Abstract
The present study tested the involvement of the opioid system in the acquisition and expression of prenatal ethanol-related memories. We evaluated how this prenatal experience modulates ethanol self-administration in newborn rats, and preweanling’s ingestion of the drug.
During Gestational Days (GDs) 17-20, four groups of dams were treated with ethanol (2 g/Kg) or water, followed immediately by naloxone (10 mg/Kg) or saline administration. A fifth group received a similar dose of naloxone 20 min before ethanol administration. On PD 1, pups were tested on an operant learning procedure to obtain milk or 3% ethanol. One hour later, an extinction session was performed. At Postnatal Days (PDs) 14 and 15, preweanlings representing each prenatal treatment were evaluated in an intake test with infusions of 5% ethanol or water. Prior to the intake test on PD14, preweanlings were administered naloxone (1 mg/Kg), saline or remained untreated. In both tests, animals representative of both genders were utilized.
One-day-old pups rapidly learned the operant behavior to gain access to milk. In contrast, only pups prenatally treated with ethanol (administered immediately before naloxone or saline injection) increased operant responding to gain access to ethanol. On an intake test at PDs 14 and 15, those animals prenatally exposed to naloxone 20 min before ethanol administration consumed significantly lower ethanol levels than the remaining prenatal ethanol groups. Postnatal treatment with naloxone diminished intake of all solutions at PD14.
These results suggest that prenatal ethanol exposure facilitates neonatal operant learning reinforced by intraoral administration of ethanol and increases ethanol consumption during PDs 14-15. The endogenous opioid system apparently is involved in the acquisition of prenatal ethanol memories, which can modulate the reinforcing attributes of the drug in neonatal and preweanling rats.
Keywords: prenatal ethanol-related memory, opioid system, neonatal operant conditioning, ethanol reinforcement
Introduction
Early experience with ethanol results in a heightened affinity for seeking and consuming ethanol in both humans and rats [1-3]. Genetically heterogeneous adult rats do not consume pharmacologically relevant levels of ethanol without extensive initiation procedures (e.g., 4), creating difficulty in assessment of ethanol reinforcement. However, naïve infant rats have been found to consume large quantities of ethanol at very high concentrations [5-8]. Absolute ethanol consumption seems to be quite high early in ontogeny and to decline gradually into adulthood [9, 10]. Additionally, preweanlings are sensitive to the pharmacologically reinforcing properties of low and moderate ethanol doses, assessed in terms of primary or second-order Pavlovian conditioning [11].
Similar to preweanlings, neonatal rats also exhibit positive responses to postabsorptive ethanol effects when very low ethanol doses (0.125 or 0.25 g/kg) are associated with a surrogate nipple [12]. Moreover, the range of ethanol doses capable of having reinforcing effects are increased (0.25, 0.5, and 0.75 g/kg) when those pups were prenatally exposed to ethanol [13].
Although heightened affinity for ethanol’s postabsorptive effects is observed throughout early ontogeny, the magnitude of these phenomena appears to be especially pronounced during the perinatal stage of development. Near-term fetuses seem to rapidly associate olfactory cues present in the amniotic fluid with postabsorptive effects of low-to-moderate ethanol doses [14, 15]. This association is expressed in terms of heightened attachment to a nipple in the presence of the olfactory cue [16]. These studies have been partially confirmed in humans. Maternal intake of ethanol during pregnancy resulted in the fetus’ detection of ethanol odor. Response patterns of 1- and 2-day-old babies to ethanol chemosensory cues appear to be modulated by levels of alcohol consumed by their mothers during pregnancy [17].
Operant paradigms have been extensively used to analyze the motivational and consummatory mechanisms underlying ethanol consumption during adulthood [18-24]. Nevertheless, the use of operant techniques to evaluate the motivational effects of ethanol during early ontogeny was not well documented until recently. Obstacles in the development of operant techniques included the limited behavioral repertoire of newborn and preweanling rats, as well as the short duration of these ontogenetic stages. Despite these limitations, a few experimental procedures have allowed operant conditioning early in ontogeny. Johanson and Hall [25] developed an instrumental conditioning procedure in which behaviors such as head probing and forelimb movements, were used by the pup to move a manipulandum that produced intraoral infusion of milk. The probability of these behaviors was increased as a function of the contingency with the nutritive reinforcer. These results were then replicated in a study in which 3- to 16-day-old pups were trained to obtain milk [26]. One disadvantage of these experimental protocols is that involves prolonged time of maternal deprivation; in some cases pups were separated from their dams for several hours before testing.
Recently, a new operant technique [27-29] for testing neonatal rats reduced significantly the maternal deprivation and evaluation times associated with the previous studies [25, 26]. In a first study, 5-day-old pups rapidly learned to emit an operant response to gain access to milk [27]. This experiment was the first to demonstrate that neonatal operant conditioning can be achieved in such a short time, with a relatively brief period of maternal deprivation [27]. Subsequently, operant conditioning mediated by ethanol reinforcement was observed in 1-day-old rats [28]. This study also confirmed that ethanol’s postabsorptive effects attained with low levels of ethanol in blood (20 mg/dl) were sufficient to promote vigorous operant responses during an extinction session [28]. Furthermore, prenatal experiences with low to moderate ethanol doses increased the probability of executing these operant responses to obtain ethanol or a compound that mimics the sensory attributes of the drug [29].
Behaviors associated with reward and reinforcement mechanisms appear to be controlled or modulated by distinct components of the endogenous opioid system [30]. Considerable information has been accumulated supporting the role of the endogenous opioid system in the mediation of seeking and ingesting ethanol [30-33]. Recent experiments have demonstrated that rats prenatally exposed to ethanol subsequently have enhanced drug intake patterns [34, 35]. But when the nonselective opioid antagonist naloxone accompanied the prenatal ethanol, neither the enhancement of subsequent ethanol ingestion [34] nor the appetitive responses to intraoral ethanol occurred [36]. In newborn rats, administration of the specific opioid antagonist CTOP (D-Pen-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2) during the first postnatal day clearly reduced the reinforcing effects of ethanol [37]. This evidence indicates that the opioid system can modulate the reinforcing effects of ethanol even during early developmental stages.
The endogenous opioid system is involved not only in the acquisition of ethanol-related memories established in utero, but also in retrieval of these memories during later postnatal stages. Chotro and Arias [34] exposed rats prenatally to ethanol and then re-exposed them to ethanol and naloxone on PD10 and PD12. These animals were later evaluated on PD14 for ethanol consumption. Reexposure to ethanol in combination with the opioid antagonist served to extinguish the expression of the prenatal ethanol memory [34].
Taking this evidence into account, the present study tested newborn and older preweanling rats for the effect of opioid activity on the acquisition and expression of an ethanol-related memory, presumably acquired prenatally. Opioid modulation of the presumed prenatal memory was tested in 1 day–old pups, in terms of an operant learning task. In addition, ethanol ingestion profiles were evaluated near the end of the second postnatal week (PDs 14 and 15). Finally, we tested whether postnatal reexposure to the drug in combination with an opioid antagonist would inhibit expression of the ethanol preference conditioned in utero.
Material and Methods
Subjects
Wistar rats, representing 56 litters, were tested. All animals employed in this study were born and reared at the vivarium of the Institute M. M. Ferreyra (INIMEC- CONICET), Córdoba, Argentina. The colony temperature was maintained at 21 to 23°C, with a 12 h light/12 h dark cycle (light onset 0800 h). Rats had continuous access to rat chow (Cargill, Buenos Aires, Argentina) and tap water delivered through automatic dispenser valves. Vaginal smears of adult female rats were microscopically analyzed daily. On the day of proestrus, females (weight, 200-300 g) were housed during the dark cycle with males (three females per male). Vaginal smears were checked the following morning (1000-1200 h), and the presence of sperm was considered the index of fecundity. The day of sperm detection was considered gestational day 0 (GD0). Females were then individually placed in standard maternity cages filled with wood shavings. The expected length of gestation in this strain is equivalent to 21.5 days [38]. The date of birth was considered postnatal day 0 (PD0).
