Abstract
The genomic organization of the IgH (Immunoglobulin heavy chain), Igκ (Immunoglobulin kappa chain), and Igλ (Immunoglobulin lambda chain) loci in the African elephant (Loxodonta africana) was annotated using available genome data. The elephant IgH locus on scaffold 57 spans over 2,974 kb, and consists of at least 112 VH gene segments, 87 DH gene segments (the largest number in mammals examined so far), six JH gene segments, a single μ, a δ remnant, and eight γ genes (α and ε genes are missing, most likely due to sequence gaps). The Igκ locus, found on three scaffolds (202, 50 and 86), contains a total of 153 Vκ gene segments, three Jκ segments, and a single Cκ gene. Two different transcriptional orientations were determined for these Vκ gene segments. In contrast, the Igλ locus on scaffold 68 includes 15 Vλ gene segments, all with the same transcriptional polarity as the downstream Jλ-Cλ cluster. These data suggest that the elephant immunoglobulin gene repertoire is highly diverse and complex. Our results provide insights into the immunoglobulin genes in a placental mammal that is evolutionarily distant from humans, mice, and domestic animals.
Introduction
The elephant is the biggest terrestrial placental mammal alive today. It belongs to the order Proboscidea and the family elephantidae, which contains only two existing species: the Asian elephant (Elephas maximus) and the African elephant (Loxodonta africana). The three lineages of this family: Loxodonta, Elephas, and Mammuthus are thought to have originated 4–6 million years ago. Whereas some species of the former two lineages are still alive today, the last representative of the Mammuthus lineage, the woolly mammoth (Mammuthus primigenius), became extinct very recently (about 3.7 thousand years ago) [1]. Phylogenetic analysis suggest that the elephant is most closely related to living mammals of Trichechus (such as the West Indian Manatee, Trichechus manatus) and Procavia (such as the Rock Hyrax, Procavia capensis) [2].
Elephants are reported to be susceptible to a wide variety of infections caused by bacteria [3], [4], viruses [5]–[13], and parasites [14]–[17]. However, there have been very few studies previously performed on the elephant immune system. In addition, little is known about the elephant immunoglobulins, except for serological testing for IgM [18], IgG [19], [20], and IgA [21]. It was reported that there were at least five subclasses of IgG in African elephant sera, with no apparent IgM or IgA [20].
Immunoglobulins are the antigen-recognition molecules of B cells of jawed vertebrates, which usually consist of two identical heavy (H) and two identical light (L) chains. In some exceptional cases, such as shark IgNAR and selected subclasses of camelid IgGs, only heavy chains are used [22]–[24]. Variable regions in the N-terminus of H/L chains are encoded by VH/VL, DH, and JH/JL genes to determine the antigen binding site and antibody specificity. However, constant regions in the C-terminus of H/L chains are encoded by IGHC/Cκ or Cλ genes and are responsible for the immunoglobulin classes and functional activities [25], [26].
In the mammals studied so far, the locus of unique immunoglobulin heavy chain genes and loci of λ and κ light chain genes are commonly organized in a “translocon” pattern [27], [28]. In the heavy chain locus, multiple VH, DH, and JH gene segments are followed by consecutive μ, δ, γ, ε, and α gene segments [29]. In the λ light chain locus, a cluster of Vλ gene segments is followed by multiple sets of clustered Jλ gene segments, each linked to a single Cλ gene. Differentially, the cluster of Vκ gene segments is followed by a cluster of Jκ gene segments, and then by a single Cκ gene [30].
IgH and IgL loci have been characterized in different mammalian species [31]–[48]. Although the genomic organization of immunoglobulin genes in mammals has remained relatively constant, variation exists in the number of variable, diversity, joining, and constant region genes. Here, we present the genomic organization of the IgH, Igκ, and Igλ loci of the African elephant, annotated on a basis of its genome data.
Materials and Methods
The elephant genome sequence
The genome sequence of the African Elephant (Loxodonta africana), provided by the Broad Institute via whole genome shotgun, can be obtained from the Ensembl database (http://www.ensembl.org). LoxAfr3, an assembly of the genome of African Elephant, has been sequenced to 7× coverage (loxAfr3, 7× coverage, July 2009). The elephant immunoglobulin gene sequences were retrieved from the UCSC genome browser (http://genome.ucsc.edu/).
Identification of the elephant Ig genes
Human immunoglobulin gene sequences were used as queries to search the elephant genome scaffolds that contained immunoglobulin genes. A conventional TBLASTN approach was used to identify constant region genes of the elephant immunoglobulins. FUZZNUC, an online software (http://embossgui.sourceforge.net/demo/fuzznuc.html) was used to find adjacent recombination signal sequences (RSSs) for identification of variable, diversity, and joining gene segments. Five or more mismatched bases were allowed to cover all genes. The locations of the annotated elephant gene sequences on the elephant genome are shown in Table S1 (S1-1∼S1-5).
Sequence alignments
Editing and comparison of sequences were carried out using the DNAstar program. Alignment of multiple sequences was performed using the Clustal W algorithm, then aligned with Clustal X software, and exported by BioEdit software with view conservation by plotting identities to a standard as a dot.
Dot matrix analysis
A dot matrix analysis (window size 30 bp and mismatch limit 9 bp) was used for comparing two sequences to identify a possible alignment of characters between the sequences.
Phylogenetic analysis
Phylogenetic studies were carried out using MrBayes3.1 and viewed with the TreeView package. All the trees were obtained with 1 million generations for the chains, a sample frequency of a 100, and a burn in of 2,500 (ngen = 1000000; Samplefreq = 100; burnin = 2,500). The site by site rate variation was set to a gamma distribution (rates = gamma) for all the Bayesian trees and a General Time-Reversible (GTR) (nst = 6) model of substitution was chosen. The sequences from other species used in phylogenetic analyses are presented in Table S2 (S2-1∼S2-2).
Definition of the VH/VL gene families
In mammals, germline VH and VL gene segments can be grouped into families based on their nucleotide sequence similarity [49]. The established criteria are that the same family members share more than 80% nucleotide similarity, those with less than 70% similarity are put into different families, and those possessing between 70% and 80% similarity are inspected on a case-by-case basis [50]. In our analysis, we placed VH and VL segments having similarity greater than 70% into the same family.
Results
Elephant immunoglobulin heavy chain genes
IgH locus
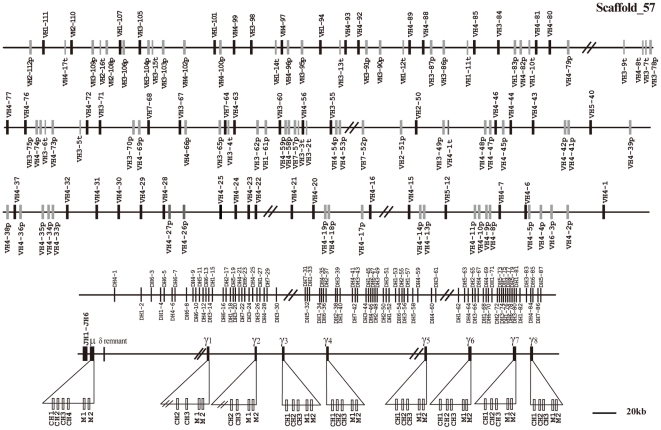
The public elephant genome assembly used in this study was loxAfr3, which is an assembly of the genome of the African Elephant (Loxodonta africana), sequenced to 7× coverage. The high genome coverage of this assembly confers a high reliability on the gene analysis. BLAST searching localized the elephant IgH locus to genomic scaffold 57. It spans approximately 2,974 kb from the most 5′ VH segment (VH2-112p) to the most 3′ γ gene (Fig. 1). A single μ and eight γ genes were identified in this scaffold. Neither ε nor α genes could be found, most likely due to sequence gaps.
