Abstract
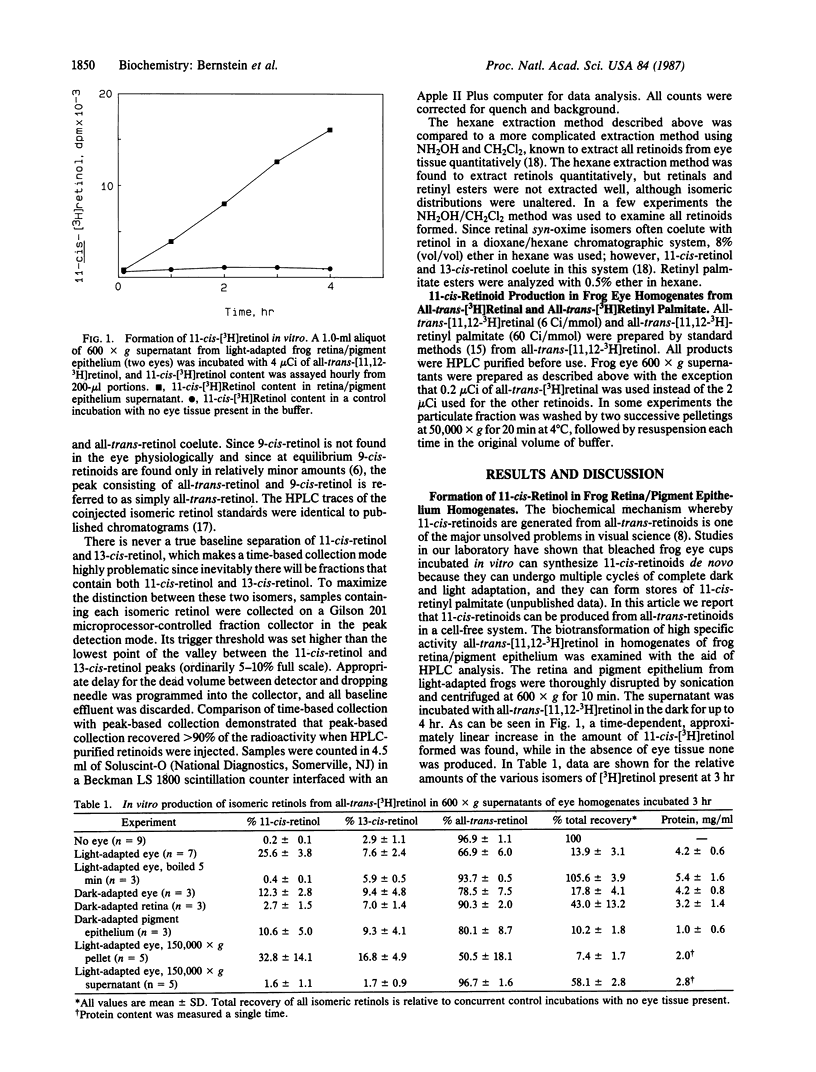
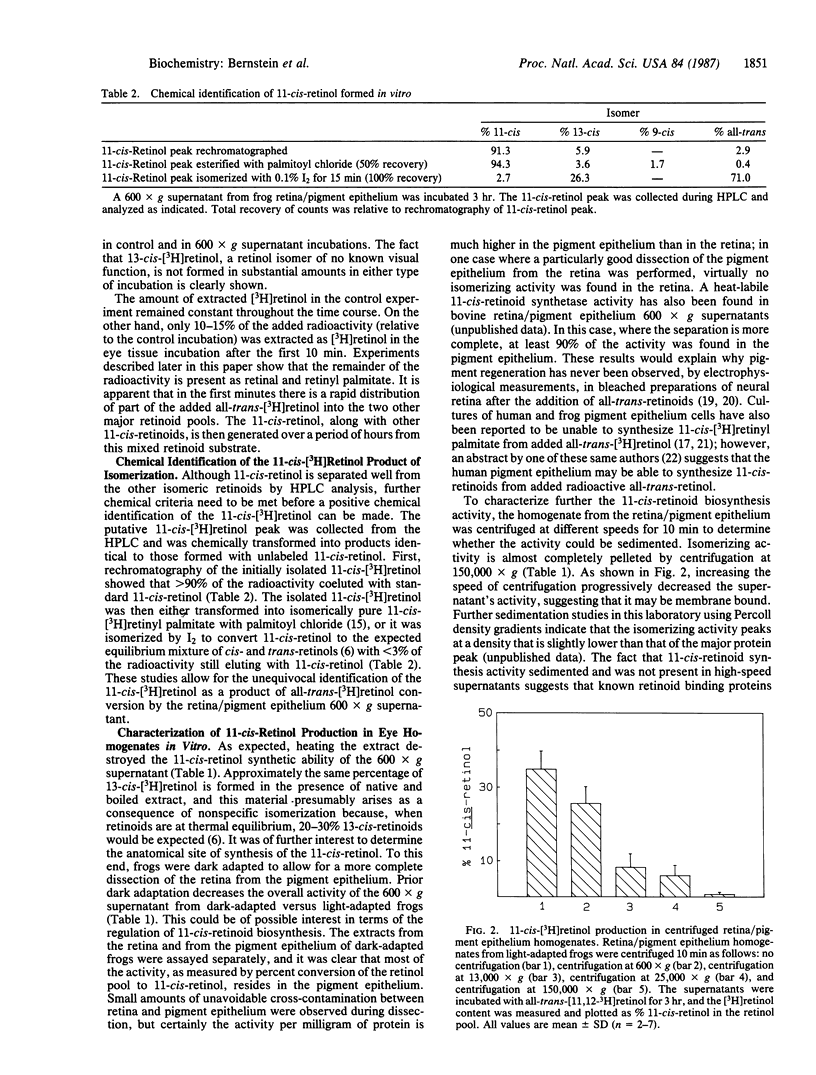
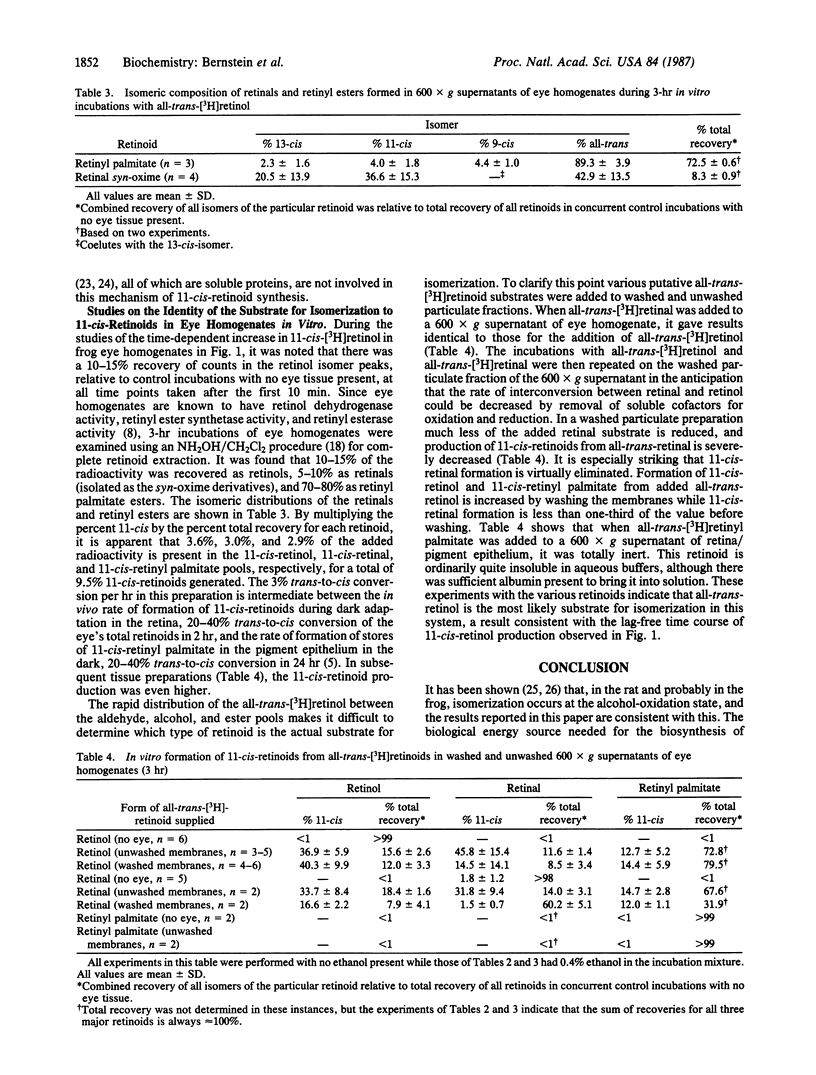
The key biochemical process of the vertebrate visual cycle required for rhodopsin regeneration, 11-cis-retinoid production from all-trans-retinoids, is shown to occur in vitro. A 600 X g supernatant from a frog retina/pigment epithelium homogenate transforms added all-trans-[3H]retinol, in a time-dependent fashion, to a mixture of 11-cis-retinol, 11-cis-retinal, and 11-cis-retinyl palmitate. 13-cis-Retinoids are formed in only minor amounts by nonspecific processes. Studies using washed particulate fractions of the 600 X g supernatant indicate that all-trans-[3H]retinol is isomerized to 11-cis-retinoids much more effectively than is all-trans-[3H]retinal or all-trans-[3H]retinyl palmitate. The 11-cis-retinoid biosynthetic activity is heat-labile, sedimentable by high-speed centrifugation, and largely found in the pigment epithelium rather than in the neural retina.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amer S., Akhtar M. Studies on a missing reaction in the visual cycle. Nat New Biol. 1972 Jun 28;237(78):266–267. doi: 10.1038/newbio237266a0. [DOI] [PubMed] [Google Scholar]
- Berman E. R., Horowitz J., Segal N., Fisher S., Feeney-Burns L. Enzymatic esterification of vitamin A in the pigment epithelium of bovine retina. Biochim Biophys Acta. 1980 Jun 5;630(1):36–46. doi: 10.1016/0304-4165(80)90135-x. [DOI] [PubMed] [Google Scholar]