At all times, animals utilized in this study were maintained and treated in accordance with the Guide for the Care and Use of Laboratory Animals [39].
Prenatal Treatment
Pregnant dams received one daily intragastric (i.g.) administration of ethanol of 0 or 2 g/kg on GD17-20. An ethanol dose of 2 g/kg resulted from the administration of a volume equivalent to 0.015 ml of a 16.8% v/v ethanol solution per gram of body weight. Dams administered the 0 g/kg dose received the same volume of only the vehicle (water). Ethanol dose was selected on the basis of prior studies demonstrating fetal chemosensory processing of the drug under similar experimental circumstances and general lack of deleterious effects of ethanol upon various infantile morphological and behavioral parameters [35, 40, 41]. Immediately after this administration, dams representing each ethanol treatment received a subcutaneous (s.c.) injection of naloxone (Sigma-Aldrich, Buenos Aires, Argentina) at a dose of 10 mg/kg (0.002 ml of a 5 mg/ml naloxone solution per gram of body weight) or a similar volume of vehicle (saline, 0.9% w/v). As a function of prenatal treatment with ethanol and naloxone, four independent prenatal groups were formed: Ethanol–Saline (E/S-0 min), Ethanol–Naloxone (E/N-0 min), Water–Saline (W/S-0 min), and Water–Naloxone (W/N-0 min). A fifth prenatal group, in which naloxone was injected s.c. 20 min before ethanol administration, was incorporated in the experimental design (Naloxone–Ethanol [N/E-20 min]). In accordance with previous literature [42-44] non-specific opioid antagonists reach peak levels in brain at 40 min postadministration time. Administering naloxone 20 min before ethanol, the premise is that opioid receptors were occupied just before ethanol achieved maximum levels in brain [45, 46].
In summary, the final experimental design resulted in five independent prenatal groups: Ethanol–Saline (E/S-0 min), Ethanol–Naloxone (E/N-0 min), Water–Saline (W/S-0 min), Water–Naloxone (W/N-0 min), and Naloxone–Ethanol (N/E-20 min).
Neonatal Operant Conditioning Test
We utilized an instrumental conditioning scheme that allowed us to evaluate the role of the operant behavior in the expression, development, and maintenance of prenatal-related memories [29].
One-day-old pups were removed from the maternal cage and were intraorally cannulated with polyethylene tubing (length, 5 cm; PE10, Clay Adams, Parsippany, NJ). The intraoral cannulation procedure has been extensively described in previous studies [26, 29, 35, 40, 47, 48]. Briefly, a flanged end of the cannula was shaped by exposure to a heat source (external diameter, 1.2 mm). A short dental needle (30G C-KJECT, CK Dental Industries, Buenos Aires, Argentina) was attached to the nonflanged end and positioned in the middle portion of the internal mucosa of the pup’s left cheek. The needle was inserted through the cheek, and the cannula was pulled through the tissue until the flanged end rested on the mouth’s mucosa. This cannulation procedure did not last more than 10 s per subject. As demonstrated by prior research, pups rapidly recover from this minor surgical intervention [49, 50].
After cannulation, pups were maintained in a heated incubator (37 ± 0.5°C; Model C-77, Isolette Air Shields, Hatboro, PA) during 90 min until the operant task started. During this time, pups remained with their littermates in a clean plastic cage inside the incubator.
Immediately prior to the commencement of operant conditioning, the anogenital area of each pup was gently stimulated through the use of a cotton swab to induce miction and defecation. Pups were then weighed (± 0.01 g precision; Ohaus Scout Pro scales (Pinebrook, NJ), and this value was taken as the preconditioning body weight. Each pup was placed in a restrictor vest (made of ultra-thin, elastic rubber, [51]) that allows head and forelimb movements and maintenance of an appropriate supine position during the conditioning procedure (this position is commonly used for pups of this age during nursing).
Conditioning occurred in an acrylic Plexiglas box (60 × 50 × 25 cm) with a smooth surface maintained at a constant temperature (35.0 ± 0.5°C) by two heating pads. The nonflanged end of the pup’s cannula was attached to another cannula (Clay Adams, PE50) connected to a 1 ml syringe mounted on an infusion pump (KD Scientific, Holliston, MA). The infusion pump was set to deliver 1 μl of fluid in 1 s directly into the oral cavity of the neonate.
Two different fluids were employed as reinforcers. One of them was milk (Sancor, bovine milk with 1.5% of fat), which is known to be a natural highly appetitive reinforcer at this age [27-29, 52, 53]. To observe whether a prenatal ethanol-related memory is able to modify operant responsiveness to milk, we included this nutrient as an alternative positive reinforcer. The second one was an ethanol solution equivalent to 3.0% v/v. This ethanol concentration is known to be an effective reinforcer in an operant learning task on PD1 [28, 29].
Newborns representative of each prenatal treatment were simultaneously trained under two different conditions. A Paired (P) pup received 1 μl of reinforcer (fixed-ratio: 1 schedule) contingent with its operant response. A Yoked (Y) control pup was reinforced only when the P pup touched the sensor. This control is particularly relevant during early ontogeny because intraoral delivery of milk or other fluids may promote overall motor activation and reflexes such as stretching that can lead to inadvertent sensor contact [53-55]. Subjects received two priming pulses of milk or ethanol at the beginning of the conditioning session (60 and 120 s). These pulses were administered only if the P pup did not display any operant response during this initial time. These priming pulses were intended to familiarize the newborn with the corresponding fluid and to minimally stimulate head and body movements.
For each litter, one same-sex pair of animals (P and its corresponding Y control) received milk while a second pair received ethanol. Each couple of subjects was placed on the conditioning surface below a circular touch-sensitive sensor (diameter, 1 cm; Model E-11× Evaluation Board, Quantum Research Group, Pittsburg, PA). The evaluation board holding the sensor was also equipped with a red light bulb. This visual stimulus and an audible tone were activated whenever a pup touched the sensor. The audiovisual signal served to determine the occurrence of an effective physical contact with the sensor. An articulated iron stand equipped with alligator clips was used to position the sensor 5 mm above the subject’s nose and forelimbs.
The operant task had two sessions. The first (Acquisition Phase) had a duration of 10 min. In this phase, sensor contact (via head probing or forelimb movements) by the P pup was reinforced. One hour later, an Extinction Phase was performed, in which behavior of the neonates did not yield any solution. Immediately after the acquisition session, the postconditioning weight of each pup was recorded. The total number of physical contacts of each pup with its respective touch-sensitive sensor throughout each conditioning session was the dependent variable. The total number of operant responses was separately analyzed as a function of the reinforcer (milk or 3% ethanol). Additionally, consumption of milk or ethanol was estimated by the percentage of body weight gain by employing the following formula: ([postconditioning weight - preconditioning weight] / preconditioning weight) × 100. When the extinction phase finished, newborns did not returned to maternal cage.
During acquisition and extinction phases, the total number of physical contacts of each pup and their respective touch-sensitive sensor were videorecorded (JVC GR-AX777 camera, Japan).
Preweanling’s Intake Test
Two hours before the test started, pups (PD14) were separated from their dam and placed in holding chambers (15 × 8 × 15 cm) maintained at 30°C with heating pads. All pups were intraorally cannulated as described above (see Neonatal Operant Conditioning Test). Pups at this developmental stage are able to control ingestion of fluids delivered via these polyethylene devices [35, 47]. This cannula was used to infuse the solutions (5% ethanol v/v or vehicle [distilled water]) directly into the oral cavity of the pup. After cannulation and immediately before commencement of the test, pups’ bladders were voided by gentle brushing of the anogenital area to induce miction and defecation; after this, pups were weighed.
