Abstract
Objectives:
Cotinine is the most widely used biomarker to distinguish active versus passive smoking. However, there is an overlap in cotinine levels when comparing light or occasional smokers versus heavily exposed passive smokers. 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) is a tobacco-specific nitrosamine measurable in urine with a much longer half-life than cotinine. The aim of the study was to determine optimal cutoff points to discriminate active versus passive smokers and to compare sensitivity and specificity for the use of cotinine, NNAL, and the ratio of the NNAL/cotinine in urine.
Methods:
Cotinine and NNAL were measured in urine of 373 active smokers and 228 passive smokers.
Results:
Geometric mean cotinine levels were 2.03 ng/ml (interquartile interval: 0.43–8.60) and 1,043 ng/ml (658–2,251) and NNAL levels were 5.80 pg/ml (2.28–15.4) and 165 pg/ml (90.8–360) pg/ml in passive and active smokers, respectively. NNAL/cotinine ratio in urine was significantly higher for passive smokers when compared with active smokers (2.85 vs. 0.16, p < .01). The receiver operating characteristics analysis determined optimal cutoff points to discriminate passive versus active smokers: 31.5 ng/ml for cotinine (sensitivity: 97.1% and specificity: 93.9%), 47.3 pg/ml for NNAL (87.4% and 96.5%), and 0.74 × 10−3 for NNAL/cotinine ratio (97.3% and 87.3%).
Conclusions:
Both urine cotinine and NNAL are sensitive and specific biomarkers for discriminating the source of tobacco smoke exposure. Cotinine is the best overall discriminator when biomarkers are measured while a person has ongoing exposure to tobacco smoke. NNAL because of its long half-life would be particularly useful when there is a delay between exposure and biomarker measurement. The NNAL/cotinine ratio provides similar sensitivity but poorer specificity at discriminating passive versus active smokers when compared with NNAL alone.
Introduction
Biomarkers are useful in assessing both active and passive smokers’ exposure to tobacco smoke. Self-reported exposure such as cigarettes smoked per day by active smokers and hours per day exposed to secondhand smoke (SHS) in passive smokers is imprecise. Exposure patterns differ significantly from person to person due to differences in how cigarettes are smoked, differences in tobacco products, room ventilation, proximity of smokers to nonsmokers, and many other environmental factors (N. L. Benowitz, 1996).
Biomarkers are used to discriminate active from passive smokers in epidemiological studies, population surveillance of tobacco use, and research on the health risks of active and passive smoking. Determination of optimal biomarker cutoff points to distinguish active versus passive smokers for use in epidemiological studies is important to minimize misclassification (N. L. Benowitz, Bernert, Caraballo, Holiday, & Wang, 2009). Two biomarkers of tobacco smoke exposure are specific to tobacco and can be measured with adequate sensitivity to assess SHS exposure. One is cotinine and the other is 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL).
Measurement of cotinine, the major proximate metabolite of nicotine, in blood, saliva, or urine has supported epidemiological findings of a causal relationship between SHS and lung cancer, cardiovascular disease, and aggravation of chronic obstructive pulmonary disease (COPD) in adults and asthma in children (Boffetta et al., 2006; Strachan, Jarvis, & Feyerabend, 1990; Whincup et al., 2004). Moreover, cotinine is widely used to distinguish smokers from nonsmokers in epidemiology studies and smoking cessation trials. The optimal cutoff point for cotinine to discriminate active smokers from nonsmokers varies from study to study. There are substantial differences between proposed urine cotinine cutoff points in various studies ranging from 20 to 550 ng/ml (Zielinska-Danch, Wardas, Sobczak, & Szoltysek-Boldys, 2007). The most widely cited cotinine cutoff points of 14 ng/ml for serum and 50 ng/ml for urine were provided by Jarvis, Tunstall-Pedoe, Feyerabend, Vesey, and Saloojee (1987). Recently, N. L. Benowitz et al. (2009) proposed optimal serum cotinine cutoff points of 3.08 and 2.99 ng/ml for adults and adolescents, respectively, and estimated an optimal urine cotinine cutoff point of 15 ng/ml. Although plasma and saliva cotinine are considered to be the best biomarkers for both active and passive smoking, the concentration in individuals exposed to SHS is very low. Urine concentrations of cotinine are much higher than in blood or saliva, and in many studies, urine is easier to collect than plasma or saliva.