Figure 1. The elephant IgH locus.
The elephant IgH locus is localized to scaffold 57. The length is approximately 2,974 kb from the most 5′ VH segment (VH2-112p) to the most 3′γ segment. Filled bars: potentially functional VH genes; open bars: VH pseudogenes: open bars and V-p; thin bars: truncated VH segments and V-t; DH: diversity genes; JH: joining genes; μ: IgM coding gene; δ: IgD remnant; and γ: IgG coding gene. VH and DH genes are numbered based on the families to which they belong and their positions in the locus. The number before the dash (VHn or DHn) indicates the family, while the number after dash represents the genomic position. JH genes are numbered based on the order of their locations in the locus. Double slashes indicate gaps >10 kb. Alpha and epsilon genes are not displayed on the map, but this does not imply their absence in the elephant IgH locus.
Constant region genes
Like other mammalian species, the elephant μ gene contains four CH and two transmembrane exons. A sequence comparison of μ genes among thirteen vertebrate species demonstrated that the critical amino acids for immunoglobulin folding, Cysteine (C) and Tryptophan (W) [51], were highly conserved in elephants (Figure S1). In addition, the elephant IgM constant region showed the highest amino acid sequence identity to human (63.8%), and the least to echidna (50.8%).
Most mammals also express a δ gene, which is always situated immediately downstream of the μ, and the distance between μ and δ usually does not exceed 7 kb. A BLAST search against the elephant whole genome using both DNA and amino acid sequences of the δ genes of other mammalian species showed no intact δ gene. However, approximately 10 kb downstream of the elephant µ (no sequence gaps for 90 kb downstream), we identified a short fragment encoding a polypeptide (Figure S2) homologous to the IgD CH3 domain of other mammals. This was done by a thorough examination of amino acid sequences encoded by the DNA sequences between μ and γ1 (based on translation of all reading frames of both sense and anti-sense sequences). An alignment of the elephant IgD remnant and the IgD CH3 domains of several mammalian species is presented in Figure S2. This indicates that the gene has been highly mutated and pseudogenized in the elephant.
In addition to the eight γ genes (γ1 to γ8) in scaffold 57 (Fig. 1), an additional γ gene (tentatively named as γ9) was identified in scaffold 495 (data not shown), which spans 77 kb. Scaffold 495 is not assembled together with scaffold 57; therefore, γ9 could potentially be either an additional subclass encoding gene or an allelic variant. The identification of multiple IgG subclass-encoding genes is in accordance with a previous report, which indicated that there were at least five subclasses of IgG in African elephant sera [20]. Sequence analysis showed no additional Ig genes in genomic scaffold 495, except for the γ9 gene. The greatest variation among mammalian IgG subclasses is usually concentrated in their hinge regions [52]–[54]. However, no elephant IgG cDNA sequences have been sequenced, it is very difficult to accurately assess the hinge regions of the elephant IgG heavy chains. The hinge region is usually encoded on a separate exon that could not be identified in the elephant due to the low level of conservation and the absence of cDNA sequences. An amino acid alignment of the nine elephant IgG subclasses is presented in Fig. 2. The first exons (CH1) of γ1 and γ2 are both missing because of gaps. The CH3 exon of γ3 is pseudogenized because of a premature stop codon (marked with a star in Fig. 2), and a frame-shift mutation (marked with shadowing in Fig. 2) caused by nucleotide (adenine) insertions at positions 148 and 158, respectively. To clarify the relationship among γ chains from mammalian species, a phylogenetic tree of IgG CH2 and CH3 exons was constructed and is shown in Figure S3. The elephant γ genes form a distinct cluster. This is consistent with previous analysis, which showed that the divergence of IgG subclasses occurred after speciation [52].
Figure 2. Alignment of the amino acid sequences of the nine elephant γ genes.
The deduced amino acid sequences of three γ chain-coding exons and two membrane exons of nine elephant γ genes are aligned. The first exons (CH1) of γ1 and γ2 are both missing due to gaps. A premature stop codon is marked with a star and a frameshift mutation is marked by shadowing in the CH3 exon of γ3. In each panel, the amino acid residues that are identical to the top sequences are represented by dots. The dashes were used to adjust the alignment. The hinge exons are not included in the figure.
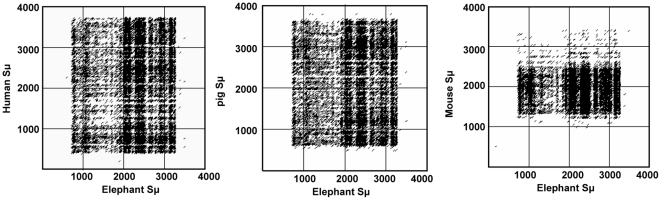
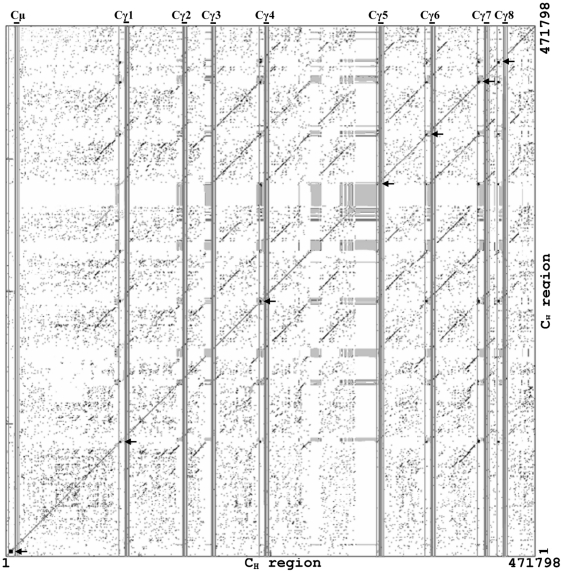
Dot matrix analysis of the elephant IgH locus showed there are switch regions upstream of the μ gene and six γ genes (γ1, γ4, γ5, γ6, γ7, and γ8), as in humans and mice [55], [56]. The switch regions of γ2, γ3, and γ9 could not be identified, most likely due to sequence gaps. Structurally, the switch regions, as in other species, are all composed of pentameric repeats (GGGCT and GAGCT). The elephant Sμ region shows substantial nucleotide similarity with those of human, mouse, and pig (Fig. 3). The six elephant Sγ regions are similar, but share little sequence similarity with human and mouse Sγ (Fig. 4 and data not shown).
Figure 3. Dot plot comparison of the elephant Sμ with human, pig, and mouse Sμ regions.
The dots represent homologies with a search length of 30 bp and maximum of 9 bp mismatches. The elephant Sμ region shows substantial nucleotide sequence homology with those of human, mouse, and pig.
Figure 4. Dot plot comparison of the elephant CH (μ and γ) region.
A dot matrix representing repetitive sequences of elephant CH (μ and γ) genes. Switch regions are indicated by black-squared boxes and marked with arrowheads, and gaps are indicated by grey-squared boxes. Positions of CH genes are indicated as vertical lines. The dots represent homologies with a search length of 30 bp and maximum of 9 bp mismatches.