To analyze opioid system modulation upon expression of a prenatal ethanol-related memory, a postnatal treatment was performed. Thirty minutes before the intake test [34], pups from each prenatal treatment received a subcutaneous injection of naloxone at 1 mg/kg (0.01 ml of a 1 mg/ml of naloxone solution per gram of body weight) or a similar volume of vehicle (saline solution). A third group of pups had no treatment at this time (naive subjects).
Rats were then placed into individual Plexiglas chambers (15 × 7 × 15cm) lined with a cotton floor. Intraoral infusions were performed by using a 10-syringe infusion pump (KD Scientific, Holliston, MA) connected to the oral cannula of each pup by a polyethylene catheter (Clay Adams, PE 50). The total volume administered to each subject was equivalent to 5.5% of the subject’s body weight and was infused at a constant rate over 15 min directly into the subject’s mouth. Pups could either consume or reject the infused solution. In the chambers, preweanlings had 2 min of habituation prior to 15 min of intake. At the end of this session, each pup’s weight was again recorded. Consumption was determined by the percentage of body weight gained (% BWG) with the following formula: ([postinfusion weight - preinfusion weight] / preinfusion weight) × 100. % BWG was used as the dependent variable under analysis.
On PD15, the procedure was the same as that used for the intake test during PD14, with the exception that the preweanlings had no naloxone treatment prior to the intake test. During both evaluation days, behavior during the intake test was videorecorded (JVC GR-AX777 camera, Japan).
Experimental Design and Data Analysis
To avoid genetic overrepresentation, each group was represented once per litter, with the male-to-female ratio remaining equivalent whenever possible [56].
Data obtained during the operant task were analyzed with a 5 × 2 × 2 mixed analysis of variance (ANOVA). Prenatal treatment (E/S-0 min, E/N-0 min, N/E-20 min, W/S-0 min, or W/N-0 min) represented the between-subjects factor. Phases of evaluation (acquisition and extinction) and learning condition of the neonates (paired or yoked) represented within-subjects factors.
Consumption during the intake test was analyzed with a 5 × 3 × 2 × 2 mixed ANOVA that included prenatal treatment (E/S-0 min, E/N-0 min, N/E-20 min, W/S-0 min, or W/N-0 min), postnatal treatment (naloxone [Nal], saline [Sal], or naive), and solution infused (5% ethanol or distilled water) as between-subjects factors. The day of evaluation (PD14 or PD15) was a within-group factor.
In the case of significant three- or four-way interactions, follow-up ANOVAs were employed before the use of post hoc tests. This procedure served to minimize the probability of Type I errors arising from multiple group comparisons. The loci of significant main effects or two-way interactions were further analyzed with Newman-Keuls post hoc comparisons. A rejection criterion of p < 0.05 was adopted for all statistical analysis in the present study. Table 1 summarizes the final number of subjects evaluated in each group during neonatal operant conditioning or preweanling intake test.
TABLE 1.
Final number of subjects employed in Neonatal Operant Conditioning and PD14-15’s Intake test.
| Prenatal Treatment |
Operant Conditioning PD1* |
Intake Test PD14-15 | ||||||
|---|---|---|---|---|---|---|---|---|
| Solution Infused | ||||||||
| Ethanol | Water | |||||||
| Reinforcer | Postnatal Treatment | |||||||
| Milk | Ethanol | Naloxone | Saline | Naive | Naloxone | Saline | Naive | |
| E/S-0 min | 8 | 8 | 13 | 13 | 13 | 13 | 13 | 13 |
| E/N-0 min | 10 | 9 | 9 | 9 | 9 | 9 | 9 | 9 |
| N/E-20 min | 9 | 7 | 6 | 6 | 6 | 6 | 6 | 6 |
| W/S-0 min | 10 | 7 | 10 | 10 | 10 | 10 | 10 | 10 |
| W/N-0 min | 10 | 10 | 11 | 11 | 11 | 11 | 11 | 11 |
Note: Each final group in Operant Conditioning represents a couple of same-sex subjects (paired and yoked).
Results
Maternal Body Weight Gain During Gestational Days 17-20, Litter Size, and Pup’s Weight
The percentage of body weight gain of dams across gestational days was calculated using the following formula: ([maternal body weight at GD20 - maternal body weight at GD17]/maternal body weight at GD17) × 100. A one-way ANOVA showed that prenatal treatments had no significant effects on this weight index. Neither the litter size of prenatal groups nor pups’ weights on PD1 was significantly affected by prenatal treatment. These results suggest that prenatal manipulations had no gross teratological effects, consistent with previous reports [16, 29].
In preliminary analysis of the data sex was included as variable. It consistently failed to exert any significant main effect, or to interact with prenatal and/or postnatal treatments. For this reason, further statistical analysis was performed by collapsing sex across prenatal (Neonatal operant conditioning test) or prenatal and/or postnatal (Preweanling’s consumption scores) treatment conditions.
Neonatal Operant Conditioning Test
Operant responding for milk
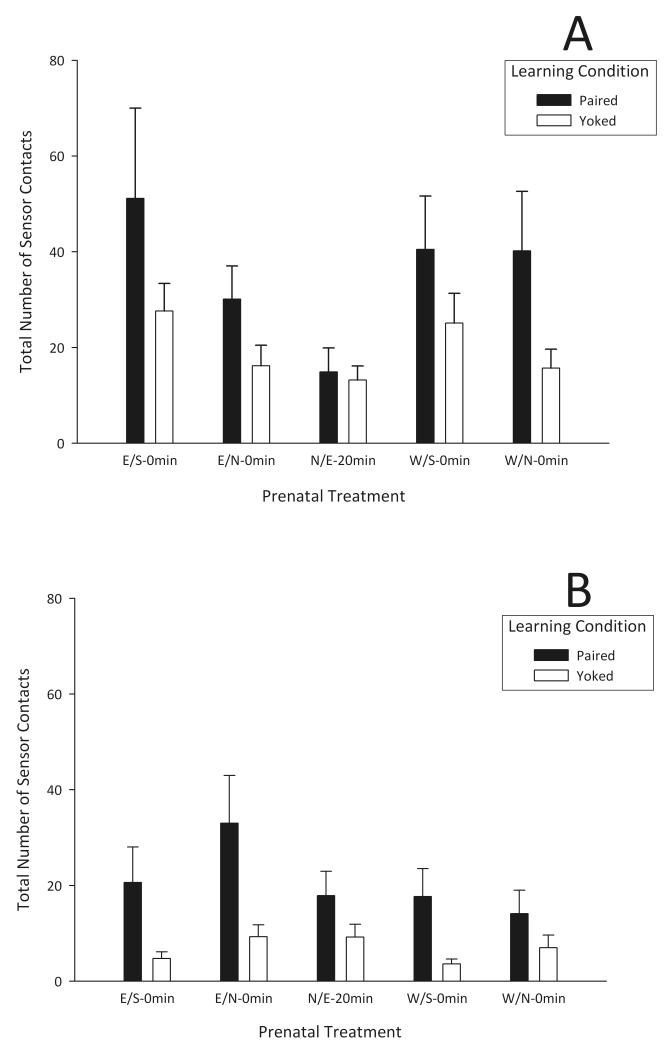
A three-way mixed ANOVA (prenatal treatment × evaluation phase [acquisition vs. extinction] × learning condition [P vs. Y]) was performed. The analysis revealed a significant main effect of evaluation phase (F1,42 = 15.73, p < 0.01) and learning condition (F1,42 = 34.85, p < 0.01). As expected, pups executed significantly fewer target behaviors during the extinction phase than during the acquisition session. Also as expected, P neonates exhibited a significantly greater number of operant responses than their corresponding Y controls. These effects were independent of prenatal treatment (Fig. 1). Although in Figure 1A there is a tendency for the P N/E-20 min group to differ from the remaining acquisition groups, this difference was not statistically significant.
