Abstract
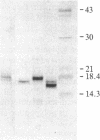
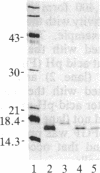
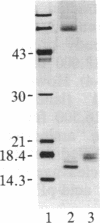
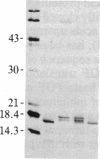
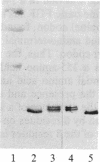
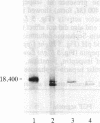
Basic fibroblast growth factor (FGF) was purified by heparin-Sepharose chromatography from two sources, brain and hepatoma cells. Brain cell-derived basic FGF (brFGF) and hepatoma cell-derived basic FGF (heFGF) were found to exist in multiple forms whose molecular weights depended on whether they were extracted from their respective tissue or cells at neutral or acid pH. When extracted at pH 7.0 brFGF and heFGF comigrated on NaDodSO4/PAGE with a Mr of approximately 18,400. When extracted at pHs 3.5-4.5, acid proteinases cleaved brFGF and heFGF to lower molecular weight forms but to different extents. brFGF was cleaved to a Mr 18,000 form at acid pH by a brain-derived acid proteinase that could be inhibited by pepstatin. heFGF was cleaved mostly to a Mr 16,500 form at acid pH by a hepatoma cell-derived acid proteinase that was inhibited by leupeptin. Electrophoretic transfer blot analysis using site-specific anti-FGF antibodies suggested that the cleavages occurred at the amino-terminal ends of brFGF and heFGF. Cleavage to lower molecular weight forms of brFGF and heFGF did not affect growth factor activity or chromatographic behavior on heparin-Sepharose columns.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Baird A., Esch F., Böhlen P., Ling N., Gospodarowicz D. Isolation and partial characterization of an endothelial cell growth factor from the bovine kidney: homology with basic fibroblast growth factor. Regul Pept. 1985 Nov 7;12(3):201–213. doi: 10.1016/0167-0115(85)90061-8. [DOI] [PubMed] [Google Scholar]
- Berger E. A., Shooter E. M. Evidence for pro-beta-nerve growth factor, a biosynthetic precursor to beta-nerve growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3647–3651. doi: 10.1073/pnas.74.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess W. H., Mehlman T., Friesel R., Johnson W. V., Maciag T. Multiple forms of endothelial cell growth factor. Rapid isolation and biological and chemical characterization. J Biol Chem. 1985 Sep 25;260(21):11389–11392. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Dart L. L., Smith D. M., Meyers C. A., Sporn M. B., Frolik C. A. Transforming growth factors from a human tumor cell: characterization of transforming growth factor beta and identification of high molecular weight transforming growth factor alpha. Biochemistry. 1985 Oct 8;24(21):5925–5931. doi: 10.1021/bi00342a035. [DOI] [PubMed] [Google Scholar]
- Esch F., Baird A., Ling N., Ueno N., Hill F., Denoroy L., Klepper R., Gospodarowicz D., Böhlen P., Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F., Ueno N., Baird A., Hill F., Denoroy L., Ling N., Gospodarowicz D., Guillemin R. Primary structure of bovine brain acidic fibroblast growth factor (FGF). Biochem Biophys Res Commun. 1985 Dec 17;133(2):554–562. doi: 10.1016/0006-291x(85)90942-8. [DOI] [PubMed] [Google Scholar]
- Frey P., Forand R., Maciag T., Shooter E. M. The biosynthetic precursor of epidermal growth factor and the mechanism of its processing. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6294–6298. doi: 10.1073/pnas.76.12.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez-Gallego G., Rodkey J., Bennett C., Rios-Candelore M., DiSalvo J., Thomas K. Brain-derived acidic fibroblast growth factor: complete amino acid sequence and homologies. Science. 1985 Dec 20;230(4732):1385–1388. doi: 10.1126/science.4071057. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Baird A., Cheng J., Lui G. M., Esch F., Bohlen P. Isolation of fibroblast growth factor from bovine adrenal gland: physicochemical and biological characterization. Endocrinology. 1986 Jan;118(1):82–90. doi: 10.1210/endo-118-1-82. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Bialecki H., Greenburg G. Purification of the fibroblast growth factor activity from bovine brain. J Biol Chem. 1978 May 25;253(10):3736–3743. [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Esch F., Bohlen P. Corpus luteum angiogenic factor is related to fibroblast growth factor. Endocrinology. 1985 Dec;117(6):2383–2391. doi: 10.1210/endo-117-6-2383. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Kawasaki T., Munechika H. Enzyme immunoassay of blasticidin S with high sensitivity: a new and convenient method for preparation of immunogenic (hapten-protein) conjugates. J Biochem. 1982 Aug;92(2):585–590. doi: 10.1093/oxfordjournals.jbchem.a133967. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Langer R., Levenson R., Smith S., Lillehei C. The stimulation of DNA synthesis and cell division in chondrocytes and 3T3 cells by a growth factor isolated from cartilage. Exp Cell Res. 1977 Mar 1;105(1):99–108. doi: 10.1016/0014-4827(77)90155-0. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Sasse J., Sullivan R., Smith J. A. Human tumor cells synthesize an endothelial cell growth factor that is structurally related to basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2448–2452. doi: 10.1073/pnas.83.8.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsbrun M., Shing Y. Heparin affinity of anionic and cationic capillary endothelial cell growth factors: analysis of hypothalamus-derived growth factors and fibroblast growth factors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):805–809. doi: 10.1073/pnas.82.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lobb R., Sasse J., Sullivan R., Shing Y., D'Amore P., Jacobs J., Klagsbrun M. Purification and characterization of heparin-binding endothelial cell growth factors. J Biol Chem. 1986 Feb 5;261(4):1924–1928. [PubMed] [Google Scholar]
- Nakane P. K. Simultaneous localization of multiple tissue antigens using the peroxidase-labeled antibody method: a study on pituitary glands of the rat. J Histochem Cytochem. 1968 Sep;16(9):557–560. doi: 10.1177/16.9.557. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982 May 10;257(9):5154–5160. [PubMed] [Google Scholar]
- Schreiber A. B., Kenney J., Kowalski J., Thomas K. A., Gimenez-Gallego G., Rios-Candelore M., Di Salvo J., Barritault D., Courty J., Courtois Y. A unique family of endothelial cell polypeptide mitogens: the antigenic and receptor cross-reactivity of bovine endothelial cell growth factor, brain-derived acidic fibroblast growth factor, and eye-derived growth factor-II. J Cell Biol. 1985 Oct;101(4):1623–1626. doi: 10.1083/jcb.101.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing Y., Folkman J., Sullivan R., Butterfield C., Murray J., Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984 Mar 23;223(4642):1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno N., Baird A., Esch F., Ling N., Guillemin R. Isolation of an amino terminal extended form of basic fibroblast growth factor. Biochem Biophys Res Commun. 1986 Jul 31;138(2):580–588. doi: 10.1016/s0006-291x(86)80536-8. [DOI] [PubMed] [Google Scholar]