Abstract
Context
Dietary carbohydrates have been associated with dyslipidemia, a lipid profile known to increase cardiovascular disease risk. Added sugars (caloric sweeteners used as ingredients in processed or prepared foods) are an increasing and potentially modifiable component in the US diet. No known studies have examined the association between the consumption of added sugars and lipid measures.
Objective
To assess the association between consumption of added sugars and blood lipid levels in US adults.
Design, Setting, and Participants
Cross-sectional study among US adults (n=6113) from the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Respondents were grouped by intake of added sugars using limits specified in dietary recommendations (<5% [reference group], 5%–<10%, 10%–<17.5%, 17.5%–<25%, and ≥25% of total calories). Linear regression was used to estimate adjusted mean lipid levels. Logistic regression was used to determine adjusted odds ratios of dyslipidemia. Interactions between added sugars and sex were evaluated.
Main Outcome Measures
Adjusted mean high-density lipoprotein cholesterol (HDL-C), geometric mean triglycerides, and mean low-density lipoprotein cholesterol (LDL-C) levels and adjusted odds ratios of dyslipidemia, including low HDL-C levels (<40 mg/dL for men; <50 mg/dL for women), high triglyceride levels (≥150 mg/dL), high LDL-C levels (≥130 mg/dL), or high ratio of triglycerides to HDL-C (>3.8). Results were weighted to be representative of the US population.
Results
A mean of 15.8% of consumed calories was from added sugars. Among participants consuming less than 5%, 5% to less than 17.5%, 17.5% to less than 25%, and 25% or greater of total energy as added sugars, adjusted mean HDL-C levels were, respectively, 58.7, 57.5, 53.7, 51.0, and 47.7 mg/dL (P<.001 for linear trend), geometric mean triglyceride levels were 105, 102, 111, 113, and 114 mg/dL (P<.001 for linear trend), and LDL-C levels modified by sex were 116, 115, 118, 121, and 123 mg/dL among women (P=.047 for linear trend). There were no significant trends in LDL-C levels among men. Among higher consumers (≥10% added sugars) the odds of low HDL-C levels were 50% to more than 300% greater compared with the reference group (<5% added sugars).
Conclusion
In this study, there was a statistically significant correlation between dietary added sugars and blood lipid levels among US adults.
Increased carbohydrate consumption has been associated with lower high-density lipoprotein cholesterol (HDL-C) levels, higher tri-glyceride levels, and higher low-density lipoprotein cholesterol (LDL-C) levels1—a lipid profile associated with cardiovascular disease risk.2 In the United States, total consumption of sugar has increased substantially in recent decades, largely owing to an increased intake of “added sugars,”3 defined as caloric sweeteners used by the food industry and consumers as ingredients in processed or prepared foods4 to increase the desirability of these foods.5 Dietary data from 1994–1996 demonstrate that US individuals aged 2 years or older consume nearly 16% of their daily energy as added sugars.3 Today, the most commonly consumed added sugars are refined beet or cane sugar (sucrose) and high-fructose corn syrup.6
While chemically there appears to be little difference between naturally occurring sugars and those added to foods, in 2000 the US Dietary Guidelines began to use the term added sugars to help consumers identify foods that provide energy but few micronutrients or phytochemicals.7 Consumption of foods high in added sugars has been associated with increased obesity,8 diabetes,9 and dental caries10 and with decreased diet quality.11 Dietary guidelines for added sugars vary widely. The Institute of Medicine suggests a limit of 25% of total energy,12 the World Health Organization advises less than 10% of total energy,13 and recent recommendations from the American Heart Association advise limiting added sugars to fewer than 100 calories daily for women and 150 calories daily for men (approximately 5% of total energy).14
Although consumption of added sugars represents an important and potentially modifiable component of the diet, no known studies have examined the correlation between consumption of added sugars and lipid measures. The purpose of this study was to assess this association among US adults.
METHODS
Participants
Study participants included US adults older than 18 years who participated in the National Health and Nutrition Examination Survey (NHANES) 1999–2006. NHANES is a continuous survey of the US civilian, noninstitutionalized population designed to obtain nationally representative estimates on diet and health indicators.15 The sample for NHANES is selected using a complex, multistage sampling design. Study protocols for NHANES 1999–2006 were approved by the institutional review board at the National Center for Health Statistics.16 Signed informed consent was obtained from all participants.
A total of 8495 adults older than 18 years provided fasting blood samples for NHANES 1999–2006. Excluded were pregnant respondents (n=495); respondents reporting an unreliable or implausible dietary intake (<600 or >4000 kcal/d) (n=403); those with extreme triglyceride levels (>400 mg/dL [to convert to mmol/L, multiply by 0.0113]) (n=206) or extreme body mass index (BMI [>65, calculated as weight in kilograms divided by height in meters squared]) (n = 1); and those taking cholesterol-lowering medications (n=887). Because insulin resistance is known to alter lipid metabolism and persons known to have diabetes are likely to change their dietary practices, those with diagnosed diabetes (n = 390) were also excluded. After these exclusions, the total sample for this study included 6113 adults (3088 women, 3025 men).
Added Sugars and Other Dietary Intake
An interviewer-assisted 24-hour dietary recall (midnight to midnight of the previous day) was used to assess dietary intake from all respondents. Because associations between nutrient intake assessed using a single 24-hour recall and health outcomes can be attenuated owing to the inability to account for day-to-day variations in intake,17 we repeated our analysis among a subsample of respondents from whom 2 dietary recalls were collected (respondents participating in NHANES 2003–2006).