VH gene segments
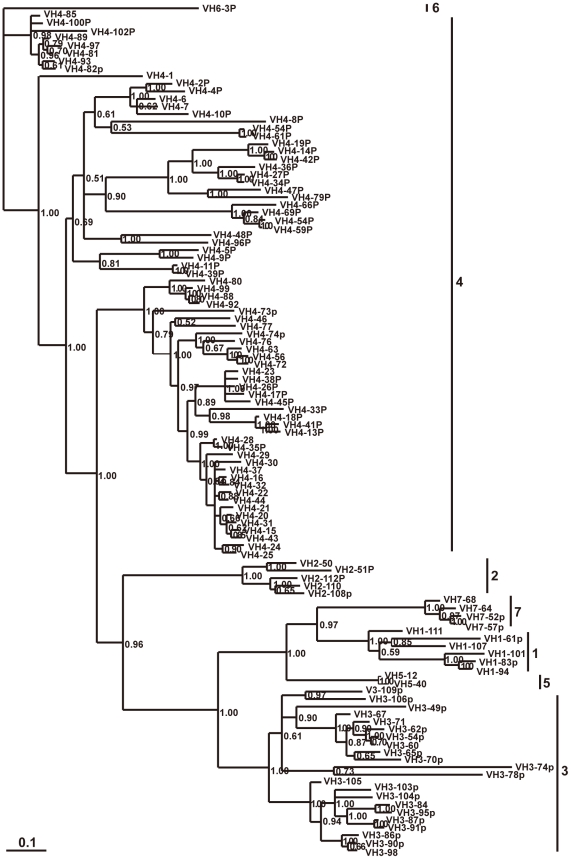
A total of 112 VH segments were identified in the elephant IgH locus. 51 of them appear to be potentially functional, because they have leader exons, normal open reading frames (ORF), downstream RSSs, and V gene domain (framework regions (FRs) and complementarity determining regions (CDRs)). The remaining 61 segments contain either in-frame stop codons or frameshifts, and are thus designated as pseudogenes. In addition, there are 17 partial segments of about 200 bp in length, which are regarded as truncated VH sequences. There are gaps above 10 kb in the elephant genome among the VH gene segments (Fig. 1), suggesting that there might be more VH segments. To examine the relationships among the elephant germline VH segments, pseudogenes as well as functional genes were used to construct a phylogenetic tree (Fig. 5). The seven identified VH gene families (1, 2, 3, 4, 5, 6, and 7) were confirmed to be homologous with the corresponding human VH gene families. The elephant VH4 family contains the most members (72 VH segments), which could be further divided into three groups (Fig. 5). We chose representative VH sequences from elephant and other mammals, covering almost all VH families identified, to construct phylogenetic trees (Fig. 6). The elephant VH genes clearly fall into the three previously known VH clans.
Figure 5. Phylogenetic analysis of the 112 elephant VH genes.
A phylogenetic tree of nucleotide sequences of 112 elephant VH segments was constructed. The seven identified VH gene families are labeled with Arabic numerals. The credibility value for each node is shown.
Figure 6. Phylogenetic analysis of mammalian VH genes.
Representatives of the seven elephant VH families are clustered with their human counterparts. The three mammalian VH clans are labeled with Roman numerals. The credibility value for each node is shown.
DH gene segments
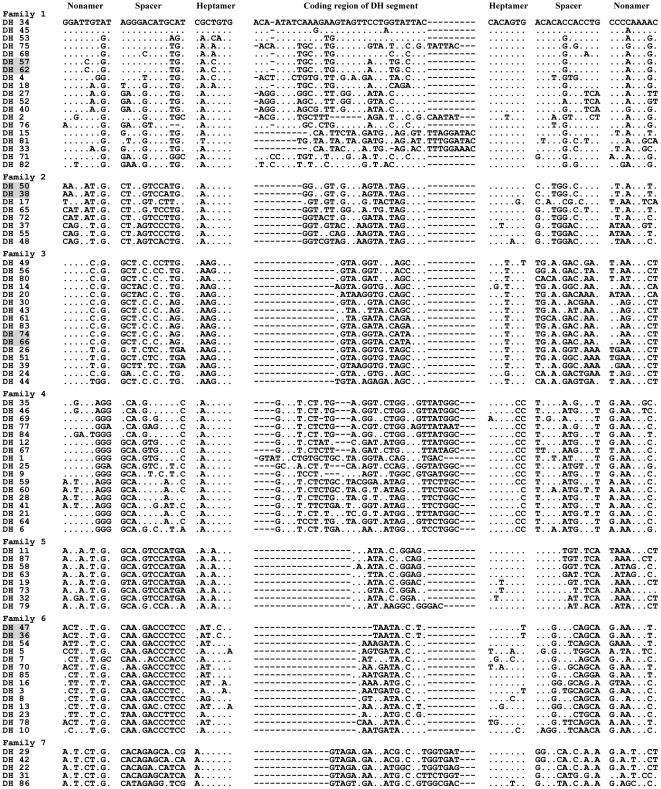
In the elephant IgH locus, 87 DH segments were identified and are presented in Figure S4 (S4-1∼S4-10). It should be noted that there might be more DH segments because of the existence of sequence gaps. Except for DH76, which has a 10 bp spacer, all the DH segments are flanked by characteristic heptamers and nonamers separated by 12-bp spacers. The potential coding regions of DH segments are 10–37 bp in length (Figure S4, S4-1∼S4-10). It has been suggested that coding regions of DH segments of humans can be described by the characteristics of their amino acids [57]. Inspection showed that a great number of polar/hydrophobic amino acids or stop codons occur widely in elephant DH coding regions (data not shown). In humans and mice, the germline DH segments can be classified into families based on the extent of sequence similarity [58], [59]. Analysis of nucleotide similarity in the coding regions and flanking RSSs indicated that the 87 elephant DH segments could be divided into seven families. Members within the same family share at least 70% nucleotide identity (data not shown), while some members in a family have completely identical sequences (these are shadowed in Fig. 7). We present the sequence alignment of the seven families in Fig. 7, which shows that each family contains characteristic sequence intervals that are distinct from other families.
Figure 7. Alignment of nucleotide sequences of seven elephant germline DH families.
Seven families representing elephant 87 germline DH segments are aligned. Nucleotides that are the same as the top segment, DH34, are indicated with dots. Dashes mean gaps introduced to make the alignment. DH57 and 62, DH50 and 38, DH74 and 66, and DH47 and 36 are shadowed as they share identical sequences. Coding regions of DH segments are separated from recombination signal sequences (RSSs) (nonamer, spacer, and heptamer).
JH gene segments
There were six germline JH gene segments found in the elephant IgH locus (Fig. 8). All the JH segments had conserved nucleotide sequences at the 3′ end. JH1 was pseudogenized by replacement of a Tryptophan (W) residue by a stop codon.
Figure 8. The six elephant germline JH gene segments.
Nucleotide and deduced amino acid sequences of six JH segments, along with RSSs, are shown. The amino acid residue W is replaced by a stop codon in the JH1 segment.
