutyric acid type A receptor. Anesthesiology. 1998;88:775–780. doi: 10.1097/00000542-199803000-00030. [DOI] [PubMed] [Google Scholar]
- 16.Rampil IJ, Mason P, Singh H. Anesthetic potency (MAC) is independent of forebrain structures in the rat. Anesthesiology. 1993;78:707–712. doi: 10.1097/00000542-199304000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds DS, Rosahl TW, Cirone J, O'Meara GF, Haythornthwaite A, Newman RJ, Myers J, Sur C, Howell O, Rutter AR, Atack J, Macaulay AJ, Hadingham KL, Hutson PH, Belelli D, Lambert JJ, Dawson GR, McKernan R, Whiting PJ, Wafford KA. Sedation and anesthesia mediated by distinct GABA(A) receptor isoforms. J Neurosci. 2003;23:8608–8617. doi: 10.1523/JNEUROSCI.23-24-08608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 19.Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 20.Skvorak K, Vissel B, Homanics GE. Production of conditional point mutant knockin mice. Genesis. 2006;44:345–353. doi: 10.1002/dvg.20222. [DOI] [PubMed] [Google Scholar]
- 21.Sonner JM, Antognini JF, Dutton RC, Flood P, Gray AT, Harris RA, Homanics GE, Kendig J, Orser B, Raines DE, Rampil IJ, Trudell J, Vissel B, Eger EI. Inhaled anesthetics and immobility: mechanisms, mysteries, and minimum alveolar anesthetic concentration. Anesth Analg. 2003;97:718–740. doi: 10.1213/01.ANE.0000081063.76651.33. [DOI] [PubMed] [Google Scholar]
- 22.Sonner JM, Werner DF, Elsen FP, Xing Y, Liao M, Harris RA, Harrison NL, Fanselow MS, Eger EI, Homanics GE. Effect of isoflurane and other potent inhaled anesthetics on minimum alveolar concentration, learning, and the righting reflex in mice engineered to express alpha1 gamma-aminobutyric acid type A receptors unresponsive to isoflurane. Anesthesiology. 2007;106:107–113. doi: 10.1097/00000542-200701000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Tobler I, Kopp C, Deboer T, Rudolph U. Diazepam-induced changes in sleep: role of the alpha 1 GABA(A) receptor subtype. Proc Natl Acad Sci U S A. 2001;98:6464–6469. doi: 10.1073/pnas.111055398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong SM, Cheng G, Homanics GE, Kendig JJ. Enflurane actions on spinal cords from mice that lack the beta3 subunit of the GABA(A) receptor. Anesthesiology. 2001;95:154–164. doi: 10.1097/00000542-200107000-00026. [DOI] [PubMed] [Google Scholar]
- 25.Zar JH. Biostatistical Analysis. Prentice-Hall, Inc; Englewood Cliffs, N.J.: 1974. Data Transformations; pp. 182–189. [Google Scholar]
- 26.Zhang Y, Sonner JM, Eger EI, Stabernack CR, Laster MJ, Raines DE, Harris RA. Gamma-aminobutyric acidA receptors do not mediate the immobility produced by isoflurane. Anesth Analg. 2004;99:85–90. doi: 10.1213/01.ANE.0000118108.64886.42. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Stabernack C, Sonner J, Dutton R, Eger EI. Both cerebral GABA(A) receptors and spinal GABA(A) receptors modulate the capacity of isoflurane to produce immobility. Anesth Analg. 2001;92:1585–1589. doi: 10.1097/00000539-200106000-00047. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Wu S, Eger EI, Sonner JM. Neither GABA(A) nor strychnine-sensitive glycine receptors are the sole mediators of MAC for isoflurane. Anesth Analg. 2001;92:123–127. doi: 10.1097/00000539-200101000-00024. [DOI] [PubMed] [Google Scholar]