Nutrient content of the foods consumed was determined by NHANES using the Food and Nutrient Database for Dietary Studies, which uses food composition data from the US Department of Agriculture National Nutrient Database for Standard Reference.18 Because that database does not include information on the added sugar content of many foods, individual food files from NHANES were merged with the most recently released MyPyramid Equivalents Database files (1999–2000, 2001–2002, and 2003–2004).19 The MyPyramid Equivalents Database translates the amounts of foods eaten in the dietary intake component of the NHANES into the number of equivalents of the MyPyramid food groups using recommended serving sizes from the US Department of Agriculture Food Guide Pyramid. Added sugars are one of the 30 food groups and subgroups used in the pyramid. A description of the MyPyramid database20 and the methods used to calculate the sugar content of foods are available elsewhere.21
Because MyPyramid serving size equivalents have been released only for the foods reported in NHANES through the 2003–2004 cycle, we used the available data to estimate the added sugar content of foods consumed by participants in NHANES 2005–2006 to include the most recent NHANES data in our analysis. In the 2005–2006 NHANES cycle, respondents reported foods represented by 5308 unique USDA food code and modification code combinations. Added sugar content for 4971 of these foods was available from the MyPyramid Equivalents 2003–2004 database, leaving 337 foods for which the added sugar content had to be estimated. The majority of these, 213 of 337 (63%), were slightly modified forms of foods for which added sugar content was available on the MyPyramid database. To these foods, the added sugar content of the unmodified form was assigned. The added sugar values for the majority of the remaining foods were imputed using values obtained from similar foods. For example, “sweetpotato, canned in syrup, w/fat added” was reported in the 2005–2006 dietary recall but did not have a corresponding MyPyramid database equivalent. The added sugar content of this food was assigned the same value as “sweetpotato, canned, ns (not specific) as to syrup.” This substitution method was used for 92 USDA food code and modification code combinations. The added sugar values for the remaining 32 items were calculated directly from nutrition label information available on food industry Web sites.
Lipid Measures
Dyslipidemia is commonly characterized by 3 lipid abnormalities: elevated triglyceride levels, elevated levels of small LDL-C particles, and reduced HDL-C levels.2 We used the cutoffs for plasma lipids as established by the Adult Treatment Panel III guidelines published by the National Institutes of Health.2 These include low HDL-C level (<40 mg/dL for men; <50 mg/dL for women [to convert to millimoles per liter, multiply by 0.0259]), high LDL-C level (≥130 mg/dL [to convert to millimoles per liter, multiply by 0.0259]), or high triglyceride level (≥150 mg/dL). In addition, the ratio of triglycerides to HDL-C was used as a measure of dyslipidemia, because a ratio greater than 3.8 has been shown to correlate well with the LDL-C phenotype (type B) associated with the small LDL-C particles most strongly linked with risk of cardiovascular disease.22 Standardized laboratory procedures used to obtain serum or plasma HDL-C and triglyceride measures have been described elsewhere.23 Levels of LDL-C were calculated by NHANES using the Friedewald formula:
| 23 |
Covariates
Intake of added sugars was examined in relation to known risk factors for cardiovascular disease.24 Variables that have been demonstrated to be associated with intake of carbohydrates as well as lipid outcome measures were included in regression models to evaluate and, as necessary, control for possible confounding. These include measures obtained by NHANES staff using standardized protocols, including BMI, waist circumference, and blood pressure. Self-reported measures included participant’s age, sex, leisure-time physical activity over the previous month, cigarette use, alcohol consumption, history of attempted weight loss in the previous year, weight change (calculated as the difference between reported current weight in pounds and reported weight 1 year previous), and use of antihypertensive medication. Because intake of added sugars25 and blood lipid response to diet26 have both been shown to vary by race/ethnicity, self-identified race/ethnicity15 was included as a covariate.
Dietary covariates included the energy-adjusted nutrient residuals for fiber, other carbohydrates (other than added sugars and fiber), saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, and cholesterol. These nutrient residuals were calculated using linear regression models with total calorie intake as the predictor and the absolute intake of each nutrient of interest (in grams) as the outcome.
Data Analysis
Statistical Analysis Software version 9.2 (SAS Institute Inc, Cary, North Carolina) was used for all analyses. Procedures that account for the complex sampling methods used in NHANES were applied. Sample weights for the 6 years of data were calculated using the formula [½ × wtsfa4yr (fasting sample weight for NHANES 1999–2002)] + [¼ × wtsfa2yr (fasting sample weight for NHANES 2003–2004)] + [¼ × wtsaf2yr (fasting sample weight for NHANES 2005–2006)],27 and these weights were used to ensure results were representative of the US population. Respondents were grouped according to their consumption of added sugars (<5% [reference group], 5%–<10%, 10%–<17.5%, 17.5%–<25%, and ≥25% of total energy intake). These groupings incorporate the limits for added sugars specified in existing dietary guidelines. All P values were 2-sided; P< .05 was considered statistically significant.
To determine the amount of added sugars consumed, we multiplied the total amount of each food consumed in grams (as provided in the NHANES database) by the amount of added sugars in each of these foods (teaspoons/100 g) (as provided in the MyPyramid database). The results for all foods were summed to obtain the total intake of added sugars for each respondent in teaspoons. These intakes were converted to grams by multiplying by 4.2 g/tsp.28 The result in grams was multiplied by 4 to obtain the total calories from added sugars. The result was then divided by total energy intake (kilocalories per day) to obtain the percent of total energy from added sugars.
Weighted frequencies, means, and confidence intervals (CIs) were calculated to describe the sample population by added sugar consumption level. The distribution of triglyceride levels was skewed; therefore, values were log transformed and geometric means are presented. Because differences in the postprandial lipoprotein response have been shown between men and women,29,30 we first tested for the presence of an interaction (P< 1.0) by including a multiplicative term between percent total energy from added sugars and sex in each of the linear regression models. Estimate statements in linear regression models, with intake of added sugars (categorized by consumption level) as the predictor, were used to determine the adjusted mean of each of the lipid measures with increased consumption of added sugars.
Logistic regression models were used to estimate the adjusted odds of dyslipidemia among respondents who consumed higher levels of added sugars compared with the reference group (those consuming <5% energy from added sugars). The presence of a linear trend was tested by defining a linear contrast in each of the linear and logistic regression models.
A sensitivity analysis was performed using dietary data from a second 24-hour recall collected from a 40% sub-sample of the respondents (those participating in NHANES 2003–2004 and NHANES 2005–2006) (n=2506). In this analysis, we used the mean intake of added sugars from the 2 dietary recalls (% total energy from added sugars) and controlled for the same dietary covariates (using the mean of the 2 dietary recalls for each) and other covariates as specified in the primary analyses.
RESULTS
A description of the study sample by intake of added sugars is provided in Table 1. As intakes of added sugars increase, respondents are more likely to be younger, non-Hispanic black, and have low income. Intake of added sugars was correlated positively with the number of cigarettes smoked and negatively with being hypertensive. Self-reported weight change over the previous year tended to be greater among respondents consuming more added sugars: a mean gain of 2.8 pounds was observed among those with 25% or greater total energy from added sugars compared with a mean loss of 0.3 pounds among those consuming less than 5% total energy from added sugars (P<.001 for linear trend). No significant trends were seen between consumption of added sugars and BMI or waist circumference.