Elephant immunoglobulin light chains
κ chain
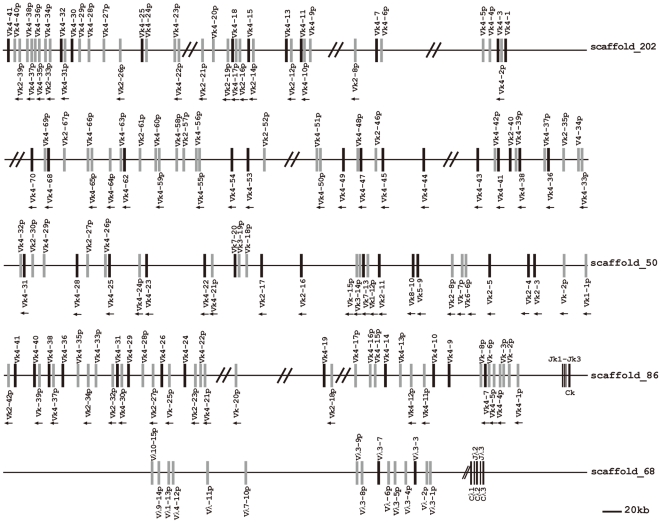
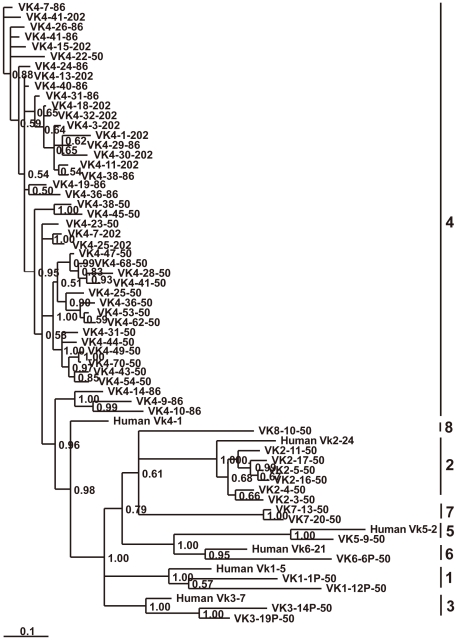
Immunoglobulin κ chain genes of elephant were identified on three scaffolds: 202, 50, and 86. A schematic diagram is shown in Fig. 9. Of the 153 germline Vκ segments from the three scaffolds, 53 were regarded as potentially functional genes and 100 as pseudogenes. Based on sequence similarity analysis, 142 of the Vκ segments can be assigned to eight families (Vκ1∼Vκ8) (Table S3), which contain 2, 31, 2, 102, 1, 1, 2, and 1 members, respectively. The remaining 11 Vκ pseudogenes could not be assigned to any family because they share less than 70% nucleotide similarity with any other Vκ gene segment. A phylogenetic tree of the elephant Vκ functional genes is shown in Fig.10. The six elephant Vκ families (Vκ1∼Vκ6) correspond to the six human Vκ gene families. In addition, scaffold 86 includes 24 Vκ segments showing the same transcriptional orientation as the Jκ and Cκ, and 18 Vκ segments showing a reverse transcriptional direction. Three Jκ segments and one Cκ gene on scaffold 86 are displayed in Figure S5. In addition, Vκ segments located on scaffolds 202 and 50 also possess two different transcriptional directions.
Figure 9. The elephant IgL locus.
The elephant Igκ locus is distributed over three scaffolds (202, 50, and 86), and the Igλ locus is located on scaffold 68. Overall configurations are drawn approximately to scale. The potentially functional Vκ and Vλ genes are shown as filled bars, while pseudogenes are represented by open bars and indicated with the letter p. Double slashes indicate gaps >10 kb. The unidirectional arrowheads below Vκ gene segments on scaffold 86 indicate that their transcriptional direction is opposite to downstream Jκ segments. However, the unidirectional arrowheads on scaffolds 202 and 50 do not represent different transcriptional directions from the identified Jκ gene segment; they merely indicate a transcriptional direction different from that of the remaining Vκ gene segments in the scaffold.
Figure 10. Phylogenetic analysis of the 58 elephant Vκ genes.
A phylogenetic tree of the nucleotide sequences of 58 elephant Vκ segments was constructed. The eight Vκ gene families are labeled with Arabic numerals. The credibility value for each node is shown.
λ chain
Scaffold 68 was determined to contain the elephant λ light gene complex (Fig. 9). Sequences analysis revealed that the 12 elephant Vλ gene segments belonged to six families (Fig. 11), which were homologous with the human Vλ 1, 3, 4, 7, 9 and 10 families. The remaining three Vλ pseudogenes could not be assigned to any family because they share less than 70% nucleotide similarity with any other Vλ gene segment. The three elephant Vλ families consists of seven members. In contrast to Vκ, all the Vλ segments possess an identical transcriptional polarity to the downstream Jλ segments. In addition, only Vλ3-3 and Vλ3-7 are identified as potentially functional genes. At the 3′ end of the locus, three constant region genes are organized in tandem, where both Cλ2 and Cλ3 are preceded by a Jλ. The Jλ segment before Cλ1 is missing because of a sequence gap. Three Cλ genes show approximately 90% amino acid identity. The sequences of two Jλ segments and three Cλ genes are presented in Figure S5.
Figure 11. Phylogenetic analysis of the 12 elephant Vλ genes.
A phylogenetic tree of the nucleotide sequences of the 12 elephant Vλ segments was constructed. The 12 elephant Vλ gene segments belong to six families, which are clustered with the human Vλ 1, 3, 4, 7, 9, and 10 families, respectively. The credibility value for each node is shown.
Discussion
In this study, we have made a preliminary analysis of the immunoglobulin genes in the elephant using the recently released elephant genome, revealing that the elephant IgH locus conforms to the “translocon” pattern. Compared with human IgH locus, which occupies a 1.25 Mb region [60], elephant IgH locus appears to span larger genomic region (approximately 3 Mb).
We translated the nucleotide sequences between the μ and γ1 genes in all three reading frames in both the positive and negative directions. By blasting the nucleotide and corresponding amino acid sequences against the NCBI database, only the IgD-CH3 remnant was identified.
With the exception of marsupials [61], [62], most placental and even monotreme mammals studied so far have been shown to have multiple IgG subclasses encoded by independent sets of exons [63]. The elephant genome contains nine IgG genes, although it is not known whether all of them are functional. This number is larger than that in any placental mammals so far examined (ranging from 1 to 7) [43], [64]–[69], providing another remarkable example for IgH chain constant region diversity in mammals.
Our analysis also suggested a high degree of complexity in the elephant IgVH locus. At least 112 VH segments constitute the elephant germ-line VH repertoire. According to the number of VH gene families, placentals studied so far could be divided into two groups. The multiple gene families group includes mice (16 families), human (seven families), and horse (seven families). The few gene families or single gene family group includes dog (three families), rabbits (one family), cattle (one family), camel (one family), and swine (one family) [70]–[78]. The elephant, having 7 VH gene families, should be put into the first group. The mammalian VH families can be further classified into three clans: I, II, and III, which have co-existed in the genome for more than 400 Myr [79]. Similar to those of humans, the elephant VH families also conform to three clans: families 1, 5, and 7 form clan I, families 2, 4, and 6 form clan II, and family 3 forms clan III. The largest group of elephant VH genes is the VH4 family of clan II. It has been demonstrated that the unique VH family identified in cattle belonged to clan II [75], [77]. In sheep, most VH genes are also categorized into clan II [80]. Based on a recent report, clan II also appeared to be the largest group in the horse [41], indicating that the herbivore animals may prefer to use the clan II VH genes.
Close attention should also be paid to the elephant DH locus, where at least 87 germline D segments could be mapped to a 450-kb DNA region; the largest number in mammals examined so far. The presence of more DH segments may greatly increase the Ig diversity generated through DNA rearrangement. The size of the elephant DH coding regions ranges from 10 to 37 bp, similar to that of human (11 to 37 bp) [57]. Further inspection revealed that the elephant DH segments were translated in three reading frames abundant in polar/hydrophobic amino acids, which is different to dog [78], horse [41], mouse [81], rabbit [82], and chicken [83], which show preferences for neutral (polar/hydrophilic) amino acids.