Tobacco-specific nitrosamines are formed from nicotine and other tobacco alkaloids during tobacco curing and burning. These compounds are potent animal and human carcinogens (Hecht et al., 1999). 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induces adenocarcinoma of the lung in rodents (Hecht, 1998). NNK is metabolized in human body to NNAL, and this metabolite is further conjugated with glucuronic acid. The level of NNAL in urine of smokers is associated with future risk of lung cancer. NNAL can be measured in urine, whole blood, plasma, and toenails (Bernert et al., 2005; Carmella, Han, Villalta, & Hecht, 2005; Hecht et al., 1999; 2002; Jacob et al., 2008). The half-life of NNAL (10–16 days) is much longer than that of cotinine (16 hr), suggesting that NNAL might be a better measure over tobacco exposure over time and that NNAL might be a better biomarker when sampling cannot be done in temporal proximity to tobacco smoke exposure (such as in hospitalized patients; Goniewicz et al., 2009). We are unaware of any published analysis of the optimal cutoff point to distinguish active versus passive smoking using urine NNAL.
As SHS ages, concentrations of nicotine decline faster than many other gaseous and particulate components of smoke due to absorption of nicotine on surfaces such as walls and carpets (Singer, Hodgson, Guevarra, Hawley, & Nazaroff, 2002). In addition, NNK levels increase as SHS ages, presumably related to the reaction of tobacco-specific alkaloid nicotine with nitric oxide in SHS (Schick & Glantz, 2007; Sleiman et al., 2010). Consistent with these changes in the composition of SHS over time, we recently reported that cotinine measurement leads to an underestimation of exposure to NNK from SHS compared with active smoking (N. Benowitz et al., 2010). Thus, we hypothesized that the ratio of NNAL/cotinine would be a better discriminator of active versus passive smoking compared with either NNAL or cotinine alone.
The aims of our study were to determine optimal cutoff points to discriminate active versus passive smokers and to compare sensitivity and specificity for the use of cotinine, NNAL, and the ratio of the NNAL/cotinine in urine.
Materials and Methods
Study Design and Subjects
Subjects from the United States, Poland, and Mexico included 376 smokers and 261 nonsmokers with evidence of exposure to SHS (passive smokers). Characteristics of study groups are presented in Table 1. Subjects were classified as active or passive smokers according to their self-declared smoking pattern or exposure to tobacco smoke. Each subject provided urine for measurement of the concentration of NNAL and cotinine. For the analysis of optimal cutoff points, subjects who did not have an NNAL level higher than the level of quantitation were excluded as we wanted people who definitely had some exposure to SHS. Subjects with a cotinine level below the limit of quantitation were excluded so that we could compute an NNAL/cotinine ratio. After these exclusions, data from 373 smokers and 228 passive smokers were used for cutoff point analysis.
Table 1.