Table 1.
Demographic and Dietary Characteristics of Adults (>18 Years) in NHANES 1999–2004 by Percent Total Energy Intake From Added Sugara,b,c
| Characteristic | % Total Energy From Added Sugar |
||||
|---|---|---|---|---|---|
| <5 (n = 893) | 5–<10 (n = 1124) | 10–<17.5 (n = 1751) | 17.5–<25 (n = 1210) | ≥25 (n = 1135) | |
| Age, mean (SD) [95% CI], yd | 45.9 (18.1) [44.7 to 47.1] |
45.7 (22.1) [44.0 to 47.0] |
44.5 (20.3) [43.5 to 45.5] |
42.6 (19.2) [41.5 to 43.7] |
38.1 (16.4) [37.1 to 39.1] |
| Men, No. (%) [95% CI] | 444 (48) [44 to 53] |
551 (46) [42 to 49] |
855 (46) [43 to 49] |
628 (52) [49 to 55] |
588 (47) [43 to 50] |
| Race/ethnicity, No. (%) [95% CI] | |||||
| Non-Hispanic whitee | 465 (71) [68 to 78] |
619 (75) [72 to 79] |
897 (73) [69 to 77] |
555 (68) [64 to 72] |
493 (70) [64 to 75] |
| Non-Hispanic blackd | 144 (8.2) [6 to 10] |
174 (8.0) [6 to 1] |
316 (10) [8 to 12] |
276 (14) [11 to 17] |
313 (15) [11 to 18] |
| Hispanic | 229 (11) [8 to 15] |
289 (12) [9 to 15] |
475 (13) [10 to 16] |
355 (15) [12 to 18] |
301 (13) [9 to 16] |
| Income below poverty, No. (%) [95% CI]d,f | 194 (14) [11 to 17] |
187 (18) [14 to 21] |
415 (18) [16 to 20] |
287 (18) [15 to 20] |
318 (23) [20 to 26] |
| Physical activity, mean (SE) [95% CI]e,g | 5217 (423) [4370 to 6064] |
4984 (379) [4226 to 5742] |
5205 (351) [4503 to 5908] |
5553 (494) [4564 to 6541] |
3957 (323) [3309 to 4605] |
| Alcohol consumption, mean (SE) [95% CI], drinks/d | 2.3 (0.1) [2.0 to 2.5] |
1.8 (0.1) [1.6 to 2.0] |
1.8 (0.1) [1.7 to 2.0] |
1.7 (0.1) [1.6 to 1.9] |
2.0 (0.1) [1.8 to 2.2] |
| Smoking, mean (SE) [95% CI], cigarettes/dd | 3.2 (0.3) [2.6 to 4.0] |
2.5 (0.3) [1.9 to 3.1] |
3.5 (0.4) [2.7 to 4.3] |
3.7 (0.6) [2.8 to 4.5] |
6.2 (0.9) [4.5 to 8.0] |
| Waist circumference, mean (SE) [95% CI], cm | 95.5 (0.8) [93.8 to 97.2] |
94.9 (0.7) [93.5 to 96.7] |
94.0 (0.5) [92.9 to 95.0] |
94.5 (0.6) [92.2 to 94.4] |
95.0 (0.6) [93.9 to 96.1] |
| BMI, mean (SE) [95% CI]h | 27.9 (0.3) [27.2 to 28.5] |
27.8 (0.3) [27.2 to 28.3] |
27.3 (0.2) [26.9 to 27.7] |
27.7 (0.2) [27.3 to 28.2] |
28.0 (0.2) [27.6 to 28.5] |
| Weight change, mean (SE) [95% CI], lbd | −0.3 (0.7) [−1.6 to 1.1] |
−0.2 (0.5) [−1.2 to 0.8] |
30.9 (0.4) [0.19 to 1.7] |
31.5 (0.5) [0.5 to 2.4] |
32.8 (0.6) [1.6 to 4.0] |
| Attempted weight loss, No. (%) [95% CI] | 266 (37) [33 to 41] |
353 (38) [35 to 42] |
502 (37) [35 to 40] |
346 (33) [29 to 37] |
332 (35) [32 to 39] |
| Hypertensive, No. (%) [95% CI]i,j | 200 (19) [15 to 23] |
244 (21) [17 to 24] |
319 (15) [13 to 17] |
205 (14) [11 to 16] |
174 (14) [11 to 17] |
| Total energy, mean (SE) [95% CI], kcal/dd | 2038 (33) [1975 to 2100] |
2172 (27) [2119 to 2226] |
2235 (21) [2194 to 2277] |
2315 (31) [2252 to 2377] |
2312 (35) [2242 to 2382] |
| % energy from carbohydrates, mean (SE) [95% CI]d | 40.9 (0.8) [39.8 to 42.0] |
45.5 (0.4) [44.7 to 46.2] |
48.4 (0.3) [47.8 to 49.0] |
52.3 (0.3) [51.6 to 53.0] |
59.8 (3.2) [59.1 to 60.4] |
| Added sugar, mean (SE) [95% CI], gd | 13.6 (0.4) [12.7 to 14.5] |
41.4 (0.6) [40.1 to 42.6] |
76.7 (0.7) [75.2 to 78.2] |
122 (1.6) [118 to 125] |
192 (3.3) [185 to 199] |
| Fiber, mean (SE) [95% CI], gd | 16.2 (0.5) [15.2 to 17.1] |
17.6 (0.4) [16.7 to 18.4] |
16.1 (0.3) [15.5 to 16.6] |
15.0 (0.1) [14.2 to 15.9] |
12.0 (0.3) [11.4 to 12.5] |
| % Energy from protein, mean (SE) [95% CI]d | 18.1 (0.3) [17.6 to 18.7] |
16.6 (0.2) [16.3 to 17.0] |
15.5 (0.1) [15.3 to 15.8] |
14.2 (0.1) [13.9 to 14.5] |
11.8 (0.1) [11.6 to 12.1] |
| % Energy from fats, mean (SE) [95% CI]d | 35.6 (0.5) [34.5 to 36.7] |
34.9 (0.4) [34.1 to 35.7] |
34.3 (0.3) [33.8 to 34.8] |
33.2 (0.3) [32.6 to 33.7] |
28.9 (0.2) [28.4 to 29.4] |
| Saturated fatty acidsd | 11.3 (0.2) [10.8 to 11.7] |
11.3 (0.2) [10.9 to 11.7] |
11.4 (0.1) [11.1 to 11.7] |
11.0 (0.1) [10.7 to 11.3] |
9.7 (0.1) [9.4 to 9.9] |
| Polyunsaturated fatty acidsd | 7.8 (0.2) [7.4 to 8.2] |
7.5 (0.1) [7.2 to 7.8] |
7.2 (0.1) [6.9 to 7.3] |
6.9 (0.1) [6.6 to 7.2] |
5.8 (0.1) [5.7 to 6.0] |
| Monounsaturated fatty acidsd | 13.3 (0.2) [12.8 to 13.7] |
12.9 (0.2) [12.6 to 13.3] |
12.7 (0.1) [12.4 to 12.9] |
12.3 (0.1) [12.1 to 12.5] |
10.8 (0.1) [10.6 to 11.0] |
| Cholesterol intake, mean (SE) [95% CI], gd | 312 (10) [291 to 333] |
293 (9.2) [275 to 312] |
308 (7.2) [293 to 322] |
295 (6.7) [282 to 309] |
238 (7.7) [222 to 253] |
Abbreviations: BMI, body mass index; CI, confidence interval; NHANES, National Health and Nutrition Examination Survey.