For the light chain genes, elephant Vκ germline genes are more abundant than Vλ (53 functional Vκ genes vs. 2 functional Vλ genes). Different mammalian species possess different ratios of Vκ and Vλ. In humans, roughly 60% of the variable light chain repertoire is κ (40 functional Vκ genes vs. 30 functional Vλ genes). The germline Vκ genes of mice are dominant by as much as 95% or more [84]. It has been proposed that the preferential use of light chain isotypes at the protein level may be correlated with the overall number of V gene segments [84]. It is thus possible that the κ chain predominates over the λ chain at the protein level in elephants.
Interestingly, a great number of pseudogenes exist in the elephant VH (61/112), Vκ (100/153), and Vλ (13/15) loci. In some species, the base-pair changes could be inferred using an existing pseudogene or germline gene as a template, and therefore pseudogenes in the V loci constitute a potential donor pool for gene conversion to generate immunoglobulin diversity [85]–[88]. A great number of V pseudogenes may contribute to the immunoglobulin diversity in elephants.
The study of structure and organization of the immunoglobulin gene loci is vital to the understanding of the nature of antibody molecules. This study provides information for comparative studies of mammalian Ig genes, as well as data for further studies of the elephant immunoglobulin genes.
Supporting Information
Alignment of IgM amino acid sequences from several vertebrate species. Elephant IgM was compared with a panel of vertebrate IgM sequences. Dots indicate similar residues as in elephant μ, whereas dashes indicate gaps introduced for optimal alignment. The cysteine residues C and W important for intra-domain disulfide bonds are shown on the first line of the alignment.
(TIF)
Alignment of the elephant IgD remnant with the IgD CH3 domains of several mammalian species. Amino acid residues that are identical to the top counterpart in every panel are shown as dots; Gaps and missing data are indicated by hyphens. Stop codons are indicated by stars.
(TIF)
Phylogenetic tree of the immunoglobulin gamma heavy chains of some mammalian species. The phylogenetic tree was constructed from the amino acid sequences of the CH3 exons of the immunoglobulin gamma heavy chains of various mammalian species. The credibility value for each node is shown.
(TIF)
87 Elephant germline DH segments. The nonamers (9-mer) and heptamers (7-mer) are displayed. Heptamer components that are different from the consensus (5′: CACTGTG and 3′: CACAGTG) are shadowed. The deduced amino acids of all three reading frames of the coding region of D segments are shown. Except DH, which has a 10 bp spacer, all the DH segments were attached by 12 bp spacer.
(TIF)
The alignment of amino acid sequences of J and C genes from elephant IgL chains. A, alignment of the deduced amino acid sequences of the three elephant Jκ gene segments. B, alignment of the amino acid sequences of the Cκ proteins from several mammalian species. C, alignment of the deduced amino acid sequences of the two elephant Jλ gene segments. D, alignment of the deduced amino acid sequences of three elephant Cλ genes and several mammalian species Cλ genes. Amino acid residues that are identical to the top counterpart in every panel are shown as dots; Gaps and missing data are indicated by hyphens.
(TIF)
The elephant immunoglobulin heavy chain and light chain DNA segments located in scaffolds 57, 202, 50, 86, and 68.
(RAR)
GenBank accession numbers or references of the gene sequences from other species used in this paper.
(RAR)
The eight elephant Vκ gene families from scaffolds 202, 50, and 86.
(TIF)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by the National Science Fund for Distinguished Young Scholars (30725029), the Taishan Scholar Foundation of Shandong Province, the National Basic Research Program of China (973 Program-2010CB945300) and Program for New Century Excellent Talents in University of China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Thomas MG, Hagelberg E, Jone HB, Yang Z, Lister AM. Molecular and morphological evidence on the phylogeny of the Elephantidae. Proc Biol Sci. 2000;267:2493–2500. doi: 10.1098/rspb.2000.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy WJ, Eizirik E, Johnson WE, Zhang YP, Ryder OA, et al. Molecular phylogenetics and the origins of placental mammals. Nature. 2001;409:614–618. doi: 10.1038/35054550. [DOI] [PubMed] [Google Scholar]
- 3.Mikota SK, Peddie L, Peddie J, Isaza R, Dunker F, et al. Epidemiology and diagnosis of Mycobacterium tuberculosis in captive Asian elephants (Elephas maximus). J Zoo Wildl Med. 2001;32:1–16. doi: 10.1638/1042-7260(2001)032[0001:EADOMT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Lyashchenko KP, Greenwald R, Esfandiari J, Olsen JH, Ball R, et al. Tuberculosis in elephants: antibody responses to defined antigens of Mycobacterium tuberculosis, potential for early diagnosis, and monitoring of treatment. Clin Vaccine Immunol. 2006;13:722–732. doi: 10.1128/CVI.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howell PG, Young E, Hedger RS. Foot-and-mouth disease in the african elephant (Loxodonta africana). Onderstepoort J Vet Res. 1973;40:41–52. [PubMed] [Google Scholar]
- 6.Davies FG, Otieno S. Elephants and zebras as possible reservoir hosts for African horse sickness virus. Vet Rec. 1977;100:291–292. doi: 10.1136/vr.100.14.291. [DOI] [PubMed] [Google Scholar]
- 7.Metzler AE, Ossent P, Guscetti F, Rubel A, Lang EM. Serological evidence of herpesvirus infection in captive Asian elephants (Elephas maximus). J Wildl Dis. 1990;26:41–49. doi: 10.7589/0090-3558-26.1.41. [DOI] [PubMed] [Google Scholar]
- 8.Turnbull PC, Bell RH, Saigawa K, Munyenyembe FE, Mulenga CK, et al. Anthrax in wildlife in the Luangwa Valley, Zambia. Vet Rec. 1991;128:399–403. doi: 10.1136/vr.128.17.399. [DOI] [PubMed] [Google Scholar]
- 9.Binepal VS, Wariru BN, Davies FG, Soi R, Olubayo R. An attempt to define the host range for African horse sickness virus (Orbivirus, Reoviridae) in east Africa, by a serological survey in some Equidae, Camelidae, Loxodontidae and Carnivore. Vet Microbiol. 1992;31:19–23. doi: 10.1016/0378-1135(92)90137-i. [DOI] [PubMed] [Google Scholar]
- 10.Formenty P, Domenech J, Lauginie F, Ouattara M, Diawara S, et al. [Epidemiologic study of bluetongue in sheep, cattle and different species of wild animals in the Ivory Coast]. Rev Sci Tech. 1994;13:737–751. [PubMed] [Google Scholar]
- 11.Bhat MN, Manickam R, Kumanan K. Serological evidence of bovine herpesviruses 1 and 2 in Asian elephants. J Wildl Dis. 1997;33:919–920. doi: 10.7589/0090-3558-33.4.919. [DOI] [PubMed] [Google Scholar]
