Abstract
The preparation of electrospun polymer microfibers with nitric oxide (NO)-release capabilities is described. Polymer solutions containing disodium 1-[2-(carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate (PROLI/NO), a low molecular weight NO donor, were electrospun to generate fibers ranging from 100–3000 nm in diameter capable of releasing NO upon immersion in aqueous solutions under physiological conditions (pH 7.4, 37 °C), with kinetics depending on polymer composition and fiber diameter. The NO release half-life for PROLI/NO-doped electrospun fibers was 2–200 times longer than that of PROLI/NO alone. The influence of polymer concentration, applied voltage, capillary diameter, solution conductivity, flow rate, and additives on fiber properties are reported and discussed with respect to potential applications.
Keywords: nitric oxide, controlled release, electrospinning, microfibers, PROLI/NO, biomaterials
Introduction
Nitric oxide (NO) is an endogenously produced free radical essential to numerous physiological functions including wound healing,1 vasodilation,2 and angiogenesis.3 As such, the therapeutic potential of administering exogenous NO as a treatment for certain disease states is a popular area of research.4–6 Harnessing the therapeutic potential of this free radical however has proven challenging due to concentration dependent effects and NO’s high reactivity.4, 5 Although low molecular weight or small molecule nitric oxide donors such as N-diazeniumdiolates and S-nitrosothiols have been shown to be efficient scaffolds for storing and delivering NO to physiological loci, well-tuned control of long-term NO release has remained elusive.4, 7
The incorporation of NO donor functionalities into macromolecular scaffolds by physical immobilization is a promising method to prolong durations of NO release based on diffusion-mediated control of NO release from the material. However, more precise control over temporal NO release often require chemical modifications.8–10 The development of therapeutic materials with well-defined ranges of NO release often necessitates the use of extensive synthetic processes and the preparation of numerous chemical compounds.11, 12 It is thus desirable to prepare materials with well-defined structural features that are able to control both the rates of water uptake and NO diffusion out of the material.
Electrospinning is a popular method for the preparation of well-defined micro- and nanomaterials.13, 14 In this process, an electric field is applied to a liquid droplet at the tip of a capillary. As the surface tension of the liquid is overcome by electrostatic repulsion due to charge accumulation, the deformed liquid droplet erupts at a critical point to form a viscoelastic jet, that accelerates toward a grounded target with its path determined by both the evaporation of solvent and electrostatic repulsion within the fiber.13, 14 A range of material morphologies (e.g., fibers, spheres, and rings) and dimensions may be achieved by fine-tuning a number of parameters, including solution concentration, conductivity, flow rate, viscosity, applied voltage, and target distance.13–15 Additionally, nonwoven mats, aligned fibers and twisted yarns may all be fabricated by altering the collection method of the fibers.13
High surface areas, facile functionalization, and tunable mechanical characteristics make electrospun materials attractive for several applications including those for medical purposes.16 Electropsun fibers have been investigated as template for tissue engineering,16–19 drug delivery,16, 20 wound dressings,16, 21, 22 and enzyme immobilization.16 By combining the already attractive characteristics of these materials with NO release, the ability to fabricate a library of therapeutic materials may emerge. Indeed, Liu and Balkus fabricated poly(lactic acid) fibers containing zeolites with tunable NO release based on the heat treatment of fibers.22 Furthermore, well-tuned, diffusion-mediated NO release may be achieved by simply controlling fiber size and polymer composition, without chemical modification to the incorporated NO donor.
Herein, we report the preparation of NO-releasing microfibers prepared by electrospinning polymer solutions of Tecoflex polyurethane, Tecophilic polyurethane, and poly(vinyl chloride) containing disodium 1-[2-(carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate (PROLI/NO), a well characterized small molecule NO donor with fast NO release kinetics.23 Fiber diameter and NO release are examined as a function of polymer type and solution concentration, and dopant amount. Potential medical applications for the fabricated materials are discussed.
Experimental Section
Materials
High molecular weight poly(vinyl chloride) was obtained from Fluka (Buchs, Switzerland). Poly(methyl methacrylate) (typical MW 120,000), proline, and sodium methoxide were purchased from Aldrich Chemical Co. (Milwaukee, WI). Tecoflex SG-85A polyurethane and Tecophilic HP-93A-100 polyurethane were gifts from Thermedics (Woburn, MA). All laboratory grade salts and solvents were purchased from Fisher Scientific. Water was purified using a Millipore Milli-Q Gradient A-10 purification system (Bedford, MA). Nitrogen, argon, and nitric oxide gases were purchased from National Welders Supply (Durham, NC).