n=4605; excluded pregnant respondents and respondents with implausible diet, diagnosed diabetes, triglyceride level greater than 400 mg/dL (to convert to mmol/L, multiply by 0.0113), or receiving treatment for elevated cholesterol levels.
Results were weighted and adjusted to account for NHANES complex sampling methodology.
Analysis of contrasts used to test trends, χ2 tests for categorical variables, and Wald F tests for continuous variables.
P<.001 for linear trend.
P<.05 for linear trend.
Income level was dichotomized based on poverty-income ratio (ratio of annual family income to federal poverty line). Below poverty indicates income at or below 130% of poverty.
Leisure-time physical activity over the previous month, defined as the sum of the duration (minutes)× frequency× metabolic equivalent intensity level (MET score) for each activity.
Calculated as weight in kilograms divided by height in meters squared.
P<.01 for linear trend.
Systolic blood pressure of 130 mm Hg or greater and diastolic blood pressure of 85 mm Hg or greater or taking antihypertensive medication.
Daily consumption of added sugars averaged 89.8 g (21.4 tsp [359 kcal). This represents 15.8% (95% CI, 15.3%–16.4%) of total daily caloric intake (total energy) and 30.7% (95% CI, 29.7%–31.7%) of total carbohydrate intake (not shown).
Total energy and percent total energy from carbohydrates increased as the proportion of energy from added sugars increased from less than 5% total energy to 25% or greater (P <.001 for linear trend for both) (Table 1). Intake of added sugars was negatively correlated with percent total energy from total, polyunsaturated, monounsaturated, and saturated fats; protein; fiber; and cholesterol (P<.001 for linear trend for all).
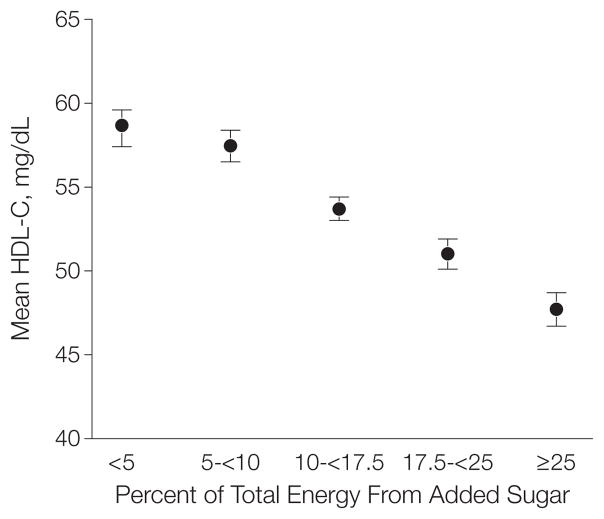
In the linear regression models we found no significant modification by sex for HDL-C level (P = .14), log-transformed triglyceride level (P=.89), or ratio of triglycerides to HDL-C (P=.93), but we did find that sex significantly modifies the correlation of added sugars and LDL-C levels (P=.01). Adjusted mean HDL-C levels were lower among respondents consuming higher amounts of added sugars: 58.7 (95% CI, 57.4–60.0) mg/dL among those consuming less than 5% energy from added sugars, 57.5 (95% CI, 56.5–58.4) mg/dL among those consuming 5% to less than 10%, 53.7 (95% CI, 53.0–54.4) mg/dL among those consuming 10% to less than 17.5%, 51.0 (95% CI, 50.1–51.9) mg/dL among those consuming 17.5% to less than 25%, and 47.7 (95% CI, 46.7–48.8) mg/dL among those consuming 25% or greater (P <.001 for linear trend) (Figure 1).
Figure 1. Multivariable-Adjusted Mean HDL-C Levels by Level of Added Sugar Intake Among US Adults, NHANES 1999–2006.
Participants grouped by percentage of total energy intake from added sugar; <5% comprises the reference group. P<.001 for linear trend. Error bars indicate 95% confidence intervals. HDL-C indicates high-density lipoprotein cholesterol; NHANES, National Health and Nutrition Examination Survey. To convert values to mmol/L, multiply by 0.0259. The 3 highest categories (10–<17.5, 17.5–<25, and ≥25) were significantly lower than the referent group (P<.001).