Fig. 1.
Overall neonatal operant behaviors (sensor contacts) during 10 min Acquisition (A) and Extinction (B) phases in response to milk as a function of prenatal treatment (Ethanol–Saline [E/S-0 min], Ethanol–Naloxone [E/N-0 min], Water–Saline [W/S-0 min], Water–Naloxone [W/N-0 min], and Naloxone–Ethanol [N/E-20 min]) and learning condition (Paired and Yoked). Vertical lines represent standard error of the mean.
In summary, 1-day-old pups rapidly learned to display operant responses when this behavior led to an infusion of a natural reinforcer such as milk. These results are consistent with previous studies indicating that milk rapidly acts as a primary reinforcer in newborn rats [27-29]. Specifically, the infusion of milk elicited higher operant responses compared with the execution of this behavior without reinforcement. Finally, independent of prenatal experience with ethanol and an opioid antagonist, all pups were capable of responding in an operant task when the reinforcer was milk.
Operant responding for ethanol
Four neonates (4.4% of a total of 90 pups) were excluded from analysis because they failed to exhibit any operant responses. The behavioral scores of these neonates were not included in the statistical analyses.
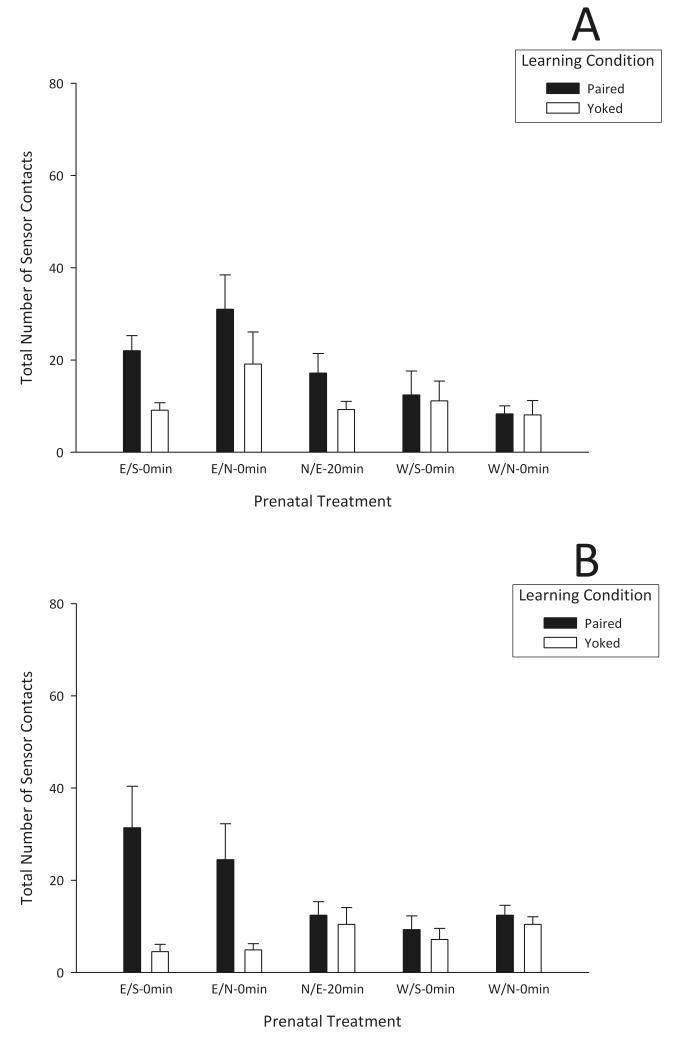
A three-way mixed ANOVA (prenatal treatment × evaluation phase × learning condition) indicated significant main effects of prenatal treatment and learning condition (F4,36 = 3.68, p < 0.025; F1,36 = 26.82, p < 0.01, respectively). A significant interaction between prenatal treatment and learning condition was also observed (F4,36 = 5.93, p < 0.01). Post hoc comparisons revealed that only P pups from the E/N-0 min and E/S-0 min groups executed significantly more operant responses than their respective yoked controls. Additionally, P pups in these prenatal groups showed significantly more operant responses than P pups in the remaining prenatal treatments (W/S-0 min, W/N-0 min, and N/E-20 min). When comparing operant responses displayed by Y neonates, we failed to observe significant differences across prenatal groups (Fig. 2).
Fig. 2.
Overall neonatal operant behaviors (sensor contacts) during 10 min Acquisition (A) and Extinction (B) phases in response to 3% ethanol as a function of prenatal treatment (Ethanol–Saline [E/S-0 min], Ethanol–Naloxone [E/N-0 min], Water–Saline [W/S-0 min], Water–Naloxone [W/N-0 min], and Naloxone–Ethanol [N/E-20 min]) and learning condition (Paired and Yoked). Vertical lines represent standard error of the mean.
In summary, 3% ethanol failed to support operant responding in neonates, except in those groups given ethanol prenatally, whether the dam’s ethanol during GDs 17-20 was immediately followed by injection of naloxone or saline (E/S-0 min and E/N-0 min). For the offspring of dams injected with the opioid antagonist 20 min before prenatal ethanol administration (N/E-20 min), however, operant responding for ethanol was not significant. The efficacy of ethanol as a reinforcer in prenatal groups E/S-0 min and E/N-0 min was also evident during the extinction phase conducted 60 min after acquisition. In contrast to milk reinforcement, P pups in the E/S-0 min and E/N-0 min groups maintained their rate of responding for ethanol throughout extinction. Consistent with previous studies [29], these results indicate that prenatal ethanol experiences facilitate neonatal operant behavior directed toward ethanol. Moreover, similar to observations in older preweanling rats [34], the opioid system is involved in modulating the reinforcing attributes of the drug.
Consumption during Neonatal Operant Conditioning
A two-way mixed ANOVA (prenatal treatment × learning condition) indicated no differences in milk consumption. All pups (experimental and controls) from all prenatal groups consumed similar levels of milk across the acquisition phase (Table 2). Nonetheless, prenatal ethanol experience determined ethanol consumption during operant conditioning. The two-way mixed ANOVA (prenatal treatment × learning conditioning) indicated a significant main effect of prenatal treatment (F4,36 = 3.80, p < 0.025). This analysis confirms that E/N-0 min and E/S-0 min prenatal groups consumed more ethanol than neonates from prenatal water-treated groups (but they do not significantly differ from N/E-20 min, p = 0.1). A main effect of learning condition (F1,36 = 5.86, p < 0.025) confirmed that P subjects consumed significantly more ethanol than Y controls (Table 2).
TABLE 2.
Mean percentage of body weight gained (± SEM) during neonatal operant conditioning.
| Prenatal Treatment |
Milk | 3% Ethano | ||
|---|---|---|---|---|
| Paired | Yoked | Paired | Yoked | |
| E/S-0 min | 0,31 ± 0,08 | 0,24 ± 0,10 | 0,38 ± 0,07 | 0,27 ± 0,05 |
| E/N-0 min | 0,29 ± 0,07 | 0,26 ± 0,03 | 0,38 ± 0,04 | 0,31 ± 0,05 |
| N/E-20 min | 0,26 ± 0,09 | 0,26 ± 0,08 | 0,26 ± 0,07 | 0,14 ± 0,07 |
| W/S-0 min | 0,29 ± 0,09 | 0,32 ± 0,07 | 0,14 ± 0,07 | 0,09 ± 0,06 |
| W/N-0 min | 0,42 ± 0,09 | 0,34 ± 0,08 | 0,14 ± 0,06 | 0,14 ± 0,05 |
These results indicate that although the infused volume of each pulse of reinforcement was quite small (1 μl), significant differences were found in amount of ethanol ingested. For milk consumption, the percentage of body weight gain did not differ between prenatal groups or learning conditions. For ethanol, however, the amounts consumed by pups varied as a function of prenatal experience with the drug. Pups in both the E/S-0 min and E/N-0 min groups consumed higher levels of the drug than pups in the remaining water prenatal conditions. Additionally, prenatal treatment with naloxone before drug administration not only inhibited operant responses when the ethanol was available (see Operant Responding for Ethanol), but also tended to decrease the volume of ethanol consumed compared with the remaining prenatally ethanol-treated pups. Finally, less ethanol was consumed by Y pups than by P pups.