Characteristics of Study Groups
| All subjects |
||||||
| Active smokers (n = 376) |
Passive smokers (n = 261) |
|||||
| Study | A | B | C | D | E | F |
| Demographic data | ||||||
| Sample size | 130 | 187 | 59 | 72 | 108 | 81 |
| Subjects with detectable cotinine levels (%) | 129 (99) | 187 (100) | 58 (98) | 54 (75) | 108 (100) | 80 (99) |
| Subjects with detectable NNAL levels (%) | 128 (98) | 187 (100) | 59 (100) | 48 (67) | 106 (98) | 77 (95) |
| Valid subjects | 128 | 187 | 58 | 45 | 106 | 77 |
| Sex (male), % | 74 (58) | 83 (44) | 26 (45) | 19 (42) | 50 (47) | 27 (35) |
| Race–ethnicity (White, non-Hispanic), % | 67 (52) | 187 (100) | 33 (57) | 42 (93) | 106 (100) | NA |
| Age (mean ± SD) | 38.2 ± 10.9 | 36.3 ± 13.8 | NA | 64.6 ± 5.8 | 34.6 ± 16.6 | 25.7 ± 7.4 |
| Nationality | USA | Poland | USA | USA | Poland | Mexico |
| Cigarettes/day (mean ± SD) | 18.4 ± 8.2 | 15.0 ± 8.4 | 6.9 ± 7.1 | – | – | – |
| Subjects included in final analysis |
||||||
| Analytic data | Active smokers (n = 373) |
Passive smokers (n = 228) |
||||
| Cotinine (ng/ml) | Geometric mean (95% CI): 1,043 (919–1,183) | Geometric mean (95% CI): 2.03 (1.59–2.61) | ||||
| IQI: 658–2,251 | IQI: 0.43–8.60 | |||||
| Range: 4.05–10,788 | Range: 0.06–470 | |||||
| NNAL (pg/ml) | Geometric mean (95% CI): 165 (146–187) | Geometric mean (95% CI): 5.80 (4.90–6.87) | ||||
| IQI: 90.8–360 | IQI: 2.28–15.4 | |||||
| Range: 3.36–2,319 | Range: 0.31–153 | |||||
| NNAL/cotinine ratio (×10−3) | Geometric mean (95% CI): 0.16 (0.15–0.17) | Geometric mean (95% CI): 2.85 (2.44–3.33) | ||||
| IQI: 0.09–0.26 | IQI: 1.32–6.14 | |||||
| Range: 0.01–18.2 | Range: 0.16–361 | |||||
Note. IQI = interquartile interval; NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol.
Adult daily smokers were recruited from three different studies (Studies A–C). Study A was a study of tobacco smoke biomarkers comparing Black and White smokers in San Francisco, USA (N = 128). Study B assessed the relationship between smoking topography and tobacco biomarkers among daily smokers recruited in Silesia region, Poland (N = 187, BK and AS). Study C compared urine biomarkers in daily (N = 36) compared with intermittent (nondaily; N = 22) smokers in Pittsburgh, USA (Saul Shiffman). Both daily and nondaily smokers were qualified as active smokers.
Nonsmokers exposed to SHS had participated in two previously published studies (Studies D and F) and one unpublished study (Study E) in which urine was collected for the assessment of SHS exposure. The first study (Study D) was a U.S. cohort study of nonsmoking adults with chronic obstructive lung disease (COPD), who collected samples in their homes and mailed samples to the investigators (N = 72, MDE; Eisner, Jacob, Benowitz, Balmes, & Blanc, 2009; Eisner et al., 2006). Among these subjects, none were current smokers, but almost half of them had history of smoking. Study E was a Polish cohort study of nonsmoking adults to assess SHS exposure in home and work environments. Subjects provided morning spot samples during screening medical tests (N = 108, WZ-D). Study F included volunteers from central Mexico who provided a urine sample before and after visiting a discotheque in which smoking was ongoing for at least 1 hr (N = 81, EL-P; Lazcano-Ponce et al., 2007).
Biomarkers Analysis
Cotinine was analyzed by liquid chromatography–atmospheric pressure chemical ionization tandem mass spectrometry (LC-MS/MS) as described by Bernert et al. (2005). Total NNAL (NNAL and NNAL-gluc) was analyzed by LC-MS/MS as described by Jacob et al. (2008). The limits of quantification were 0.05 ng/ml and 0.25 pg/ml for cotinine and NNAL, respectively.
Data Analysis
Nonparametric Kruskal–Wallis analysis of variance was used to compare biomarker levels and their ratio among study groups. Receiver operating characteristics (ROC) curves were generated for three subsets of data (two biomarkers separately and NNAL/cotinine ratio). For generating ROC curves and estimating optimal cutoff points, we assumed 21% population smoking prevalence and that both sensitivity and selectivity had the same importance. To compare diagnostic effectiveness of cotinine, NNAL, and their ratio, we compared their areas under the curve (AUCROC) using statistical test provided by software system in ROC module. All analyses were performed using STATISTICA data analysis software system v. 8 (StatSoft, Inc., Tulsa, OK).
Results
Characteristics of subjects and the geometric mean data on urine cotinine, NNAL, and NNAL/cotinine ratio are presented in Table 1. As noted previously, after excluding subjects whose cotinine or NNAL were below the limit of quantitation, we had a population of 373 active smokers and 228 passive smokers for ROC analysis.