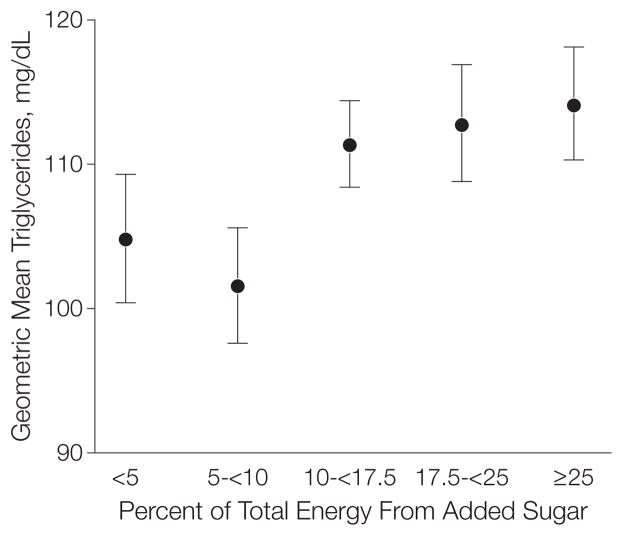
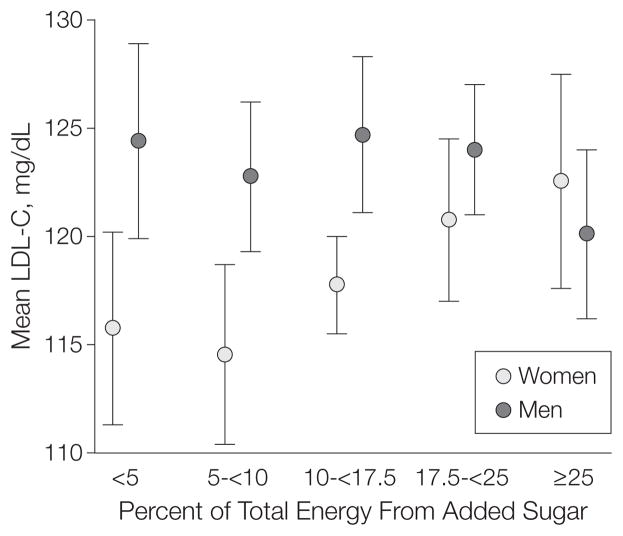
Geometric mean triglyceride levels were 105 (95% CI, 100–109) mg/dL among respondents consuming less than 5% energy from added sugars, 102 (95% CI, 98–106) mg/dL among those consuming 5% to less than 10%, 111 (95% CI, 108–114) mg/dL among those consuming 10% to less than 17.5%, 113 (95% CI, 109–117) mg/dL among those consuming 17.5% to less than 25%, and 114 (95% CI, 110–118) mg/dL among those consuming 25% or greater (P=.02 for linear trend) (Figure 2). Among these same consumption groups, ratios of triglycerides to HDL-C were 2.4 (95% CI, 2.2–2.5), 2.3 (95% CI, 2.2–2.4), 2.6 (95% CI, 2.5–2.7), 2.8 (95% CI, 2.6–2.9), and 3.1 (95% CI, 2.9–3.2), respectively (P <.001 for trend) (not shown); and LDL-C levels among women were 116 (95% CI, 111–120) mg/dL, 115 (95% CI, 110–118) mg/dL, 118 (95% CI, 116–120) mg/dL, 121 (95% CI, 117–124) mg/dL, and 123 (95% CI, 118–128) mg/dL, respectively (Figure 3) (P=.047 for linear trend). There were no significant linear (P=.17) or nonlinear (P=.39) trends between intake of added sugars and LDL-Cs among men.
Figure 2. Multivariable-Adjusted Geometric Mean Triglyceride Levels by Level of Added Sugar Intake Among US Adults, NHANES 1999–2006.
Participants grouped by percentage of total energy intake from added sugar; <5% comprises the reference group. P=.02 for linear trend. Error bars indicate 95% confidence intervals. NHANES indicates National Health and Nutrition Examination Survey. To convert values to mmol/L, multiply by 0.0113. The categories 10–<17.5 and 17.5–<25 were significantly higher than the referent group at P<.05, and the category ≥25 was significantly higher at P<.01.
Figure 3. Multivariable-Adjusted Mean LDL-C Levels by Level of Added Sugar Intake Among US Men and Women, NHANES 1999–2006.
Participants grouped by percentage of total energy intake from added sugar; <5% comprises the reference group. Linear trend: P=.047 (women) and P=.17 (men). Error bars indicate 95% confidence intervals. LDL-C indicates low-density lipoprotein cholesterol; NHANES, National Health and Nutrition Examination Survey. To convert values to mmol/L, multiply by 0.0259.
The odds of having a low HDL-C level were greater with higher consumption of added sugars (Table 2). Compared with respondents consuming less than 5% energy from added sugars, the adjusted odds ratios (ORs) were 1.0 (95% CI, 0.8–1.4) among those consuming 5% to less than 10% energy from added sugars, 1.5 (95% CI, 1.2–1.9) among those consuming 10% to less than 17.5%, 1.9 (95% CI, 1.5–2.6) among those consuming 17.5% to 25%, and 3.1 (95% CI, 2.3–4.3) among those consuming 25% or greater (P<.001 for linear trend).
Table 2.
Adjusted Odds Ratios of Dyslipidemia Among US Adults (>18 Years) Associated With Consumption of Added Sugara
| Dyslipidemia Measure | %Total Energy From Added Sugar |
||||
|---|---|---|---|---|---|
| <5 (n = 893) | 5–<10 (n = 1124) | 10–<17.5 (n = 1751) | 17.5–<25 (n = 1210) | ≥25 (n = 1135) | |
| Low HDL-C (<50 mg/dL [women]; <40 mg/dL [men]) | |||||
| Prevalence, % | 22.4 | 22.6 | 28.2 | 31.7 | 43.9 |
| Adjusted OR (95% CI) | |||||
| Model 1b,c | 1 [Reference] | 1.0 (0.7–1.4) | 1.3 (1.0–1.7) | 1.6 (1.2–2.0) | 2.6 (2.0–3.4) |
| Model 2c,d | 1 [Reference] | 1.0 (0.8–1.4) | 1.5 (1.2–1.9) | 1.9 (1.5–2.6) | 3.1 (2.3–4.3) |
| High triglycerides (>150 mg/dL) | |||||
| Prevalence, % | 26.4 | 22.9 | 27.0 | 28.7 | 28.0 |
| Adjusted OR (95% CI) | |||||
| Model 1b,e | 1 [Reference] | 0.8 (0.7–1.1) | 1.1 (0.9–1.3) | 1.2 (0.9–1.4) | 1.3 (1.0–1.7) |
| Model 2d,e | 1 [Reference] | 0.8 (0.7–1.1) | 1.1 (0.9–1.4) | 1.3 (1.0–1.6) | 1.2 (0.9–1.6) |
| High LDL-C (>130 mg/dL) | |||||
| Prevalence, % | 37.3 | 35.1 | 36.9 | 37.0 | 35.5 |
| Adjusted OR (95% CI) | |||||
| Model 1b | 1 [Reference] | 0.9 (0.7–1.2) | 1.0 (0.8–1.3) | 1.1 (0.8–1.3) | 1.1 (0.9–1.5) |
| Model 2d | 1 [Reference] | 0.9 (0.7–1.2) | 1.1 (0.9–1.3) | 1.1 (0.9–1.5) | 1.2 (0.9–1.7) |
| High triglycerides–HDL-C ratio (>3.8) | |||||
| Prevalence, % | 19.9 | 15.3 | 19.7 | 23.4 | 24.9 |
| Adjusted OR (95% CI) | |||||
| Model 1b,c | 1 [Reference] | 0.7 (0.5–1.0) | 1.0 (0.8–1.3) | 1.2 (0.9–1.6) | 1.5 (1.1–2.0) |
| Model 2c,d | 1 [Reference] | 0.7 (0.5–1.0) | 1.1 (0.8–1.4) | 1.5 (1.1–2.0) | 1.6 (1.1–2.3) |
Abbreviations: CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio.