Preweanling Intake Test
Preweanling body weight on PD14
To test whether an effect of prenatal experience with ethanol and/or naloxone persisted until the second postnatal week, preinfusion body weights of 14-day-old pups were analyzed using one-way ANOVA. This analysis indicated no significant difference in body weights of preweanlings across all prenatal treatments.
Preweanling’s consumption scores
An initial three-way ANOVA included the following independent factors: prenatal treatment (E/S-0 min, E/N-0 min, W/N-0 min, W/S-0 min, or N/E-20min), postnatal treatment (naive, saline, or naloxone), and solution infused (5% ethanol or water). Analysis of the amount consumed on PD14 indicated that fluid consumption did not differ among postnatal control conditions (naive animals or saline-injected pups), so these postnatal control groups were collapsed within each prenatal condition.
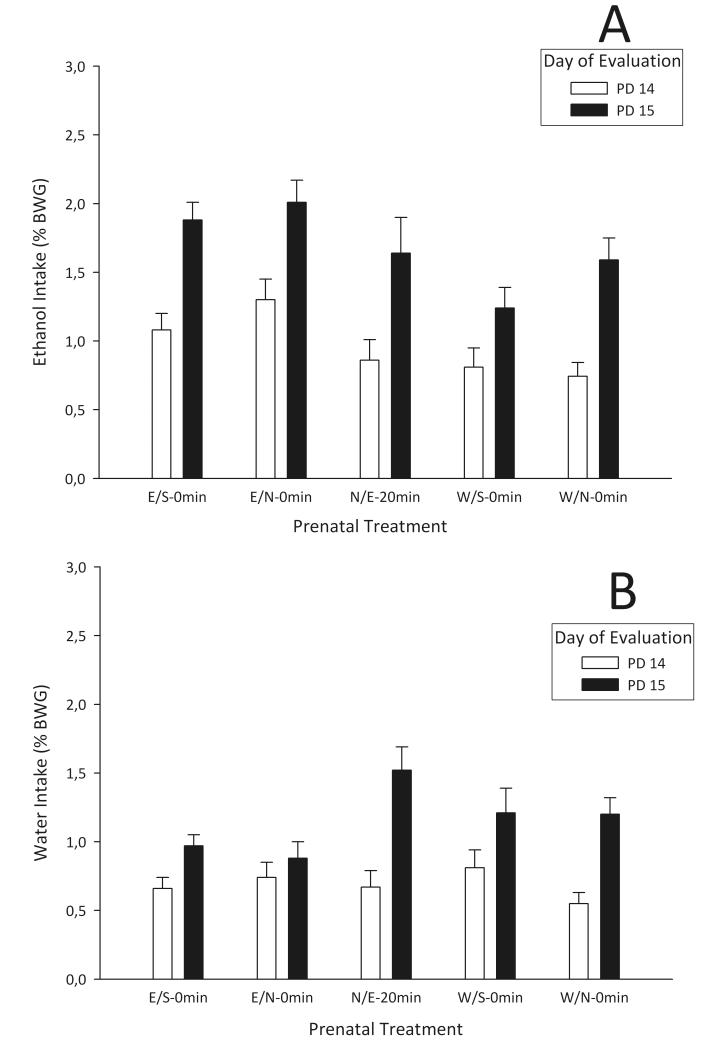
Statistical analysis of consumption scores during PDs 14-15 began with a four-way mixed ANOVA (prenatal treatment [E/S-0 min, E/N-0 min, W/S-0 min, W/N-0 min, or N/E-20 min] × postnatal treatment [naloxone or control] × solution infused [5% ethanol or water] × evaluation day (PD14 or PD15]). This analysis revealed significant main effects of postnatal treatment (F1,176 = 39.83, p < 0.01), solution infused (F1,176 =37.02, p < 0.01], and evaluation day (F1,176 = 137.87, p < 0.01). The analysis also revealed the following significant two-way interactions: prenatal treatment × solution infused (F4,176 = 6.05, p < 0.01), prenatal treatment × evaluation day (F4,176 = 2.46, p < 0.05), solution infused × evaluation day (F1,176 = 5.75, p < 0.025), and postnatal treatment × evaluation day (F1,176 = 16.76, p < 0.01). Of major importance, post hoc comparisons of conditions associated with the prenatal treatment × solution infused interaction revealed that ethanol consumption depended on prenatal experience with the drug. Particularly, pups from E/S-0 min and E/N-0 min groups consumed more ethanol than those from the other prenatal conditions (although E/S-0 min did not differ significantly from N/E-20 min group, p = 0.1; Figure 3A). Water intake scores did not differ across prenatal treatments (Figure 3B).
Fig. 3.
Mean percentage of body weight gain (%BWG) of 5% ethanol (A) or water (B) during PD14 and PD15 as a function of prenatal treatment (Ethanol–Saline [E/S-0 min], Ethanol–Naloxone [E/N-0 min], Water–Saline [W/S-0 min], Water–Naloxone [W/N-0 min], and Naloxone–Ethanol [N/E-20 min]). Vertical lines represent standard error of the mean.
A three-way interaction between postnatal treatment × solution infused × evaluation day (F1,176 = 4.24, p < 0.05) was found to be significant. To better analyze this significant interaction, two follow-up ANOVAs were conducted, one for consumption of ethanol and the other for water consumption. For ethanol consumption, the two-way ANOVA indicated a significant main effect of postnatal treatment (F1,96 = 12.32, p < 0.01) and evaluation day (F1,96 = 99.47, p < 0.01). The interaction of these two factors was also significant (F1,96 = 20.60, p < 0.01). Post hoc comparisons showed that all pups increased ethanol consumption across evaluation days. Postnatal treatment with naloxone significantly reduced ethanol consumption on PD14, although on PD15, this reduction in intake was not observed compared with control pups (ethanol consumption scores: Nal-PD14: 0.61 ± 0.06; PD15: 1.65 ± 0.10; Control-PD14: 1.32 ± 0.08; PD15: 1.70 ± 0.11 [%BWG ± SEM]). For water consumption, the two-way ANOVA indicated a significant main effect of postnatal treatment (F1,96 = 28.12, p < 0.01) and evaluation day (F1,96 = 42.78, p < 0.01). This analysis indicates that postnatal treatment with naloxone decreased water intake on PD14. In addition, both postnatal groups increased water consumptions profiles across evaluation days (water consumption scores: Nal-PD14: 0.44 ± 0.06; PD15: 0.98 ± 0.08; Control-PD14: 0.93 ± 0.05; PD15: 1.27 ± 0.09 [%BWG ± SEM]).
These results confirm previous studies [34-36, 57] showing that prenatal experience with ethanol promoted higher ethanol intake during infancy. This effect was not observed by Chotro and Arias [34] when an opioid antagonist, naloxone, was simultaneously presented with maternal administration of ethanol. Under the present experimental conditions, this opioid antagonist was effective only when dams were injected 20 min before ethanol administration (N/E-20 min prenatal group). Additionally, the present results show a nonspecific acute effect of postnatal naloxone treatment on PD14, reflected in reductions of both water and ethanol intake. Postnatal exposure to this opioid antagonist failed to interact with prenatal treatments. Even when all pups increased their consumption scores across evaluation days, ethanol consumption in the E/S-0 min and E/N-0 min prenatal groups was significantly higher than that of the other prenatal groups, confirming that prenatal ethanol experiences increase postnatal ethanol intake.