- Bernstein P. S., Lichtman J. R., Rando R. R. Nonstereospecific biosynthesis of 11-cis-retinal in the eye. Biochemistry. 1985 Jan 15;24(2):487–492. doi: 10.1021/bi00323a036. [DOI] [PubMed] [Google Scholar]
- Bernstein P. S., Rando R. R. In vivo isomerization of all-trans- to 11-cis-retinoids in the eye occurs at the alcohol oxidation state. Biochemistry. 1986 Oct 21;25(21):6473–6478. doi: 10.1021/bi00369a020. [DOI] [PubMed] [Google Scholar]
- Bernstein P. S., Rando R. R. The specific inhibition of 11-cis-retinyl palmitate formation in the frog eye by diaminophenoxypentane, an inhibitor of rhodopsin regeneration. Vision Res. 1985;25(6):741–748. doi: 10.1016/0042-6989(85)90181-6. [DOI] [PubMed] [Google Scholar]
- Bownds D. Site of attachment of retinal in rhodopsin. Nature. 1967 Dec 23;216(5121):1178–1181. doi: 10.1038/2161178a0. [DOI] [PubMed] [Google Scholar]
- Bridges C. D., Alvarez R. A., Fong S. L., Gonzalez-Fernandez F., Lam D. M., Liou G. I. Visual cycle in the mammalian eye. Retinoid-binding proteins and the distribution of 11-cis retinoids. Vision Res. 1984;24(11):1581–1594. doi: 10.1016/0042-6989(84)90316-x. [DOI] [PubMed] [Google Scholar]
- Bridges C. D., Alvarez R. A., Fong S. L. Vitamin A in human eyes: amount, distribution, and composition. Invest Ophthalmol Vis Sci. 1982 Jun;22(6):706–714. [PubMed] [Google Scholar]
- Bridges C. D., Alvarez R. A. Measurement of the vitamin A cycle. Methods Enzymol. 1982;81:463–485. doi: 10.1016/s0076-6879(82)81065-3. [DOI] [PubMed] [Google Scholar]