SI conversion factors: To convert HDL-C and LDL-C values to mmol/L, multiply by 0.0259; triglycerides values to mmol/L, multiply by 0.0113.
All results are weighted and adjusted to account for NHANES complex sampling methodology.
Adjusted for age, race/ethnicity, sex.
P<.001 by χ2 test for trend.
Adjusted for age; sex; race/ethnicity; poverty; body mass index; waist circumference; weight change; physical activity; total energy intake; nutrient residuals for intake of mono-unsaturated fatty acids, polyunsaturated fatty acids, saturated fatty acids, cholesterol, fiber, and other carbohydrates (excluding fiber and added sugars); hypertension; cigarette smoking; and alcohol use.
P<.05 by χ2 test for trend.
The trends in adjusted ORs with higher intake of added sugars were also positive for triglyceride levels (P=.02) and for ratio of triglycerides to HDL-C (P<.001 for linear trend) (Table 2). Adjusted ORs of high triglyceride levels among these same consumption groups were 0.8 (95% CI, 0.7–1.1), 1.1 (95% CI, 0.9–1.4), 1.3 (95% CI, 1.0–1.6), and 1.2 (95% CI, 0.9–1.6), respectively, compared with the reference group, and adjusted ORs of high ratio of triglycerides to HDL-C were 0.7 (95% CI, 0.5–1.0), 1.1 (95% CI, 0.8–1.4), 1.5 (95% CI, 1.1–2.0), and 1.6 (95% CI, 1.1–2.3), respectively. There was no significant trend in adjusted ORs of high LDL-C level with greater intake of added sugars.
The adjusted mean HDL-C level, geometric mean triglyceride level, and mean ratio of triglycerides to HDL-C obtained when using the mean intake of added sugars from the subsample with two 24-hour dietary recalls as the exposure were similar in magnitude (≤10%) and in trend to those obtained in the full sample using a single 24-hour recall (linear trend: HDL-C level, P <.001; log triglyceride level, P <.02; ratio of triglycerides to HDL-C, P <.001) (not shown). Among women in the subsample there was no longer a positive linear trend in LDL-C levels with greater added sugar intake (P=.61).
COMMENT
The consumption of large amounts of added sugars, a prominent source of low-nutrient calories, is a relatively new phenomenon. It was not until the mid-19th century that these sweeteners became widely available and consumption began to increase dramatically.31 Individuals in the United States now consume a substantial proportion of their total energy as added sugars. The adults in our study consumed nearly one-sixth (15.8%) of their daily calories from added sugars. This represents a substantial increase from 1977–1978, when added sugars contributed only 10.6% of the calories consumed by adults.32
Monitoring trends in consumption and understanding the effect added sugars have on risk of cardiovascular and other diseases is critically important, because added sugars are a potentially modifiable source of calories. While it has been known for some time that carbohydrates can increase the risk of cardiovascular disease by altering lipid profiles, this knowledge has been difficult to translate effectively into improvement in dietary practices. This is likely owing to lack of data identifying clear points for consumption limits and because carbohydrates and sugars are found in a wide variety of foods ranging from fruits, vegetables, and whole grains to soft drinks. Unlike most other carbohydrates, added sugars alone contribute no nutrients other than energy. Added sugars are food additives that can be recognized by consumers and have been proposed for specific labeling on food and beverage packaging. The results of our study demonstrate that increased added sugars are associated with important cardiovascular disease risk factors, including lower HDL-C levels, higher triglyceride levels, and higher ratios of triglycerides to HDL-C.
The mechanism through which the dysmetabolic effects of carbohydrates occur is not completely understood. Studies suggest that these effects could be mediated by fructose, a monosaccharide found in large quantities in nearly all added sugars. Fructose has been shown to increase de novo lipogenesis in the liver, hepatic triglyceride synthesis, and secretion of very low-density lipoproteins. Fructose also appears to decrease the peripheral clearance of lipids.14
Our results support the importance of dietary guidelines that encourage consumers to limit their intake of added sugars. The 2005 US Dietary Guidelines do not provide a quantified intake guideline for added sugars, suggesting only that consumers “choose and prepare foods and beverages with little added sugars or caloric sweeteners.” The new Food Guide Pyramid (the federal nutrition education tool designed to translate the US Dietary Guidelines into kinds and amounts of food to eat each day) includes calories consumed as added sugars as part of “discretionary calories,” ie, those not required to meet nutrient needs. Most discretionary calorie allowances are small (between 100 and 300 calories), especially for individuals who are not physically active—a level of added sugars substantially lower than that currently consumed by adults in the United States. New guidelines from the American Heart Association encourage adults to limit added sugars more than any of the previously issued guidelines.12–14 Women are advised to limit their added sugars to fewer than 100 calories daily and men to fewer than 150 calories daily (approximately 5% of total energy intake).