Discussion
One-day-old rats with a brief period of maternal deprivation rapidly gained access to milk by operant behavior in the present study. This operant response was acquired in a short period of time. Regardless of prenatal ethanol exposure, these results generally are to be the expected when operant behaviors during perinatal stages are reinforced with a natural reinforcer such as milk [27-29].
Corresponding results with ethanol as reinforcer were quite different. Consistent with previous studies [29], ethanol (3%) served as a positive reinforcer only in pups that had previous experience with the drug during late gestation (prenatal groups exposed to naloxone or saline immediately after ethanol administration; E/S-0 min and E/N-0 min groups). This difference in rate of responding as a function of prenatal treatment does not appear to be attributable to hyperactivity caused by prenatal ethanol exposure [29] because all prenatal pup groups displayed similar learning patterns when a natural reinforcer (milk) was contingent upon their respective operant behavior. Similarly, and in agreement with previous studies using the same ethanol dose and administration procedures for dams during GDs 17-20, we systematically failed to observe detrimental effects of the drug on either neonatal body weight or a variety of alternative morphological parameters [29, 35, 40]. Also, in agreement with previous studies [29], differences observed between prenatal groups in the operant learning task when ethanol acts as reinforcer suggest that, after birth, pups express a conditioned ethanol memory acquired as fetuses.
Important for the aims of the present study, when the opioid system was blocked with naloxone 20 min prior to maternal ethanol administration (prenatal group N/E-20 min), effective operant responding for ethanol clearly did not occur in neonates. These results appear reasonable when considering that naloxone reaches maximum blood levels between 60 and 120 min [42] after injection. These levels are similar to those encountered in brain [43]. Zagon et al. [58] found that, after administering the mother with a nonspecific opioid antagonist -naltrexone, 50 mg/kg-, a 40% of this dose was found in fetal brain after 60 min. Several other studies also indicate that peak ethanol blood and brain concentrations are achieved at 30-60 min postadministration times [14, 35, 44, 45]. Therefore, under the present prenatal manipulations – the N/E-20 min prenatal group in particular -- when ethanol was administered to the dam, naloxone probably was actively blocking opioid receptors. Further refinement of temporal parameters between presentation of the opioid antagonist and ethanol, and further knowledge of their respective pharmacokinetic features, are needed to fully understand the relationship between the present results (i.e., no effect of naloxone given essentially simultaneously with ethanol) and those previously reported [34].
In the present study, small amounts of milk or ethanol were available upon completion of a response requirement. The maintenance of response during the extinction, displayed by P neonates, may be treated as a simple measure of seeking of the reinforcer [59, 60]. Results from the present neonatal operant learning task indicated that ethanol-seeking behavior was very similar to the behavior that accompanied consummatory behavior during acquisition (E/S-0 min and E/N-0 min prenatal groups). In this sense it seems that, under these experimental conditions, mechanisms of ethanol’ seeking [59, 60] and wanting (consummatory) [61] behaviors would be in the same direction. Future research approaches need to be conducted to analyze “liking behaviors” [61, 62] that underlies the consummatory responses elicited by ethanol in this model of neonatal operant paradigm. Alternatively it is well established, however, that persistence during extinction or reversal is increased by prenatal ethanol [63, 64], which might be a simpler explanation.
Administration of a moderate dose of ethanol (2 g/kg) to dams during the last part of GDs 17-20 enhanced ethanol consumption in the offspring on PD14 and PD15. Ethanol consumption was also affected by prenatal treatment with naloxone. When this opioid antagonist was injected 20 min before ethanol administration to the dam and fetuses, the usual enhancement of ethanol intake did not occur in the offspring. Yet when naloxone was given immediately after ethanol on GDs 17-20, in contrast with the effect observed by Chotro and Arias [34], no reduction in ethanol intake was observed on PD14-15 (E/N-0 min vs. E/S-0 min).
The intention of the postnatal naloxone treatment was to block the expression of an ethanol-related memory comprising the chemosensory and pharmacological attributes of ethanol acquired as a fetus. This effect was not seen under the present experimental conditions, which differed from those of Chotro and Arias [34] in the following ways. First, in the study by Chotro and Arias, pups had experiences with ethanol under naloxone’s effects on PD10 and PD12, prior to a test for ethanol intake on these days as well as the test on PD14. In the present one, however, a single naloxone injection was followed by an intake test on PD14 and then another on PD15. Perhaps multiple trials facilitate habituation to sources of stimulation (e.g., intraoral infusion, handling, s.c. injection) that can compete with preweanling’s perception of low amounts of ethanol [65]. Second, in the study conducted by Chotro and Arias [34], postnatal naloxone treatment occurred earlier in ontogeny (PD10 and PD12). Third, ethanol consumption scores were higher than in the present study, probably accompanied by higher blood ethanol levels. Consequently, the present postabsorptive effects of ethanol could be more pronounced and therefore more sensitive to naloxone’s antagonism of the opioid system. These three factors may have masked effects of postnatal naloxone on ethanol intake in the present study.
In summary, these results provide further evidence that moderate prenatal ethanol exposure has a profound effect upon neonatal and preweanling predisposition to express appetitive responses to the drug. The study also provides new evidence about the consequences of ethanol-seeking behavior after birth. Prenatal exposure to the drug facilitates neonatal operant learning supported by intraoral administration of a low ethanol concentration. Moreover, prenatal antagonism of opioid receptors inhibits operant responding for ethanol, but not operant responding for a natural reinforcer, milk. These findings agree with previous studies in suggesting a facilitative effect of prenatal ethanol on later ethanol intake as well as an apparent importance of conditioning processes established in utero. This prenatal associative learning [9, 15, 66] apparently can exert lingering, long-lasting effects on ethanol affinity. Exposure to ethanol during gestation has been observed to induce heightened ethanol intake in preweanling [67] and adolescent [34] rats, results recently confirmed by unpublished experiments conducted in our laboratory (Godino et al.; Fabio et al., unpublished data).
These effects seem to depend on a fully functional opioid system at the time prenatal ethanol is presented to the fetus, and perhaps also when the effects are expressed later. Opioid involvement might be important not only for ethanol’s motivational effects but also for the acquisition of ethanol memories in utero. These possible relationships will require further study.
ACKNOWLEDGEMENTS
This work was supported by the Agencia Nacional de Promoción Científica y Tecnológica (PICT 38080, P.A.), PICT 254 (J.C.M.), Secretaría de Ciencia y Tecnología (SECyT, P.A.), the U.S. National Institute on Alcohol Abuse and Alcoholism (AA11960, AA015992, and AA013098), the U.S. National Institute of Mental Health (MH035219) (N.E.S), and a fellowship from CONICET (to R.S.M.M.). R.S.M.M. is a student of the Ph.D. program of Doctorado en Ciencias Biológicas (F.C.E.F. y N. – U.N.C.). We also gratefully acknowledge Aceitera General Deheza S.A.