- 12.Evans A, Gakuya F, Paweska JT, Rostal M, Akoolo L, et al. Prevalence of antibodies against Rift Valley fever virus in Kenyan wildlife. Epidemiol Infect. 2008;136:1261–1269. doi: 10.1017/S0950268807009806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oni O, Wajjwalku W, Boodde O, Chumsing W. Canine distemper virus antibodies in the Asian elephant (Elaphas maximus). Vet Rec. 2006;159:420–421. doi: 10.1136/vr.159.13.420. [DOI] [PubMed] [Google Scholar]
- 14.Hove T, Dubey JP. Prevalence of Toxoplasma gondii antibodies in sera of domestic pigs and some wild game species from Zimbabwe. J Parasitol. 1999;85:372–373. [PubMed] [Google Scholar]
- 15.Riemann GP, Burridge MJ, Behymer DE, Franti CE. Toxoplasma gondii antibodies in free-living African mammals. J Wildl Dis. 1975;11:529–533. doi: 10.7589/0090-3558-11.4.529. [DOI] [PubMed] [Google Scholar]
- 16.Tuntasuvan D, Mohkaew K, Dubey JP. Seroprevalence of Toxoplasma gondii in elephants (Elephus maximus indicus) in Thailand. J Parasitol. 2001;87:229–230. doi: 10.1645/0022-3395(2001)087[0229:SOTGIE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Dangolla A, Ekanayake DK, Rajapakse RP, Dubey JP, Silva ID. Seroprevalence of Toxoplasma gondii antibodies in captive elephants (Elephus maximus maximus) in Sri Lanka. Vet Parasitol. 2006;137:172–174. doi: 10.1016/j.vetpar.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 18.King DP, Sanders JL, Nomura CT, Stoddard RA, Ortiz CL, et al. Ontogeny of humoral immunity in northern elephant seal (Mirounga angustirostris) neonates. Comp Biochem Physiol B Biochem Mol Biol. 1998;121:363–368. doi: 10.1016/s0305-0491(98)10118-9. [DOI] [PubMed] [Google Scholar]
- 19.Kelly PJ, Tagwira M, Matthewman L, Mason PR, Wright EP. Reactions of sera from laboratory, domestic and wild animals in Africa with protein A and a recombinant chimeric protein AG. Comp Immunol Microbiol Infect Dis. 1993;16:299–305. doi: 10.1016/0147-9571(93)90159-3. [DOI] [PubMed] [Google Scholar]
- 20.Kelly PJ, Carter SD, Azwai SM, Cadman HF. Isolation and characterisation of immunoglobulin g and IgG subclasses of the African elephant (Loxodonta africana). Comp Immunol Microbiol Infect Dis. 1998;21:65–73. doi: 10.1016/s0147-9571(97)80053-7. [DOI] [PubMed] [Google Scholar]
- 21.Marquez ME, Slobodianik NH, Ronayne de Ferrer PA, Carlini AR, Vergani DF, et al. Immunoglobulin A levels in southern elephant seal (Mirounga leonina) milk during the suckling period. Comp Biochem Physiol B Biochem Mol Biol. 1995;112:569–572. doi: 10.1016/0305-0491(95)00101-8. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg AS, Avila D, Hughes M, Hughes A, McKinney EC, et al. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature. 1995;374:168–173. doi: 10.1038/374168a0. [DOI] [PubMed] [Google Scholar]
- 23.Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 24.Roux KH, Greenberg AS, Greene L, Strelets L, Avila D, et al. Structural analysis of the nurse shark (new) antigen receptor (NAR): molecular convergence of NAR and unusual mammalian immunoglobulins. Proc Natl Acad Sci. 1998;95 doi: 10.1073/pnas.95.20.11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edelman GM, Gall WE. The antibody problem. Annu Rev Biochem. 1969;38:415–466. doi: 10.1146/annurev.bi.38.070169.002215. [DOI] [PubMed] [Google Scholar]
- 26.Porter RR. The structure of the heavy chain of immunoglobulin and its relevance to the nature of the antibody-combining site. The Second CIBA Medal Lecture. Biochem J. 1967;105:417–426. doi: 10.1042/bj1050417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honjo T, Frederick A, Neuberger M, editors. Molecular Biology of B Cells: Elsevier Academic Press. 2004. 600
- 28.Zhang YA, Nie P, Luo HY, Wang YP, Sun YH, et al. Characterization of cDNA encoding immunoglobulin light chain of the mandarin fish (Siniperca chuatsi). Vet Immunol Immunopathol. 2003;95:81–90. doi: 10.1016/s0165-2427(03)00105-3. [DOI] [PubMed] [Google Scholar]
- 29.Thomas RK, Re D, Wolf J, Diehl V. Part I: Hodgkin's lymphoma–molecular biology of Hodgkin and Reed-Sternberg cells. Lancet Oncol. 2004;5:11–18. doi: 10.1016/s1470-2045(03)01319-6. [DOI] [PubMed] [Google Scholar]
- 30.Qin T, Ren L, Hu X, Guo Y, Fei J, et al. Genomic organization of the immunoglobulin light chain gene loci in Xenopus tropicalis: evolutionary implications. Dev Comp Immunol. 2008;32:156–165. doi: 10.1016/j.dci.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Gambon-Deza F, Sanchez-Espinel C, Magadan-Mompo S. The immunoglobulin heavy chain locus in the platypus (Ornithorhynchus anatinus). Mol Immunol. 2009;46:2515–2523. doi: 10.1016/j.molimm.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Chevillard C, Ozaki J, Herring CD, Riblet R. A three-megabase yeast artificial chromosome contig spanning the C57BL mouse Igh locus. J Immunol. 2002;168:5659–5666. doi: 10.4049/jimmunol.168.11.5659. [DOI] [PubMed] [Google Scholar]
- 33.Frippiat JP, Williams SC, Tomlinson IM, Cook GP, Cherif D, et al. Organization of the human immunoglobulin lambda light-chain locus on chromosome 22q11.2. Hum Mol Genet. 1995;4:983–991. doi: 10.1093/hmg/4.6.983. [DOI] [PubMed] [Google Scholar]
- 34.Hendricks J, Terpstra P, Dammers PM, Somasundaram R, Visser A, et al. Organization of the variable region of the immunoglobulin heavy-chain gene locus of the rat. Immunogenetics. 62:479–486. doi: 10.1007/s00251-010-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawasaki K, Minoshima S, Nakato E, Shibuya K, Shintani A, et al. Evolutionary dynamics of the human immunoglobulin kappa locus and the germline repertoire of the Vkappa genes. Eur J Immunol. 2001;31:1017–1028. [PubMed] [Google Scholar]
- 36.Kawasaki K, Minoshima S, Nakato E, Shibuya K, Shintani A, et al. One-megabase sequence analysis of the human immunoglobulin lambda gene locus. Genome Res. 1997;7:250–261. doi: 10.1101/gr.7.3.250. [DOI] [PubMed] [Google Scholar]
- 37.Kirschbaum T, Jaenichen R, Zachau HG. The mouse immunoglobulin kappa locus contains about 140 variable gene segments. Eur J Immunol. 1996;26:1613–1620. doi: 10.1002/eji.1830260731. [DOI] [PubMed] [Google Scholar]
- 38.Kirschbaum T, Pourrajabi S, Zocher I, Schwendinger J, Heim V, et al. The 3' part of the immunoglobulin kappa locus of the mouse. Eur J Immunol. 1998;28:1458–1466. doi: 10.1002/(SICI)1521-4141(199805)28:05<1458::AID-IMMU1458>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 39.Mage RG, Lanning D, Knight KL. B cell and antibody repertoire development in rabbits: the requirement of gut-associated lymphoid tissues. Dev Comp Immunol. 2006;30:137–153. doi: 10.1016/j.dci.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Pech M, Smola H, Pohlenz HD, Straubinger B, Gerl R, et al. A large section of the gene locus encoding human immunoglobulin variable regions of the kappa type is duplicated. J Mol Biol. 1985;183:291–299. doi: 10.1016/0022-2836(85)90001-4. [DOI] [PubMed] [Google Scholar]