Characterization
Electrospun fibers were sputter-coated with 2.5 nm Au/Pd and imaged using a Hitachi S-4700 Scanning Electron Microscope. Fiber diameters were averaged from at least 75 measurements. Solution conductivities were measured using a Malvern Nano Series Zetasizer operated in zeta potential mode using an average of 5 measurements.
Synthesis of PROLI/NO
PROLI/NO was prepared following procedures described previously in the literature.23 Briefly, 2.05 g of proline was dissolved in a solution consisting of 25 mL of methanol and 2.00 g sodium methoxide. The solution was then placed in a stainless steel reaction vessel where it was flushed with Ar a total of eight times over 45 min and charged with NO to a pressure of 5 atm for 3 d with constant stirring. A series of three additional Ar purges were performed after 3 d, before the solution was precipitated by the addition of 150 mL of diethyl ether and stored at −20 °C for 4 h to aid in precipitation. The precipitate was isolated by vacuum filtration and dried in vacuo to yield PROLI/NO as a white solid. The isolated PROLI/NO was stored at −20 °C.
Fiber Formation
Electrospun fibers were fabricated using a custom electrospinning apparatus consisting of a Series 205B High Voltage Power Supply from Bertan Associates, Inc. and a Kent Scientific Genie Plus syringe pump. Voltage was applied to standard stainless steel blunt-tip needles (Jensen Global, Santa Barbara, CA) attached to solution-filled syringes positioned atop the syringe pump. A grounded circular steel disk covered in aluminum foil was mounted perpendicular to the direction of the syringe at a distance of 15 cm. Polymer samples were dissolved in 2 mL of a 3:1:1 mixture of tetrahydrofuran N, N′ dimethylformamide: methanol. For samples containing dopant, the polymer was first dissolved in 1.6 mL of a 3:1 mixture of THF:DMF, followed by the addition of dopant dissolved in 400 μL of MeOH. Fibers were electrospun at applied voltages ranging from 10 – 20 kV, flow rates of 15 – 100 μL min−1, and spinneret diameters of 0.152 – 0.965 mm ID (30 gauge – 18 gauge blunt tip needles) with variable polymer and dopant concentrations.
Nitric Oxide Release Analysis
Nitric oxide release from the electrospun materials was investigated using a chemiluminescence Sievers Nitric Oxide Analyzer Model 280i. Electrospun samples were removed from their aluminum foil substrate and placed in a solution of deoxygenated phosphate buffered saline (PBS, pH 7.4) held at 37 °C. The reaction flask was connected to the analyzer and sparged with N2 gas at 70 mL min−1 with additional N2 flow supplied via a vessel sidearm to match the instrument collection rate of 200 mL min−1. Nitric oxide release from the samples was measured in real time at 1 s intervals. A calibration line was constructed using 26.39 ppm NO gas (balance N2) and air passed through a Sievers NO zero filter.
Results and Discussion
Fiber Formation
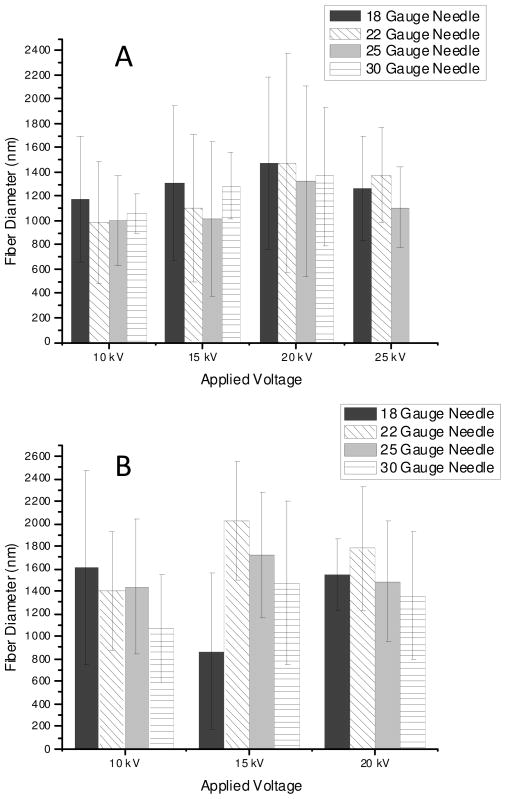
Electrospinning is an extremely complex technique that is highly dependent on several parameters including conductivity, concentration, solution composition, and additives.24, 25 As a result, optimization of methods to fabricate fibers of well-defined diameters was required before investigating the effects of NO donor incorporation on fiber formation and NO release characteristics. Although an essential component of the electrospinning process, the significance in the variation of applied voltage on fiber diameter and morphology has been debated.24, 25 Increasing the applied voltage has been shown to decrease the diameter of the charged liquid jet up to a threshold value beyond which diameters increase as the increasing electric field draws more material out of the syringe.24 Other studies have shown minimal impact of applied voltage variation on fiber diameters.25 The influence of applied voltage on Tecoflex fibers spun from solution was thus investigated by varying the magnitude of applied voltage from 10–25 kV. As shown in Figure 1, the dependence of applied voltage on the diameter of fibers electrospun from 12 and 16% Tecoflex solutions was minimal. Although local maxima of fiber diameters was noted with increasing voltage for each polymer concentration, high fiber diameter polydispersities at each voltage resulted in insignificant statistical differences.