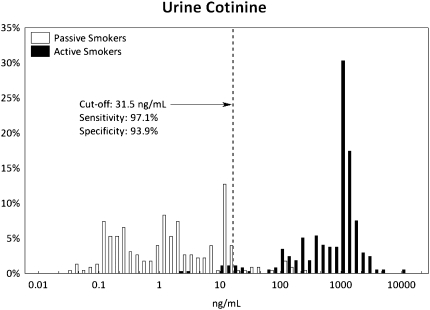
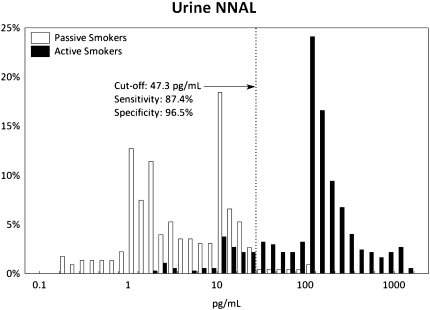
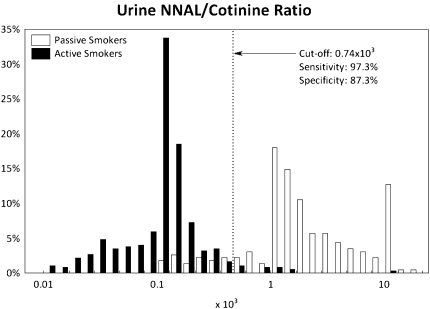
ROC curves are presented in Supplementary Figure 1, and their detailed characteristics are given in Supplementary Table 1. The ROC analyses showed that optimal cutoff points to discriminate passive versus active smokers were 31.5 ng/ml for cotinine (with a sensitivity of 97.1% and a specificity of 93.9%), 47.3 pg/ml for NNAL (87.4% and 96.5%), and 0.74 × 10−3 (NNAL [picograms per milliliter]/cotinine [nanograms per milliliter]) for NNAL/cotinine ratio (97.3% and 87.3%). The frequency distributions of cotinine, NNAL, and NNAL/cotinine ratio among active and passive smokers, with dotted lines showing the optimal ROC cutoff points, are presented in Figures 1–3, respectively.
Figure 1.
Distribution of urine cotinine concentrations with receiver operating characteristics of the optimal cutoff point among passive and active smokers. The dashed line is drawn at the optimal ROC cutoff point.
Figure 2.
Distribution of urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) concentrations with receiver operating characteristics (ROC) of the optimal cutoff point among passive and active smokers. The dashed line is drawn at the optimal ROC cutoff point.
Figure 3.
Distribution of the urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL)/cotinine ratio with receiver operating characteristics (ROC) of the optimal cutoff point among passive and active smokers. The dashed line is drawn at the optimal ROC cutoff point.
Results of AUCROC comparison are presented in Supplementary Table 2. For discriminating active versus passive smokers, there were significant differences between the AUCROC of cotinine and the AUCROC of both NNAL and NNAL/cotinine ratio (p ≤ .0001), indicating that cotinine had the superior overall discriminating performance. There was no significant difference between AUCROC of NNAL and NNAL/cotinine ratio (p = .26).
Discussion
Our study is novel in determining the optimal urine NNAL cutpoint to distinguish active versus passive smoking and comparing the performance of urine cotinine and NNAL as discriminators of active versus passive smoking. We defined passive smoke exposure as having NNAL in the urine at levels above the level of quantitation based on the fact that NNK, the precursor to NNAL, is entirely specific to tobacco.
The utility of urine cotinine to separate passive smokers from active smokers was studied by Zielinska-Danch et al. (2007), who estimated an optimal cutoff point of 550 ng/ml to distinguish the two groups in healthy Polish population. Other studies of Holl, Grabert, Heinze, and Debatin (1998), Hobbs, Wilmink, Adam, and Bradbury (2005), and Savitz, Dole, Terry, Zhou, and Thorp (2001) have used a similar cutoff point of 500 ng/ml to distinguish active versus combined group of light, nondaily, or passive smokers but did not perform an analysis to specifically determine an optimal cutoff point. The cutpoint of 500 or 550 ng/ml used in these five studies is much higher than the optimal cutoff point of 31.5 ng/ml that we have determined in the present paper.
