Since its advent in 1998, electron capture dissociation (ECD) has come to be regarded as a potentially powerful tool for elucidating protein structures. Numerous efforts to optimize ECD for protein analysis have been reported over the past decade.1,2 Less publicized has been a small number of recent attempts3–5 to overcome the limitation of the original implementation of ECD, namely, the necessity for practical purposes of having to perform it on Fourier transform ion cyclotron resonance (FTICR) instruments. Baba et al.,3 Silivra et al.,4 and Ding and Brancia5 independently succeeded in observing ECD in a linear ion trap, a three dimensional (3D) ion trap, and a digital 3D ion trap, respectively. In the first two of these demonstrations, magnetic fields were used for electron confinement, and, in the latter, a digitally generated, rectangular-trapping, electric-field waveform was used for this purpose. In all three approaches, it was necessary to use a moderating gas (He) either to convert some of the translational energy of the electrons into rotational energy about the magnetic field lines, to compensate for the unavoidable transfer of energy from the radiofrequency (RF) field to the electrons, or both. In the two 3D ion-trap demonstrations,4,5 ECD occurred in the analyzer itself, whereas, in the linear ion-trap demonstration,3 it took place in a custom-designed cell. By virtue of being analyzer-independent, the linear multipole would seem to be a more promising platform than the 3D ion trap.
A year ago, we also succeeded in observing ECD on an electrospray ionization (ESI) triple quadrupole (QqQ) mass spectrometer system by placing the octapole collision cell of the instrument inside a solenoid and mounting a ring filament (electron emitter) concentric with the ion beam axis at the entrance of the octapole.6 This design was deemed unsatisfactory, however, because the presence of the RF field did not allow for sufficient control of the electron energy and, furthermore, the solenoid-current required to generate enough magnetic flux density to confine the electrons inside the RF octapole was too high.
In order to perform ECD efficiently, the precursor ions must be forced to mingle with a dense population of low-energy electrons. Since the reagent electrons and the multiply protonated precursor ions have opposite polarities and masses that differ by more than six orders of magnitude, the conditions for simultaneously confining them in the same volume of space cannot be satisfied in a purely electrostatic cell, and can only be minimally satisfied in an RF cell. It is possible, however, to achieve practical levels of ECD by focusing the electrons with strong magnetostatic lenses and guiding the ions with an electrostatic field. Here, we report ECD of substance P in a linear, RF-free, hybrid electrostatic/magnetostatic cell.
The cell comprises five, axially polarized, N42SH-grade Nd-Fe-B ring magnets (SuperMagnetMan, Birmingham, AL, USA) that have a 3.0″ diameter, 0.5″ thickness, and 0.375″ bore. The magnets, which are held together by an aluminum housing, are arranged in the alternating-polarity-structure of an axial traveling wave tube.7 Four soft-iron rings separate the five magnets, and a soft-iron ring terminates the entire assembly at each end. All of the iron rings have 1.0″ diameter, 0.125″ thickness, and 0.125″ bore. Thin (0.010″ thick) Teflon rings insulate each iron ring from its adjacent magnets. Each of the six iron rings and the aluminum housing of the magnet are connected to an independently adjustable ±100-V channel of a seven-channel power supply (which can be floated up to 8kV) so that it can function as an electrostatic lens as well as a pole piece for a magnetostatic lens. A ring-shaped, floating filament of tungsten-rhenium wire, located concentric with the axis of the cell at the ion entrance, serves as the source of electrons.