- Bridges C. D. Vitamin A and the role of the pigment epithelium during bleaching and regeneration of rhodopsin in the frog eye. Exp Eye Res. 1976 May;22(5):435–455. doi: 10.1016/0014-4835(76)90182-2. [DOI] [PubMed] [Google Scholar]
- Flood M. T., Bridges C. D., Alvarez R. A., Blaner W. S., Gouras P. Vitamin A utilization in human retinal pigment epithelial cells in vitro. Invest Ophthalmol Vis Sci. 1983 Sep;24(9):1227–1235. [PubMed] [Google Scholar]
- Fong S. L., Bridges C. D., Alvarez R. A. Utilization of exogenous retinol by frog pigment epithelium. Vision Res. 1983;23(1):47–52. doi: 10.1016/0042-6989(83)90040-8. [DOI] [PubMed] [Google Scholar]
- HUBBARD R. Retinene isomerase. J Gen Physiol. 1956 Jul 20;39(6):935–962. doi: 10.1085/jgp.39.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD R., WALD G. Cis-trans isomers of vitamin A and retinene in the rhodopsin system. J Gen Physiol. 1952 Nov;36(2):269–315. doi: 10.1085/jgp.36.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok-Keung Fung B., Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A. 1980 May;77(5):2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers G. M., Olson J. A. Statistical solvent optimization for the separation of geometric isomers of retinol by high-performance liquid chromatography. J Chromatogr. 1984 May 18;291:51–57. doi: 10.1016/s0021-9673(00)95006-4. [DOI] [PubMed] [Google Scholar]