A urine cotinine cutoff of 15 ng/ml was recently estimated by N. L. Benowitz et al. (2009) based on extrapolation from optimal serum levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnics groups in the United States between 1999 and 2004, using data from average ratios of cotinine in urine versus plasma. The differences between the cutoff points for urine cotinine observed in the present study and the N. L. Benowitz et al. (2009) study compared with those reported by WZ-D and other in the literature are likely due to different levels of secondhand exposure patterns among examined populations (higher passive exposure results in the higher value of cutoff point) and/or to misclassification of intermittent or light smokers as nonsmokers (such misclassification also leads to higher cutoff points). We believe that our estimate is generalizable to populations of SHS-exposed people broadly since it was estimated based on three subpopulations of active and passive smokers with various patterns of tobacco smoke exposure and smoking behaviors.
Our study presents the first formal analysis of the cutoff point for urine NNAL to distinguish passive versus active smokers. Our optimal cutoff point of 47.3 pg/ml allows discrimination of active versus passive smokers with very high specificity of 96.5% but only moderate sensitivity of 87.4%. To compare the results of our analysis to data from other published studies on active and passive smokers when urine NNAL data were provided, we summarized finding from other studies in Supplementary Table 3. All studies on passive smokers found urine NNAL levels to be lower than our proposed cutoff point value. We identified only one study of active smokers showing that some smokers in Shanghai have lower urine NNAL levels than our cutoff point of 47.3 pg/ml (Yuan et al., 2009). The other studies of active smokers showed NNAL much higher than our proposed cutoff point value. It should be noted that the proposed cutoff point for NNAL is based on discriminating known active versus known passive smokers, the latter defined by the presence of NNAL in the urine. If one uses urine NNAL to discriminate active smokers from all nonsmokers (both those exposed and not exposed to SHS, many of whom would have undetectable levels of NNAL in urine), the cutoff point would be lower. We recommend the following two-step approach to using urine NNAL to classify smoke exposure. First, the presence of NNAL at levels above the limit of quantitation indicates tobacco exposure—either active or passive smoke exposure (or smokeless tobacco exposure, which is not considered in the present analysis). Second, considering only individuals with detectable NNAL, the cutoff of 47.3 pg/ml is used to determine if a person is an active or a passive smoker.
Our analysis finds that overall urine cotinine performs better than NNAL or the ratio of NNAL/cotinine in discriminating active versus passive tobacco smoke exposure. Cotinine provides better sensitivity (97.1% vs. 87.4%) but slightly worse specificity (93.9% vs. 96.5%) than NNAL. This finding suggests that when cotinine is used in the epidemiological studies, fewer active smokers are misclassified as passive, whereas using NNAL leads to fewer passive smokers misclassified as active.
However, since NNAL has a long elimination half-life, urine NNAL represents a cumulative measure of exposure over time. The half-life of NNAL averages 10–16 days, so NNAL can be measured in the urine for a month or longer after the last exposure (Goniewicz et al., 2009). Cotinine has an average half-life of 16 hr, so cotinine is eliminated from the body within 3–4 days after the last exposure (N. L. Benowitz, 1996). Thus, for epidemiological studies of long-term intermittent exposure to SHS or in studies in which biomarker measurements cannot be made in temporal proximity to exposure, such as in hospitalized patients, the use of NNAL is expected to be of greater utility than cotinine.
Recently, we published a paper showing that cotinine measurements lead to an underestimation of exposure to the carcinogen NNK from SHS when compared with active smoking (N. Benowitz et al., 2010). We showed that passive smoking is associated with a much higher ratio of NNAL/cotinine in the urine compared with active smoking. Thus, in the present analysis, we hypothesized that the ratio of NNAL/cotinine would be a better discriminator than either biomarker alone. This was not the case. The ratio had comparable sensitivity but less specificity compared with cotinine. Overall, the ratio of NNAL/cotinine performed no better than NNAL itself.