- 41.Sun Y, Wang C, Wang Y, Zhang T, Ren L, et al. A comprehensive analysis of germline and expressed immunoglobulin repertoire in the horse. Dev Comp Immunol. 2010;34:1009–1020. doi: 10.1016/j.dci.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Tang L, Sampson C, Dreitz MJ, McCall C. Cloning and characterization of cDNAs encoding four different canine immunoglobulin gamma chains. Vet Immunol Immunopathol. 2001;80:259–270. doi: 10.1016/s0165-2427(01)00318-x. [DOI] [PubMed] [Google Scholar]
- 43.Wagner B, Miller DC, Lear TL, Antczak DF. The complete map of the Ig heavy chain constant gene region reveals evidence for seven IgG isotypes and for IgD in the horse. J Immunol. 2004;173:3230–3242. doi: 10.4049/jimmunol.173.5.3230. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Olp JJ, Miller RD. On the genomics of immunoglobulins in the gray, short-tailed opossum Monodelphis domestica. Immunogenetics. 2009;61:581–596. doi: 10.1007/s00251-009-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye J. The immunoglobulin IGHD gene locus in C57BL/6 mice. Immunogenetics. 2004;56:399–404. doi: 10.1007/s00251-004-0712-z. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, Cui H, Whittington CM, Wei Z, Zhang X, et al. Ornithorhynchus anatinus (platypus) links the evolution of immunoglobulin genes in eutherian mammals and nonmammalian tetrapods. J Immunol. 2009;183:3285–3293. doi: 10.4049/jimmunol.0900469. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Y, Kacskovics I, Rabbani H, Hammarstrom L. Physical mapping of the bovine immunoglobulin heavy chain constant region gene locus. J Biol Chem. 2003;278:35024–35032. doi: 10.1074/jbc.M301337200. [DOI] [PubMed] [Google Scholar]
- 48.Zhao Z, Zhao Y, Pan-Hammarstrom Q, Liu W, Liu Z, et al. Physical mapping of the giant panda immunoglobulin heavy chain constant region genes. Dev Comp Immunol. 2007;31:1034–1049. doi: 10.1016/j.dci.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Schroeder HW, Jr, Hillson JL, Perlmutter RM. Structure and evolution of mammalian VH families. Int Immunol. 1990;2:41–50. doi: 10.1093/intimm/2.1.41. [DOI] [PubMed] [Google Scholar]
- 50.Brodeur PH, Riblet R. The immunoglobulin heavy chain variable region (Igh-V) locus in the mouse. I. One hundred Igh-V genes comprise seven families of homologous genes. Eur J Immunol. 1984;14:922–930. doi: 10.1002/eji.1830141012. [DOI] [PubMed] [Google Scholar]
- 51.Lesk AM, Chothia C. Evolution of proteins formed by beta-sheets. II. The core of the immunoglobulin domains. J Mol Biol. 1982;160:325–342. doi: 10.1016/0022-2836(82)90179-6. [DOI] [PubMed] [Google Scholar]
- 52.Wagner B, Greiser-Wilke I, Wege AK, Radbruch A, Leibold W. Evolution of the six horse IGHG genes and corresponding immunoglobulin gamma heavy chains. Immunogenetics. 2002;54:353–364. doi: 10.1007/s00251-002-0458-4. [DOI] [PubMed] [Google Scholar]
- 53.Beck OE. Distribution of virus antibody activity among human IgG subclasses. Clin Exp Immunol. 1981;43:626–632. [PMC free article] [PubMed] [Google Scholar]
- 54.Roux KH, Strelets L, Michaelsen TE. Flexibility of human IgG subclasses. J Immunol. 1997;159:3372–3382. [PubMed] [Google Scholar]
- 55.Lundqvist ML, Middleton DL, Hazard S, Warr GW. The immunoglobulin heavy chain locus of the duck. Genomic organization and expression of D, J, and C region genes. J Biol Chem. 2001;276:46729–46736. doi: 10.1074/jbc.M106221200. [DOI] [PubMed] [Google Scholar]
- 56.Pan-Hammarstrom Q, Zhao Y, Hammarstrom L. Class switch recombination: a comparison between mouse and human. Adv Immunol. 2007;93:1–61. doi: 10.1016/S0065-2776(06)93001-6. [DOI] [PubMed] [Google Scholar]
- 57.Corbett SJ, Tomlinson IM, Sonnhammer EL, Buck D, Winter G. Sequence of the human immunoglobulin diversity (D) segment locus: a systematic analysis provides no evidence for the use of DIR segments, inverted D segments, "minor" D segments or D-D recombination. J Mol Biol. 1997;270:587–597. doi: 10.1006/jmbi.1997.1141. [DOI] [PubMed] [Google Scholar]
- 58.Kurosawa Y, Tonegawa S. Organization, structure, and assembly of immunoglobulin heavy chain diversity DNA segments. J Exp Med. 1982;155:201–218. doi: 10.1084/jem.155.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siebenlist U, Ravetch JV, Korsmeyer S, Waldmann T, Leder P. Human immunoglobulin D segments encoded in tandem multigenic families. Nature. 1981;294:631–635. doi: 10.1038/294631a0. [DOI] [PubMed] [Google Scholar]
- 60.Lefranc MP. Nomenclature of the human immunoglobulin heavy (IGH) genes. Exp Clin Immunogenet. 2001;18:100–116. doi: 10.1159/000049189. [DOI] [PubMed] [Google Scholar]
- 61.Bell RG, Lynch NR, Turner KJ. Immunoglobulins of the marsupial Setonix brachyurus (Quokka). Characterization of three major serum classes, IgG2, IgG1 and IgM. Immunology. 1974;27:1103–1115. [PMC free article] [PubMed] [Google Scholar]
- 62.Bell RG. Marsupial immunoglobulins: the distribution and evolution of macropod IgG2, IgG1, IgM and light chain antigenic markers within the sub-class Metatheria. Immunology. 1977;33:917–924. [PMC free article] [PubMed] [Google Scholar]
- 63.Belov K, Hellman L. Immunoglobulin genetics of Ornithorhynchus anatinus (platypus) and Tachyglossus aculeatus (short-beaked echidna). Comp Biochem Physiol A Mol Integr Physiol. 2003;136:811–819. doi: 10.1016/s1095-6433(03)00165-x. [DOI] [PubMed] [Google Scholar]
- 64.Flanagan JG, Rabbitts TH. Arrangement of human immunoglobulin heavy chain constant region genes implies evolutionary duplication of a segment containing gamma, epsilon and alpha genes. Nature. 1982;300:709–713. doi: 10.1038/300709a0. [DOI] [PubMed] [Google Scholar]
- 65.Shimizu A, Takahashi N, Yaoita Y, Honjo T. Organization of the constant-region gene family of the mouse immunoglobulin heavy chain. Cell. 1982;28:499–506. doi: 10.1016/0092-8674(82)90204-5. [DOI] [PubMed] [Google Scholar]
- 66.Knight KL, Burnett RC, McNicholas JM. Organization and polymorphism of rabbit immunoglobulin heavy chain genes. J Immunol. 1985;134:1245–1250. [PubMed] [Google Scholar]
- 67.Bruggemann M, Free J, Diamond A, Howard J, Cobbold S, et al. Immunoglobulin heavy chain locus of the rat: striking homology to mouse antibody genes. Proc Natl Acad Sci U S A. 1986;83:6075–6079. doi: 10.1073/pnas.83.16.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Knight KL, Suter M, Becker RS. Genetic engineering of bovine Ig. Construction and characterization of hapten-binding bovine/murine chimeric IgE, IgA, IgG1, IgG2, and IgG3 molecules. J Immunol. 1988;140:3654–3659. [PubMed] [Google Scholar]
- 69.Butler JE, Brown WR. The immunoglobulins and immunoglobulin genes of swine. Vet Immunol Immunopathol. 1994;43:5–12. doi: 10.1016/0165-2427(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 70.Van Dijk KW, Mortari F, Kirkham PM, Schroeder HW, Jr, Milner EC. The human immunoglobulin VH7 gene family consists of a small, polymorphic group of six to eight gene segments dispersed throughout the VH locus. Eur J Immunol. 1993;23:832–839. doi: 10.1002/eji.1830230410. [DOI] [PubMed] [Google Scholar]