REFERENCES
- [1].Chotro MG, Arias C. Ontogenetic difference in ethanol reinforcing properties: the role of the opioid system. Behav Pharmacol. 2007;18:661–6. doi: 10.1097/FBP.0b013e3282f00754. [DOI] [PubMed] [Google Scholar]
- [2].Molina JC, Spear NE, Spear LP, Mennella JA, Lewis MJ. The International Society for Developmental Psychobiology 39th Annual Meeting Symposium: Alcohol and development: beyond fetal alcohol syndrome. Dev Psychobiol. 2007;49:227–42. doi: 10.1002/dev.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Spear NE, Molina JC. Fetal or infantile exposure to alcohol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res. 2005;25:909–29. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- [4].Samson HH, Pfeffer AO, Tolliver GA. Oral ethanol self-administration in rats: models of alcohol-seeking behavior. Alcohol Clin Exp Res. 1988;12:591–8. doi: 10.1111/j.1530-0277.1988.tb00248.x. [DOI] [PubMed] [Google Scholar]
- [5].Lee JS, Crawford J, Spear NE. Characteristics and consequences of free-feeding ethanol ingestion during the first two postnatal weeks of the rat. Alcohol Clin Exp Res. 1998;22:1615–22. [PubMed] [Google Scholar]
- [6].Petrov ES, Varlinskaya EI, Spear NE. Self-administration of ethanol and saccharin in newborn rats: effects on suckling plasticity. Behav Neurosci. 2001;115:1318–31. [PubMed] [Google Scholar]
- [7].Truxell E, Spear NE. Immediate acceptance of ethanol in infant rats: ontogenetic differences with moderate but not high ethanol concentration. Alcohol Clin Exp Res. 2004;28:1200–11. doi: 10.1097/01.alc.0000134220.34842.18. [DOI] [PubMed] [Google Scholar]
- [8].Varlinskaya EI, Petrov ES, Cheslock SJ, Spear NE. A new model of ethanol self-administration in newborn rats: gender effects on ethanol ingestion through a surrogate nipple. Alcohol Clin Exp Res. 1999;23:1368–76. [PubMed] [Google Scholar]
- [9].Sanders S, Spear NE. Ethanol acceptance is high during early infancy and becomes still higher after previous ethanol ingestion. Alcohol Clin Exp Res. 2007;31:1148–58. doi: 10.1111/j.1530-0277.2007.00400.x. [DOI] [PubMed] [Google Scholar]
- [10].Truxell EM, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res. 2007;31:755–65. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- [11].Molina JC, Ponce LF, Truxell E, Spear NE. Infantile sensitivity to ethanol’s motivational effects: ethanol reinforcement during the third postnatal week. Alcohol Clin Exp Res. 2006;30:1506–19. doi: 10.1111/j.1530-0277.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- [12].Petrov ES, Varlinskaya EI, Spear NE. Reinforcement from pharmacological effects of ethanol in newborn rats. Alcohol Clin Exp Res. 2003;27:1583–91. doi: 10.1097/01.ALC.0000089960.62640.58. [DOI] [PubMed] [Google Scholar]
- [13].Nizhnikov ME, Molina JC, Varlinskaya EI, Spear NE. Prenatal ethanol exposure increases ethanol reinforcement in neonatal rats. Alcohol Clin Exp Res. 2006;30:34–45. doi: 10.1111/j.1530-0277.2006.00009.x. [DOI] [PubMed] [Google Scholar]
- [14].Abate P, Pepino MY, Dominguez HD, Spear NE, Molina JC. Fetal associative learning mediated through maternal alcohol intoxication. Alcohol Clin Exp Res. 2000;24:39–47. [PubMed] [Google Scholar]
- [15].Abate P, Pueta M, Spear NE, Molina JC. Fetal learning about ethanol and later ethanol responsiveness: evidence against “safe” amounts of prenatal exposure. Exp Biol Med. 2008;233:139–54. doi: 10.3181/0703-MR-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Abate P, Varlinkaya EI, Cheslock SJ, Spear NE, Molina JC. Neonatal activation of alcohol-related prenatal memories: impact on the first suckling response. Alcohol Clin Exp Res. 2002;26:1512–22. doi: 10.1097/01.ALC.0000034668.93601.8F. [DOI] [PubMed] [Google Scholar]
- [17].Faas AE, Spontón ED, Moya PR, Molina JC. Differential responsiveness to alcohol odor in human neonates: effects of maternal consumption during gestation. Alcohol. 2000;22:7–17. doi: 10.1016/s0741-8329(00)00103-8. [DOI] [PubMed] [Google Scholar]
- [18].Cunningham CL, Fidler TL, Hill KG. Animal models of alcohol’s motivational effects. Alcohol Res Health. 2000;24:85–92. [PMC free article] [PubMed] [Google Scholar]
- [19].Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–64. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- [20].McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–69. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- [21].Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: rodent models. Int Rev Neurobiol. 2003;54:107–53. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- [22].Samson HH, Files FJ, Denning C. Chronic ethanol self-administration in a continuous-access operant situation: the use of a sucrose/ethanol solution to increase daily ethanol intake. Alcohol. 1999;19:151–5. doi: 10.1016/s0741-8329(99)00032-4. [DOI] [PubMed] [Google Scholar]
- [23].Samson HH, Sharpe AL, Denning C. Initiation of ethanol self-administration in the rat using sucrose substitution in a sipper-tube procedure. Psychopharmacology. 1999;147:274–9. doi: 10.1007/s002130051167. [DOI] [PubMed] [Google Scholar]
- [24].Samson HH, Slawecki CJ, Sharpe AL, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcohol Clin Exp Res. 1998;22:1783–7. [PubMed] [Google Scholar]
- [25].Johanson IB, Hall WG. Appetitive learning in 1-day-old rat pups. Science. 1979;205:419–21. doi: 10.1126/science.451612. [DOI] [PubMed] [Google Scholar]
- [26].Domínguez HD, Bocco G, Chotro MG, Spear NE, Molina JC. Operant responding controlled by milk or milk contaminated with alcohol as a positive reinforcers in infant rats. Pharmacol Biochem Behav. 1993;44:403–9. doi: 10.1016/0091-3057(93)90482-9. [DOI] [PubMed] [Google Scholar]
- [27].Arias C, Spear NE, Molina A, Molina JC. Rapid acquisition of operant conditioning in 5-day-old rat pups: a new technique articulating suckling-related motor activity and milk reinforcement. Dev Psychobiol. 2007;49:576–88. doi: 10.1002/dev.20236. [DOI] [PubMed] [Google Scholar]
- [28].Bordner KA, Molina JC, Spear NE. Analysis of ethanol reinforcement in 1-day-old rats: assessment through a brief and novel operant procedure. Alcohol Clin Exp Res. 2008;32:580–92. doi: 10.1111/j.1530-0277.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- [29].March SM, Abate P, Spear NE, Molina JC. Late prenatal ethanol experience increases neonatal operant behavior towards and ethanol related reinforcers. Alcohol Clin Exp Res. 2009;33:1981–93. doi: 10.1111/j.1530-0277.2009.01037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2004;4:39–50. doi: 10.2174/1568026043451573. [DOI] [PubMed] [Google Scholar]
- [31].Bodnar RJ, Klein GE. Endogenous opiates and behavior: 2005. Peptides. 2006;27:3391–478. doi: 10.1016/j.peptides.2006.07.011. [DOI] [PubMed] [Google Scholar]
- [32].Froehlich JC, Badia-Elder NE, Zink RW, McCullough DE, Portoghese PS. Contribution of the opioid system to alcohol aversion and alcohol drinking behavior. J Pharmacol Exp Ther. 1998;287:284–92. [PubMed] [Google Scholar]
- [33].Marinelli PW, Quirion R, Gianoulakis C. An in vivo profile of β-endorphin release in the arcuate nucleus and nucleus accumbens following exposure to stress or alcohol. Neuroscience. 2004;127:777–84. doi: 10.1016/j.neuroscience.2004.05.047. [DOI] [PubMed] [Google Scholar]
- [34].Chotro MG, Arias C. Prenatal exposure to ethanol increases ethanol consumption: a condiitoned response? Alcohol. 2003;30:19–28. doi: 10.1016/s0741-8329(03)00037-5. [DOI] [PubMed] [Google Scholar]