Recommendations to reduce cardiovascular disease risk have long promoted a diet low in fat and cholesterol to lower levels of serum total cholesterol and LDL-C.33,34 Possibly as a result, the consumption of added fats and oils appears to have decreased, and intakes of refined carbohydrates appear to have increased.35 While the overall effect of these dietary trends is unclear, there is a need to review the dietary recommendations to see how they influence intake of added sugars and to develop further understanding of the role different carbohydrates and sugars play in increasing risk of chronic disease.
Our study has several important strengths. First, we used nationally representative data and, to our knowledge, this is the first study to assess the association between intake of added sugar and lipid measures among US adults. Second, we were able to control for several important confounding variables, including BMI, physical activity, total energy intake, and other dietary components. Third, the use of trained staff following standardized protocols to measure height and weight and collect laboratory and interview data increases the accuracy and validity of the data collected.
Our study also has some limitations. A single 24-hour dietary recall was used to assess diet and may not represent the usual diet of respondents. Compared with food frequency questionnaires, 24-hour recalls provide greater detail on the types and amounts of food eaten, but the inability to measure within-person variability can cause misclassification.36 The similarity between the results in the subsample analysis using the mean of 2 dietary recalls and those obtained in the full sample with a single dietary recall suggests that the effect of misclassification attributable to unmeasured variability was limited in our study. While underreporting of certain foods high in sugar, such as sodas and sweets, may occur more frequently among some groups also at increased risk of dyslipidemia, such as groups comprising overweight or obese individuals,37 systematic misclassification of this type would be expected to bias our findings toward the null. In addition, studies that use a cross-sectional design such as ours are limited in that exposures and outcomes are measured at the same time. As a result, our data can be used only to assess associations. They cannot be used to determine causality or even to assess directionality or temporality of the associations observed.
In conclusion, higher consumption of added sugars is associated with several important measures of dyslipidemia, an important risk factor for cardiovascular disease among US adults. Although long-term trials to study the effect of reducing added sugars and other carbohydrates on lipid profiles are needed, our data support dietary guidelines that target a reduction in consumption of added sugar.
Footnotes
Disclaimer: The findings reported in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author Contributions: Ms Welsh had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Welsh, Vos.
Acquisition of data: Welsh, Gillespie.
Analysis and interpretation of data: Welsh, Sharma, Abramson, Vaccarino, Gillespie, Vos.
Drafting of the manuscript: Welsh, Vos.
Critical revision of the manuscript for important intellectual content: Welsh, Sharma, Abramson, Vaccarino, Gillespie, Vos.
Statistical analysis: Welsh, Sharma, Gillespie.
Administrative, technical, or material support: Vaccarino, Vos.
Study supervision: Vos.
Financial Disclosures: Dr Vos reported that she is the author of and receives royalties from a book about childhood obesity and that she is supported in part by a career award from the National Institute of Diabetes and Digestive and Kidney Diseases (K23DK080953) and from the Children’s Digestive Health and Nutrition Foundation. No other authors reported disclosures.
References
- 1.Stanhope KL, Havel PJ. Fructose consumption: considerations for future research on its effects on adipose distribution, lipid metabolism, and insulin sensitivity in humans. J Nutr. 2009;139(6):1236S–1241S. doi: 10.3945/jn.109.106641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 3.Krebs-Smith SM. Choose beverages and foods to moderate your intake of sugars: measurement requires quantification. J Nutr. 2001;131(2S-1):527S–535S. doi: 10.1093/jn/131.2.527S. [DOI] [PubMed] [Google Scholar]
- 4.Dietary guidelines for Americans. 6. US Department of Health & Human Services; Jan, 2005. [Accessed October 16, 2009]. Web site. http://www.healthierus.gov/dietaryguidelines. [Google Scholar]
- 5.Sugars in our diet. European Food Information Council (EUFIC); [Accessed October 12, 2009]. Web site. http://www.eufic.org/page/en/nutrition/sugar. [Google Scholar]
- 6.Haley S, Ali M. Sugar backgrounder. US Department of Agriculture Economic Research Service; Jul, 2007. [Accessed March 19, 2010]. Web site. http://www.ers.usda.gov/Publications/SSS/Jul07/SSS249/ [Google Scholar]
- 7.Johnson RK, Frary C. Choose beverages and foods to moderate your intake of sugars: the 2000 dietary guidelines for Americans—what’s all the fuss about? J Nutr. 2001;131(10):2766S–2771S. doi: 10.1093/jn/131.10.2766S. [DOI] [PubMed] [Google Scholar]
- 8.Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health. 2007;97(4):667–675. doi: 10.2105/AJPH.2005.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montonen J, Jarvinen R, Knekt P, Heliovaara M, Reunanen A. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr. 2007;137(6):1447–1454. doi: 10.1093/jn/137.6.1447. [DOI] [PubMed] [Google Scholar]
- 10.Marshall TA, Eichenberger-Gilmore JM, Larson MA, Warren JJ, Levy SM. Comparison of the intakes of sugars by young children with and without dental caries experience. J Am Dent Assoc. 2007;138 (1):39–46. doi: 10.14219/jada.archive.2007.0019. [DOI] [PubMed] [Google Scholar]
- 11.Frary CD, Johnson RK, Wang MQ. Children and adolescents’ choices of foods and beverages high in added sugars are associated with intakes of key nutrients and food groups. J Adolesc Health. 2004;34(1):56–63. doi: 10.1016/s1054-139x(03)00248-9. [DOI] [PubMed] [Google Scholar]
- 12.Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. National Academies Press; 2005. [Accessed March 19, 2010]. Web site. http://www.nap.edu/openbook.php?record_id=10490. [DOI] [PubMed] [Google Scholar]