- 71.Berman JE, Mellis SJ, Pollock R, Smith CL, Suh H, et al. Content and organization of the human Ig VH locus: definition of three new VH families and linkage to the Ig CH locus. EMBO J. 1988;7:727–738. doi: 10.1002/j.1460-2075.1988.tb02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnston CM, Wood AL, Bolland DJ, Corcoran AE. Complete sequence assembly and characterization of the C57BL/6 mouse Ig heavy chain V region. J Immunol. 2006;176:4221–4234. doi: 10.4049/jimmunol.176.7.4221. [DOI] [PubMed] [Google Scholar]
- 73.Almagro JC, Martinez L, Smith SL, Alagon A, Estevez J, et al. Analysis of the horse V(H) repertoire and comparison with the human IGHV germline genes, and sheep, cattle and pig V(H) sequences. Mol Immunol. 2006;43:1836–1845. doi: 10.1016/j.molimm.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 74.Schrenzel MD, King DP, McKnight ML, Ferrick DA. Characterization of horse (Equus caballus) immunoglobulin mu chain-encoding genes. Immunogenetics. 1997;45:386–393. doi: 10.1007/s002510050220. [DOI] [PubMed] [Google Scholar]
- 75.Berens SJ, Wylie DE, Lopez OJ. Use of a single VH family and long CDR3s in the variable region of cattle Ig heavy chains. Int Immunol. 1997;9:189–199. doi: 10.1093/intimm/9.1.189. [DOI] [PubMed] [Google Scholar]
- 76.Sun J, Kacskovics I, Brown WR, Butler JE. Expressed swine VH genes belong to a small VH gene family homologous to human VHIII. J Immunol. 1994;153:5618–5627. [PubMed] [Google Scholar]
- 77.Sinclair MC, Gilchrist J, Aitken R. Bovine IgG repertoire is dominated by a single diversified VH gene family. J Immunol. 1997;159:3883–3889. [PubMed] [Google Scholar]
- 78.Bao Y, Guo Y, Xiao S, Zhao Z. Molecular characterization of the VH repertoire in Canis familiaris. Vet Immunol Immunopathol. 2010;137:64–75. doi: 10.1016/j.vetimm.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 79.Ota T, Nei M. Divergent evolution and evolution by the birth-and-death process in the immunoglobulin VH gene family. Mol Biol Evol. 1994;11:469–482. doi: 10.1093/oxfordjournals.molbev.a040127. [DOI] [PubMed] [Google Scholar]
- 80.Charlton KA, Moyle S, Porter AJ, Harris WJ. Analysis of the diversity of a sheep antibody repertoire as revealed from a bacteriophage display library. J Immunol. 2000;164:6221–6229. doi: 10.4049/jimmunol.164.12.6221. [DOI] [PubMed] [Google Scholar]
- 81.Gu H, Kitamura D, Rajewsky K. B cell development regulated by gene rearrangement: arrest of maturation by membrane-bound D mu protein and selection of DH element reading frames. Cell. 1991;65:47–54. doi: 10.1016/0092-8674(91)90406-o. [DOI] [PubMed] [Google Scholar]
- 82.Knight KL, Crane MA. Generating the antibody repertoire in rabbit. Adv Immunol. 1994;56:179–218. doi: 10.1016/s0065-2776(08)60452-6. [DOI] [PubMed] [Google Scholar]
- 83.Reynaud CA, Anquez V, Weill JC. The chicken D locus and its contribution to the immunoglobulin heavy chain repertoire. Eur J Immunol. 1991;21:2661–2670. doi: 10.1002/eji.1830211104. [DOI] [PubMed] [Google Scholar]
- 84.Almagro JC, Hernandez I, Ramirez MC, Vargas-Madrazo E. Structural differences between the repertoires of mouse and human germline genes and their evolutionary implications. Immunogenetics. 1998;47:355–363. doi: 10.1007/s002510050370. [DOI] [PubMed] [Google Scholar]
- 85.Lucier MR, Thompson RE, Waire J, Lin AW, Osborne BA, et al. Multiple sites of V lambda diversification in cattle. J Immunol. 1998;161:5438–5444. [PubMed] [Google Scholar]
- 86.Almagro JC, Dominguez-Martinez V, Lara-Ochoa F, Vargas-Madrazo E. Structural repertoire in human VL pseudogenes of immunoglobulins: comparison with functional germline genes and amino acid sequences. Immunogenetics. 1996;43:92–96. doi: 10.1007/BF00186612. [DOI] [PubMed] [Google Scholar]
- 87.Haino M, Hayashida H, Miyata T, Shin EK, Matsuda F, et al. Comparison and evolution of human immunoglobulin VH segments located in the 3' 0.8-megabase region. Evidence for unidirectional transfer of segmental gene sequences. J Biol Chem. 1994;269:2619–2626. [PubMed] [Google Scholar]
- 88.Vargas-Madrazo E, Almagro JC, Lara-Ochoa F. Structural repertoire in VH pseudogenes of immunoglobulins: comparison with human germline genes and human amino acid sequences. J Mol Biol. 1995;246:74–81. doi: 10.1006/jmbi.1994.0067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of IgM amino acid sequences from several vertebrate species. Elephant IgM was compared with a panel of vertebrate IgM sequences. Dots indicate similar residues as in elephant μ, whereas dashes indicate gaps introduced for optimal alignment. The cysteine residues C and W important for intra-domain disulfide bonds are shown on the first line of the alignment.
(TIF)
Alignment of the elephant IgD remnant with the IgD CH3 domains of several mammalian species. Amino acid residues that are identical to the top counterpart in every panel are shown as dots; Gaps and missing data are indicated by hyphens. Stop codons are indicated by stars.
(TIF)
Phylogenetic tree of the immunoglobulin gamma heavy chains of some mammalian species. The phylogenetic tree was constructed from the amino acid sequences of the CH3 exons of the immunoglobulin gamma heavy chains of various mammalian species. The credibility value for each node is shown.
(TIF)
87 Elephant germline DH segments. The nonamers (9-mer) and heptamers (7-mer) are displayed. Heptamer components that are different from the consensus (5′: CACTGTG and 3′: CACAGTG) are shadowed. The deduced amino acids of all three reading frames of the coding region of D segments are shown. Except DH, which has a 10 bp spacer, all the DH segments were attached by 12 bp spacer.
(TIF)
The alignment of amino acid sequences of J and C genes from elephant IgL chains. A, alignment of the deduced amino acid sequences of the three elephant Jκ gene segments. B, alignment of the amino acid sequences of the Cκ proteins from several mammalian species. C, alignment of the deduced amino acid sequences of the two elephant Jλ gene segments. D, alignment of the deduced amino acid sequences of three elephant Cλ genes and several mammalian species Cλ genes. Amino acid residues that are identical to the top counterpart in every panel are shown as dots; Gaps and missing data are indicated by hyphens.
(TIF)
The elephant immunoglobulin heavy chain and light chain DNA segments located in scaffolds 57, 202, 50, 86, and 68.
(RAR)
GenBank accession numbers or references of the gene sequences from other species used in this paper.
(RAR)
The eight elephant Vκ gene families from scaffolds 202, 50, and 86.
(TIF)