In conclusion, we provide novel information on the use of urine biomarkers to discriminate active versus passive smokers. Overall, urine cotinine performs best with a combination of high sensitivity and specificity. However, because cotinine has a short half-life, it is useful only when biomarker samples are taken in close temporal proximity to tobacco smoke exposure. Urine NNAL has high specificity and moderate sensitivity but because of its long half-life has the advantage that it can be used when subjects have not been exposed to tobacco smoke for some period of time, such as in studying tobacco-related disease in hospitalized patients.
Funding
Research supported by the Flight Attendants Medical Research Institute, National Institutes of Health grants NCI CA78603, P30 DA12393, NIH R01 HL677438 (MDE), K24 HL 097245 (MDE), R25CA113710, and Medical University of Silesia grants KNW-2-185/09 (WZD and AS) and KNW-2-185/09 (BK).
Declaration of Interests
Dr. NLB is a consultant to several pharmaceutical companies that market medications to aid smoking cessation and has served as a paid expert witness in litigation against tobacco companies. The other authors have no conflicts to declare.
Supplementary Material
Supplementary Figure 1 and Tables 1–3 can be found online at http://www.ntr.oxfordjournals.org
Acknowledgments
We thank Dr. Saul Shiffman for providing urine samples and data on intermittent smokers, Dr. Faith Allen, Sandy Tinetti, and Katherine Dains for assistance in data management; Lisa Yu and Tricia Mao for assistance with laboratory analysis; and Marc Olmsted for editorial assistance.
References
- Benowitz N, Goniewicz ML, Eisner MD, Lazcano-Ponce E, Zielinska-Danch W, Koszowski B, et al. Urine cotinine underestimates exposure to the tobacco-derived lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in passive compared with active smokers. Cancer Epidemiology & Biomarkers of Prevention. 2010;19:2795–2800. doi: 10.1158/1055-9965.EPI-10-0497. doi:1055-9965.EPI-10-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiology Review. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. American Journal of Epidemiology. 2009;169:236–248. doi: 10.1093/aje/kwn301. doi:kwn301. [DOI] [PubMed] [Google Scholar]
- Bernert JT, Jain RB, Pirkle JL, Wang L, Miller BB, Sampson EJ. Urinary tobacco-specific nitrosamines and 4-aminobiphenyl hemoglobin adducts measured in smokers of either regular or light cigarettes. Nicotine & Tobacco Research. 2005;7:729–738. doi: 10.1080/14622200500259762. doi:X08147535X68KH31. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Clark S, Shen M, Gislefoss R, Peto R, Andersen A. Serum cotinine level as predictor of lung cancer risk. Cancer Epidemiology Biomarkers Prevention. 2006;15:1184–1188. doi: 10.1158/1055-9965.EPI-06-0032. doi:15/6/1184. [DOI] [PubMed] [Google Scholar]
- Carmella SG, Han S, Villalta PW, Hecht SS. Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in smokers’ blood. Cancer Epidemiology & Biomarkers Prevention. 2005;14:2669–2672. doi: 10.1158/1055-9965.EPI-05-0129. doi:14/11/2669. [DOI] [PubMed] [Google Scholar]
- Eisner MD, Balmes J, Yelin EH, Katz PP, Hammond SK, Benowitz N, et al. Directly measured secondhand smoke exposure and COPD health outcomes. BioMed Central Pulmonary Medicine. 2006;6:12. doi: 10.1186/1471-2466-6-12. doi:1471-2466-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner MD, Jacob P 3rd, Benowitz NL, Balmes J, Blanc PD. Longer term exposure to secondhand smoke and health outcomes in COPD: Impact of urine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Nicotine & Tobacco Research. 2009;11:945–953. doi: 10.1093/ntr/ntp091. doi:ntp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goniewicz ML, Havel CM, Peng MW, Jacob P, 3rd, Dempsey D, Yu L, et al. Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiology & Biomarkers of Prevention. 2009;18:3421–3425. doi: 10.1158/1055-9965.EPI-09-0874. doi:18/12/3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chemical Research in Toxicology. 1998;11:559–603. doi: 10.1021/tx980005y. doi:10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Chen M, Dor Koch JF, Miller AT, Murphy SE, et al. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Research. 1999;59:590–596. [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Ye M, Le KA, Jensen JA, Zimmerman CL. et al. Quantitation of metabolites of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone after cessation of smokeless tobacco use. Cancer Research. 2002;62:129–134. [PubMed] [Google Scholar]