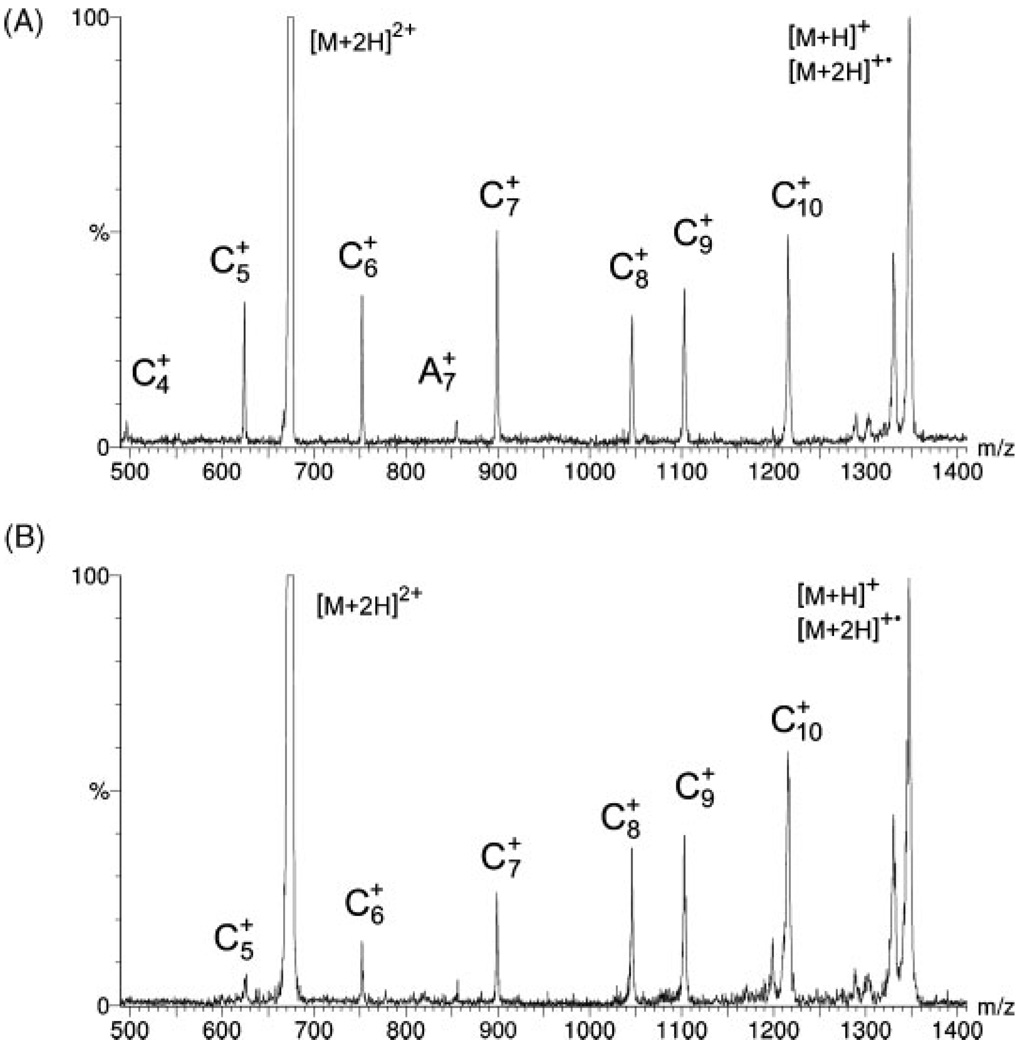
The cell was tested by using it as a replacement for the RF octapole in a commercial ESI QqQ mass spectrometer (Finnigan TSQ700; Thermo Fisher Scientific, Inc., Waltham, MA, USA). By adjusting the potentials on the electrostatic lenses of the cell, settings were easily found that allowed the electrons emitted from the ring filament to merge in sufficient numbers with the ion beam to produce ECD spectra of doubly protonated substance P (American Peptide Co., Sunnyvale, CA, USA) that appear in all respects (except, obviously, in resolution) the same as those produced on FTICR instruments1,8 (Fig. 1(A)).
Figure 1.
ECD spectra of doubly protonated substance P with total flight times through the dissociation cell of (A) ~25 µs and (B) ~12 µs.