Figure 1.
Fiber diameter as a function of applied voltage and needle gauge for A) 12 and B) 16 wt% Tecoflex.
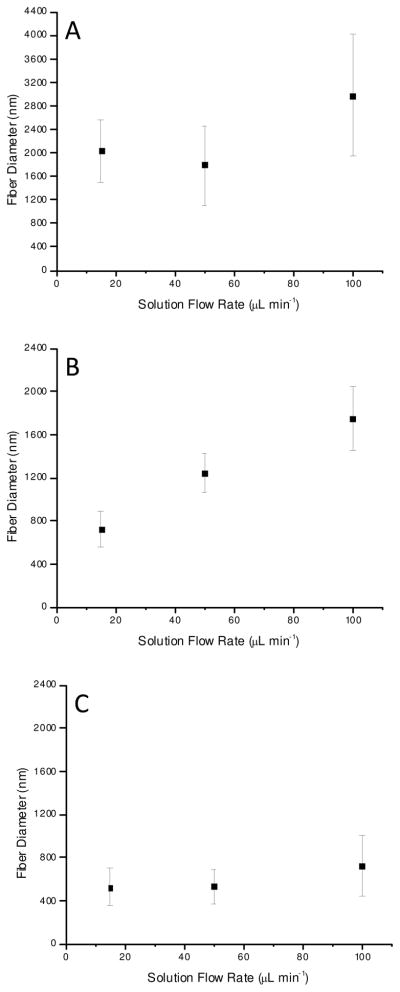
During the electrospinning process, the flight of the charged polymer jet is primarily dictated by the electrostatic charge buildup required to overcome the surface tension of the spinning solution. However, altering the amount of solution exposed to the applied electric field may influence fiber formation.24, 25 The effect of solution flow rate through the electrified capillary on fiber formation was thus also investigated (Supporting Information). Similar to previously published reports,24, 25 variation of the flow rate from 15–100 μL min−1 did not significantly influence fiber diameters at low polymer solution concentrations (8 wt%). In contrast, significant changes in average fiber diameters were observed for the higher polymer solution concentrations (12 and 16 wt%). The average fiber diameters from 16 wt% solutions of Tecoflex, Tecophilic, and PVC increased by 66, 40, and 37%, respectively, when solution flow rates were increased from 50 to 100 μL min−1 (Figure 2). Substantial increases in fiber diameters were also observed for 12 wt% solutions of Tecophilic and PVC upon doubling the solution flow rate from 50 to 100 μL min−1 (32 and 97%, respectively). Despite these changes, increasing the flow rate of lower concentration polymer solutions did not result in any significant increase in resultant fiber diameter. This behavior is likely only seen at higher polymer concentrations due to contributions from the higher solution viscosity and increased polymer chain entanglement as polymer wt% increases.
Figure 2.
Flow rate dependence on electrospun fiber diameter for 16 wt% A) Tecoflex solutions, B) Tecophilic solutions, and C) PVC solutions.
Important morphological changes were also observed as solution flow rates were altered for 8 wt% Tecoflex solutions. As shown in Figure 3, 8 wt% solutions electrospun at 15 μL min−1 resulted in the formation of beaded fibers, indicating a threshold concentration for electrospinning. Under these conditions, electrospray and electrospinning contributions may result from insufficient solution cohesion and the expulsion of charged droplets from the Taylor cone.15, 26 Upon increasing the solution flow rate to 50 and 100 μL min−1, the electrospun materials were free of beading indicating that electrospray contributions were overcome by the increased solution volume in the electric field. By forcing additional polymer through the electric field, charge accumulation is mediated via additional volume over which the accumulated charge may be spread. In turn, charge buildup necessary for the expulsion of charged droplets is avoided.24
Figure 3.
SEM images of Tecoflex polyurethane fibers electrospun from 8 wt% solutions with flow rates of A) 15, B) 50, and C) 100 μL min−1.