- Hobbs SD, Wilmink AB, Adam DJ, Bradbury AW. Assessment of smoking status in patients with peripheral arterial disease. Journal of Vascular Surgery. 2005;41:451–456. doi: 10.1016/j.jvs.2004.12.039. doi:S0741521404017136. [DOI] [PubMed] [Google Scholar]
- Holl RW, Grabert M, Heinze E, Debatin KM. Objective assessment of smoking habits by urinary cotinine measurement in adolescents and young adults with type 1 diabetes. Reliability of reported cigarette consumption and relationship to urinary albumin excretion. Diabetes Care. 1998;21:787–791. doi: 10.2337/diacare.21.5.787. [DOI] [PubMed] [Google Scholar]
- Jacob P, 3rd, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Analytical Chemistry. 2008;80:8115–8121. doi: 10.1021/ac8009005. doi:10.1021/ac8009005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Comparison of tests used to distinguish smokers from nonsmokers. American Journal of Public Health. 1987;77:1435–1438. doi: 10.2105/ajph.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazcano-Ponce E, Benowitz N, Sanchez-Zamorano LM, Barbosa-Sanchez L, Valdes-Salgado R, Jacob P., 3rd et al. Secondhand smoke exposure in Mexican discotheques. Nicotine & Tobacco Research. 2007;9:1021–1026. doi: 10.1080/14622200701495967. doi:781560731. [DOI] [PubMed] [Google Scholar]
- Savitz DA, Dole N, Terry JW, Jr, Zhou H, Thorp JM., Jr. Smoking and pregnancy outcome among African-American and white women in central North Carolina. Epidemiology. 2001;12:636–642. doi: 10.1097/00001648-200111000-00010. [DOI] [PubMed] [Google Scholar]
- Schick SF, Glantz S. Concentrations of the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in sidestream cigarette smoke increase after release into indoor air: Results from unpublished tobacco industry research. Cancer Epidemiology & Biomarkers of Prevention. 2007;16:1547–1553. doi: 10.1158/1055-9965.EPI-07-0210. doi:16/8/1547. [DOI] [PubMed] [Google Scholar]
- Singer BC, Hodgson AT, Guevarra KS, Hawley EL, Nazaroff WW. Gas-phase organics in environmental tobacco smoke. 1. Effects of smoking rate, ventilation, and furnishing level on emission factors. Environmental Science Technology. 2002;36:846–853. doi: 10.1021/es011058w. [DOI] [PubMed] [Google Scholar]
- Sleiman M, Gundel LA, Pankow JF, Jacob P, 3rd, Singer BC, Destaillats H. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proceedings of National Academy Science U S A. 2010;107:6576–6581. doi: 10.1073/pnas.0912820107. doi:0912820107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan DP, Jarvis MJ, Feyerabend C. The relationship of salivary cotinine to respiratory symptoms, spirometry, and exercise-induced bronchospasm in seven-year-old children. American Review of Respiratory Disease. 1990;142:147–151. doi: 10.1164/ajrccm/142.1.147. [DOI] [PubMed] [Google Scholar]
- Whincup PH, Gilg JA, Emberson JR, Jarvis MJ, Feyerabend C, Bryant A, et al. Passive smoking and risk of coronary heart disease and stroke: Prospective study with cotinine measurement. British Medical Journal. 2004;329:200–205. doi: 10.1136/bmj.38146.427188.55. doi:10.1136/bmj.38146.427188.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JM, Koh WP, Murphy SE, Fan Y, Wang R, Carmella SG, et al. Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Research. 2009;69:2990–2995. doi: 10.1158/0008-5472.CAN-08-4330. doi:0008-5472.CAN-08-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinska-Danch W, Wardas W, Sobczak A, Szoltysek-Boldys I. Estimation of urinary cotinine cut-off points distinguishing non-smokers, passive and active smokers. Biomarkers. 2007;12:484–496. doi: 10.1080/13547500701421341. doi:778720746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.