- [35].Domínguez HD, López MF, Molina JC. Neonatal responsiveness to alcohol odor and infant alcohol intake as a function of alcohol experience during late gestation. Alcohol. 1998;16:109–17. doi: 10.1016/s0741-8329(97)00169-9. [DOI] [PubMed] [Google Scholar]
- [36].Arias C, Chotro MG. Increased palatability of ethanol after prenatal ethanol exposure is mediated by the opioid system. Pharmacol Biochem Behav. 2005;82:434–42. doi: 10.1016/j.pbb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- [37].Nizhnikov ME, Varlinskaya EI, Petrov ES, Spear NE. Reinforcing properties of ethanol in neonatal rats: involvement of the opioid system. Behav Neurosci. 2006;120:267–80. doi: 10.1037/0735-7044.120.2.267. [DOI] [PubMed] [Google Scholar]
- [38].Molina JC, Ferreyra HF, Spear LP, Spear NE. Acute ethanol exposure during gestational day 8 in the rat: effects upon physical and behavioral parameters. Alcohol. 1984;1:459–64. doi: 10.1016/0741-8329(84)90022-3. [DOI] [PubMed] [Google Scholar]
- [39].National Institute of Health. Institute of laboratory animal resources, commission on life sciences. National Research Council . Guide for the care and use of laboratory animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- [40].Domínguez HD, López MF, Chotro MG, Molina JC. Perinatal responsiveness to alcohol’s chemosensory cues as a function of prenatal alcohol administration during gestational days 17-20 in the rat. Neurobiol Learn Mem. 1996;65:103–12. doi: 10.1006/nlme.1996.0012. [DOI] [PubMed] [Google Scholar]
- [41].Molina JC, Chotro MG, Domínguez HD. Fetal alcohol learning derived from ethanol contamination of the prenatal environment. In: Lecanuet JP, Fifer WP, Krasnegor NA, Smotherman WP, editors. Fetal development: a psychobiological perspective. Lawrence Erlbaum Associates; Hillsdale, NJ: 1995. pp. 295–315. [Google Scholar]
- [42].Gilman A Goodman, Limbird LE, Hardman JG. Las bases farmacológicas de la terapeútica. McGraw Hill Interamericana Editores; Mexico, D.F.: 1996. [Google Scholar]
- [43].Ngai SH, Berkowitz BA, Yang JC, Hempstead J, Spector S. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976;44:398–401. doi: 10.1097/00000542-197605000-00008. [DOI] [PubMed] [Google Scholar]
- [44].Burattini C, McGeehan AJ, Griffin WC, 3rd, Gass JT, Kinder JR, Janak PH, Olive MF. A microdialysis study of extracellular levels of acamprosate and naltrexone in the rat brain following acute and repeated administration. Addict Biol. 2008;13:70–9. doi: 10.1111/j.1369-1600.2008.00097.x. [DOI] [PubMed] [Google Scholar]
- [45].Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol Clin Exp Res. 2008;32:2016–27. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Varlinskaya EI, Spear LP. Ontogeny of acute tolerance to ethanol-induced social inhibition in Sprague-Dawley rats. Alcohol Clin Exp Res. 2006;30:1833–44. doi: 10.1111/j.1530-0277.2006.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pepino MY, Kraebel KS, López MF, Spear NE, Molina JC. Behavioral detection of low concentrations of ethanol in milk in the preweanling rat. Alcohol. 1998;15:337–53. doi: 10.1016/s0741-8329(97)00154-7. [DOI] [PubMed] [Google Scholar]
- [48].Pepino MY, López MF, Spear NE, Molina JC. Infant rats respond differently to alcohol after nursing from an alcohol-intoxicated dam. Alcohol. 1999;18:189–201. doi: 10.1016/s0741-8329(99)00003-8. [DOI] [PubMed] [Google Scholar]
- [49].Molina JC, Serwatka J, Enters EK, Spear LP, Spear NE. Acute alcohol intoxication disrupts brightness but not olfactory conditioning in preweanling rats. Behav Neurosci. 1987;101:846–53. doi: 10.1037//0735-7044.101.6.846. [DOI] [PubMed] [Google Scholar]
- [50].Spear LP, Specht SM, Kirstein CL, Kuhn CM. Anterior and posterior, but not cheek, intraoral cannulation procedures elevate serum corticosterone levels in neonatal rat pups. Dev Psychobiol. 1989;22:401–11. doi: 10.1002/dev.420220407. [DOI] [PubMed] [Google Scholar]
- [51].Petrov ES, Varlinskaya EI, Spear NE. Self-administration of ethanol and saccharin in newborn rats: effects on suckling plasticity. Behav Neurosci. 2001;115:1318–31. [PubMed] [Google Scholar]
- [52].Robinson SR, Arnold HM, Spear NE, Smotherman WP. Experience with milk and an artificial nipple promotes conditioned opioid activity in the rat fetus. Dev Psychobiol. 1993;26:375–87. doi: 10.1002/dev.420260702. [DOI] [PubMed] [Google Scholar]
- [53].Robinson SR, Smotherman WP. Organization of the stretch response to milk in the rat fetus. Dev Psychobiol. 1992;25:33–49. doi: 10.1002/dev.420250104. [DOI] [PubMed] [Google Scholar]
- [54].Hall WG. Feeding and behavioral activation in infant rats. Science. 1979;205:206–9. doi: 10.1126/science.451591. [DOI] [PubMed] [Google Scholar]
- [55].Robinson SR, Smotherman WP. Fundamental motor patterns of the mammalian fetus. J Neurobiol. 1992;23:1574–600. doi: 10.1002/neu.480231013. [DOI] [PubMed] [Google Scholar]
- [56].Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- [57].Arias C, Chotro MG. Increased preference for ethanol in the infant rat after prenatal ethanol exposure, expressed on intake and taste reactivity tests. Alcohol Clin Exp Res. 2005;29:337–46. doi: 10.1097/01.alc.0000156115.35817.21. [DOI] [PubMed] [Google Scholar]
- [58].Zagon IS, Hurst WJ, McLaughlin PJ. Transplacental transfer of naltrexone in rats. Life Sci. 1997;61:1261–7. doi: 10.1016/s0024-3205(97)00671-1. [DOI] [PubMed] [Google Scholar]
- [59].Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology. 1993;112:163–82. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- [60].Piasecki J, Koros E, Dyr W, Kostowski W, Danysz W, Bienkowski P. Ethanol-reinforced behaviour in the rat: effects of uncompetitive NMDA receptor antagonist, memantine. Eur J Pharmacol. 1998;354:135–43. doi: 10.1016/s0014-2999(98)00442-7. [DOI] [PubMed] [Google Scholar]
- [61].Berridge KC. Pleasures of the brain. Brain Cogn. 2003;52:106–28. doi: 10.1016/s0278-2626(03)00014-9. [DOI] [PubMed] [Google Scholar]
- [62].Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–98. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- [63].Becker HC. Effects of ethanol on the central nervous system: Fetal damage—neurobehavioral effects. In: Deitrich RA, editor. Pharmacologic Effects of Ethanol on the Nervous System. CRC Press; Boca Raton: 1996. pp. 407–440. [Google Scholar]
- [64].Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol Exposure: Comparability of effects in humans and animal models. Neurotoxicol Teratol. 1990;12:231–237. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- [65].Pueta M, Abate P, Spear NE, Molina JC. Interactions between ethanol experiences during late gestation and nursing: effects upon infantile and maternal responsiveness to ethanol. Int J Comp Psychol. 2005;18:207–24. [Google Scholar]
- [66].Chotro MG, Arias C. Exposure to low and moderate doses of alcohol on late gestation modifies infantile response to and preference for alcohol in rats. Ann Ist Super Sanita. 2006;42:22–30. [PubMed] [Google Scholar]
- [67].Youngentob SL, Molina JC, Spear NE, Youngentob LM. The effect of gestational ethanol exposure on voluntary ethanol intake in early postnatal and adult rats. Behav Neurosci. 2007;121:1306–15. doi: 10.1037/0735-7044.121.6.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]