- 13.Nishida C, Uauy R, Kumanyika S, Shetty P. The Joint WHO/FAO Expert Consultation on diet, nutrition and the prevention of chronic diseases: process, product and policy implications. Public Health Nutr. 2004;7(1a):245–250. doi: 10.1079/phn2003592. [DOI] [PubMed] [Google Scholar]
- 14.Johnson RK, Appel LJ, Brands M, et al. American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism and the Council on Epidemiology and Prevention. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120(11):1011–1020. doi: 10.1161/CIRCULATIONAHA.109.192627. [DOI] [PubMed] [Google Scholar]
- 15.National Health and Nutrition Examination Survey data. Centers for Disease Control and Prevention (CDC); [Accessed January 3, 2010]. Web site. http://www.cdc.gov/NCHS/nhanes.htm. [Google Scholar]
- 16.NCHS Research Ethics Review Board (ERB) approval. Centers for Disease Control and Prevention (CDC); [Accessed October 3, 2010]. Web site. http://www.cdc.gov/nchs/nhanes/irba98.htm. [Google Scholar]
- 17.Willett W, editor. Nutritional Epidemiology. 2. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 18.National Agricultural Library National Nutrient Database for Standard Reference. US Dept of Agriculture; [Accessed June 15, 2009]. Web site. http://fnic.nal.usda.gov/nal_display/index.php?info_center=4&tax_level=1. [Google Scholar]
- 19.MyPyramid Equivalents Database for USDA survey food codes. 1994–2002 Version 1. US Dept of Agriculture Agricultural Research Service; 2006. [Accessed July 7, 2009]. Web site. http://www.ars.usda.gov/ba/bhnrc/fsrg. [Google Scholar]
- 20.Cleveland LE, Cook DA, Krebs-Smith SM, Friday J. Method for assessing food intakes in terms of servings based on food guidance. Am J Clin Nutr. 1997;65(4 suppl):1254S–1263S. doi: 10.1093/ajcn/65.4.1254S. [DOI] [PubMed] [Google Scholar]
- 21.USDA Database for the added sugars content of selected foods. US Dept of Agriculture Agricultural Research Service; 2005. [Accessed August 19, 2009]. Web site. http://www.ars.usda.gov/nutrientdata. [Google Scholar]
- 22.Hanak V, Munoz J, Teague J, Stanley A, Jr, Bittner V. Accuracy of the triglyceride to high-density lipo-protein cholesterol ratio for prediction of the low-density lipoprotein phenotype B. Am J Cardiol. 2004;94(2):219–222. doi: 10.1016/j.amjcard.2004.03.069. [DOI] [PubMed] [Google Scholar]
- 23.Laboratory Procedure Manual. Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS); [Accessed December 12, 2009]. http://www.cdc.gov/nchs/data/nhanes.htm. [Google Scholar]
- 24.Cooney MT, Dudina AL, Graham IM. Value and limitations of existing scores for the assessment of cardiovascular risk: a review for clinicians. J Am Coll Cardiol. 2009;54(14):1209–1227. doi: 10.1016/j.jacc.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Thompson FE, McNeel TS, Dowling EC, Midthune D, Morrissette M, Zeruto CA. Interrelationships of added sugars intake, socioeconomic status, and race/ethnicity in adults in the United States: National Health Interview Survey, 2005. J Am Diet Assoc. 2009;109(8):1376–1383. doi: 10.1016/j.jada.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Horn LV, Ballew C, Liu K, et al. Diet, body size, and plasma lipids-lipoproteins in young adults: differences by race and sex: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1991;133(1):9–23. doi: 10.1093/oxfordjournals.aje.a115807. [DOI] [PubMed] [Google Scholar]
- 27.Key concepts about NHANES survey design. Centers for Disease Control and Prevention (CDC); [Accessed August 19, 2009]. Web site. http://www.cdc.gov/nchs/tutorials/Nhanes/SurveyDesign/SampleDesign/Info1.htm. [Google Scholar]
- 28.US Department of Agriculture Economic Research Service; [Accessed June 15, 2009]. National Agricultural Library National Nutrient Database for Standard Reference [grams in 1 tsp of sugar] Web site. http://www.nal.usda.gov/fnic/foodcomp/cgi-bin/measure.pl. [Google Scholar]
- 29.Couillard C, Bergeron N, Prud’homme D, et al. Gender difference in postprandial lipemia: importance of visceral adipose tissue accumulation. Arterioscler Thromb Vasc Biol. 1999;19(10):2448–2455. doi: 10.1161/01.atv.19.10.2448. [DOI] [PubMed] [Google Scholar]
- 30.Obarzanek E, Sacks FM, Vollmer WM, et al. DASH Research Group. Effects on blood lipids of a blood pressure-lowering diet: the Dietary Approaches to Stop Hypertension (DASH) Trial. Am J Clin Nutr. 2001;74(1):80–89. doi: 10.1093/ajcn/74.1.80. [DOI] [PubMed] [Google Scholar]
- 31.Ballinger RA. A History of Sugar Marketing Through 1974. Washington, DC: US Dept of Agriculture; 1978. Agricultural Economic Report No. 382. [Google Scholar]
- 32.Glinsmann WH, Irausquin H, Park YK. Evaluation of health aspects of sugars contained in carbohydrate sweeteners: report of Sugars Task Force, 1986. J Nutr. 1986;116(11 suppl):S1–S216. doi: 10.1093/jn/116.suppl_11.S1. [DOI] [PubMed] [Google Scholar]
- 33.Grundy SM, Bilheimer D, Blackburn H, et al. Report of Nutrition Committee. Rationale of the diet-heart statement of the American Heart Association. Circulation. 1982;65(4):839A–854A. [PubMed] [Google Scholar]
- 34.Carleton RA, Dwyer J, Finberg L, et al. Report of the Expert Panel on Population Strategies for Blood Cholesterol Reduction: a statement from the National Cholesterol Education Program, National Heart, Lung, and Blood Institute, National Institutes of Health. Circulation. 1991;83(6):2154–2232. doi: 10.1161/01.cir.83.6.2154. [DOI] [PubMed] [Google Scholar]
- 35.Wells HF, Buzby JC. Economic Information Bulletin No. 33. US Department of Agriculture Economic Research Service; Mar, 2008. [Accessed March 22, 2010]. Dietary Assessment of Major Trends in U.S. Food Consumption, 1970–2005. Web site. http://www.ers.usda.gov/Publications/EIB33/ [Google Scholar]
- 36.Dodd KW, Guenther PM, Freedman LS, et al. Statistical methods for estimating usual intake of nutrients and foods: a review of the theory. J Am Diet Assoc. 2006;106(10):1640–1650. doi: 10.1016/j.jada.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Krebs-Smith SM, Graubard BI, Kahle LL, Subar AF, Cleveland LE, Ballard-Barbash R. Low energy reporters vs others: a comparison of reported food intakes. Eur J Clin Nutr. 2000;54(4):281–287. doi: 10.1038/sj.ejcn.1600936. [DOI] [PubMed] [Google Scholar]