- Ostapenko I. A., Furayev V. V. 9-Cis isomerization of all-trans retinal during in vitro regeneration of visual pigment. Nat New Biol. 1973 Jun 6;243(127):185–186. doi: 10.1038/newbio243185a0. [DOI] [PubMed] [Google Scholar]
- Pepperberg D. R., Brown P. K., Lurie M., Dowling J. E. Visual pigment and photoreceptor sensitivity in the isolated skate retina. J Gen Physiol. 1978 Apr;71(4):369–396. doi: 10.1085/jgp.71.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperberg D. R., Masland R. H. Retinal-induced sensitization of light-adapted rabbit photoreceptors. Brain Res. 1978 Jul 28;151(1):194–200. doi: 10.1016/0006-8993(78)90964-2. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rotmans J. P., Daemen F. J., Bonting S. L. Biochemical aspects of the visual process. XIX. Formation of isorhodopsin from photolyzed rhodopsin by bacterial action. Biochim Biophys Acta. 1972 Jun 23;267(3):583–587. doi: 10.1016/0005-2728(72)90190-9. [DOI] [PubMed] [Google Scholar]
- Saari J. C., Bredberg L. Enzymatic reduction of 11-cis-retinal bound to cellular retinal-binding protein. Biochim Biophys Acta. 1982 May 27;716(2):266–272. doi: 10.1016/0304-4165(82)90277-x. [DOI] [PubMed] [Google Scholar]
- Wheeler G. L., Bitensky M. W. A light-activated GTPase in vertebrate photoreceptors: regulation of light-activated cyclic GMP phosphodiesterase. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4238–4242. doi: 10.1073/pnas.74.10.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]