The segmented design of the ECD cell provides additional opportunities for controlling electron-ion interactions and dissociation of precursor ions. For instance, by appropriately setting the potentials on the electrostatic lenses, the electron capture events can be forced to take place in the early entry side segments of the cell, and decomposition of the radical precursor ions can be observed as a function of time after electron capture. To demonstrate this possibility, the total flight time of [M+2H]+• radical ions through the cell was decreased (by changing the cell potential from 80V to 300 V) from ~25 µs to ~12 µs to produce spectra within which the relative strengths of the fragment signals are markedly different (Fig. 1(B)). Since no changes in the relative intensities of the fragment ions were observed when the electron energy was varied, it would seem that the majority of the decrease of the intensities of the shorter c-type ions is most likely due to the decreased residence time of the radical ions, [M+2H]+•, inside the cell before they enter the second analyzer. A more complete explanation will require a more thorough investigation; however, it is clear that the new cell makes it possible to investigate the mechanisms of ECD from previously unavailable vantage points. For example, it should be possible to test ideas like the recently proposed9 sequential formation of diagnostic c-type ions.
In summary, we have achieved ECD in a linear, RF-free, hybrid electrostatic/magnetostatic cell without the aid of a cooling gas. The design and compact construction of the cell allow it to be incorporated into virtually any type of tandem mass spectrometer, e.g., triple quadrupole, hybrid quadrupole ion trap, hybrid quadrupole time-of-flight, or even FTICR. The segmented electrostatic focusing of the cell makes it possible to straightforwardly monitor the decompositions of the radical precursor ions as a function of time. The strong magnetostatic focusing provided by the traveling wave tube configuration of the cell together with the capability for moving and trapping ions provided by the electrostatic segments of the cell could enable regular collision-induced dissociation over a much broader range of collision energies than those typically possible in ion-trap or quadrupole instruments. Finally, it should be possible to design a hybrid electrostatic/magnetostatic cell for ion mobility mass spectrometry. Investigation of the latter two possibilities is being planned.
Acknowledgements
This research was supported directly by a grant from the W. M. Keck Foundation and indirectly by an Environmental Health Sciences Center Grant (ES00210) from the National Institute of Environmental Health Science. We also thank Elsworth T. Hinke for his technical assistance.
REFERENCES
- 1.Cooper HJ, Hakansson K, Marshall AG. Mass Spectrom. Rev. 2005;24:201. doi: 10.1002/mas.20014. [DOI] [PubMed] [Google Scholar]
- 2.Bakhtiar R, Guan Z. Biotechnol. Lett. 2006;28:1047. doi: 10.1007/s10529-006-9065-z. [DOI] [PubMed] [Google Scholar]
- 3.Baba T, Hashimoto Y, Hasegawa H, Hirabayashi A, Waki I. Anal. Chem. 2004;76:4263. doi: 10.1021/ac049309h. [DOI] [PubMed] [Google Scholar]; Satake H, Hasegawa H, Hirabayashi A, Hashimoto Y, Baba T. Anal. Chem. 2007;79:8755. doi: 10.1021/ac071462z. [DOI] [PubMed] [Google Scholar]
- 4.Silivra OA, Kjeldsen F, Ivonin IA, Zubarev R. J. Am. Soc. Mass Spectrom. 2005;16:22. doi: 10.1016/j.jasms.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Ding L, Brancia L. Anal. Chem. 2006;78:1995. doi: 10.1021/ac0519007. [DOI] [PubMed] [Google Scholar]
- 6.Ji H, Voinov V, Deinzer ML, Barofsky DF. ECD in a linear RF-field without buffer gas; Proc. 55th ASMS Conf. Mass Spectrometry and Allied Topics; Indianapolis, Indiana. 2007. 1381(WPE-084) [Google Scholar]
- 7.Campbell P. Permanent Magnet Materials and Their Application. Cambridge: Cambridge University Press; 1994. pp. 201–202. [Google Scholar]
- 8.Budnik BA, Nielsen ML, Olsen JV, Haselmann KF, Hörth P, Haehnel W, Zubarev RA. Int. J. Mass Spectrom. 2002;219:283. [Google Scholar]
- 9.Leymarie N, Costello CE, O’Connor PB. J. Am. Soc. Mass. Spectrom. 2003;125:8949. doi: 10.1021/ja028831n. [DOI] [PubMed] [Google Scholar]