The Taylor cone is a deformed liquid droplet created at the tip of a capillary in the presence of an applied voltage.27, 28 It is from this feature that charged jets and droplets are expelled during electrospinning and electrospraying, respectively.14 As such, alterations in the structure of the Taylor cone may influence the electrospinning process and resulting fiber size and/or morphology. As the size of the Taylor cone is a function of the capillary diameter, the effect of capillary size on electrospinning was investigated for the three polymers. Similar to flow rate, changing the capillary diameter did not influence fiber size independently of other variables (Supporting Information). No trend in fiber size was observed by varying capillary diameter during electrospinning experiments for Tecoflex solutions. Furthermore, needle gauge variation did not inhibit beaded fiber formation for 8 wt% Tecoflex solutions. Varying the capillary diameter of Tecophilic solutions resulted in the greatest spread in fiber diameter with the largest diameter needle (18 gauge) producing the largest diameter fibers for 8, 12, and 16 wt% polymer solutions compared to fibers spun from 22 and 30 gauge needles. An increase in polymer concentration was coupled with an increase in average fiber diameters when switching from 30 to 18 gauge needles as fiber diameters from 8, 12, and 16 wt% Tecophilic increased by 13, 50, and 83%, respectively. Although diameters of fibers electrospun from 8, 12, and 16 wt% PVC solutions did not change appreciably with increasing capillary diameter, 16 wt% PVC did not result in fiber formation using a 30 gauge needle due to substantial clogging.
The alteration of electrospinning parameters to influence fiber diameter is a difficult task because of the multiple variables that are important to the development of well-defined micro- and nano-fibers. Indeed, the fine interplay between specific parameters minimizes the influence that one variable will have on ensuing fiber diameter and morphology. As such, it is expected that the variation of one electrospinning parameter may exhibit inconsistent effects when using different polymer compositions and/or solution concentrations. Although a small degree of product control is achievable by varying applied voltage, capillary diameter, and flow rate, the most easily controlled and influential electrospinning parameter for tuning fiber size and morphology remains solution concentration (Figure 4). In general, our results indicate that increasing polymer concentrations in solution will produce fibers with larger average diameters than those spun from lower concentration solutions.
Figure 4.
SEM images of Tecoflex fibers electrospun from A) 8, B) 12, and C) 16 wt% solutions at 15 kV using 15 μL min−1 flow rate and a 22 gauge needle.
Dopant Effects on Fiber Formation
The incorporation of additives into electrospun scaffolds is a popular method for imparting specific functions to these materials.16, 29, 30 However, the addition of even small amounts of dopant may influence the formation of electrospun materials due to alterations in solution behavior (e.g., viscosity, surface tension, etc.), which has been shown to dictate electrospinning capabilities.29, 30 Therefore, understanding the influence of specific dopants on electrospinning behavior is an essential parameter to investigate. Physical incorporation of PROLI/NO and proline (control) in Tecoflex resulted in smaller fiber diameters than undoped fibers, with the highest additive content generally resulting in the smallest fibers (Table 1). Significant additive concentrations paired with elevated polymer concentrations, however, resulted in increased fiber diameters (687 ± 173 nm for 12% Tecoflex, 2.4% prolino, 50 μL min−1) and in some cases capillary clogging preventing electrospinning altogether (16% Tecoflex, 3.2% proline or PROLI/NO). Unlike undoped fibers, the inclusion of proline and PROLI/NO in 8 wt% Tecoflex solutions electrospun at 15 μL min−1 led to the formation of non-beaded fibers confirming that the presence of additives also influences fiber morphology (Figure 5). Nevertheless, beading reappeared as the proline content in solution was increased to 1.6 wt%, suggesting high concentrations of dopant influenced the cohesiveness of the liquid jet. Fibers containing PROLI/NO exhibited slightly smaller fiber diameters than fibers containing the same concentration of proline, confirming that an additive’s influence on fiber diameter is contingent upon both structure and concentration. Solution conductivity measurements indicated that this decrease in fiber diameter upon PROLI/NO inclusion was an artifact of the solutions containing the ionic diazeniumdiolates versus uncharged proline (Supporting Information). Others have reported extensively on the influence of solution conductivitiy on electrospinning.13, 24, 25, 29 Of note, greater polymer concentrations did not show the same trends with PROLI/NO inclusion resulting in slightly larger diameters than their proline-containing counterparts. The degree of fiber branching also increased upon addition of higher concentrations of additives.
Table 1.
Influence of dopant type and concentrations on fiber diameter
| Polymer | Weight Percent (%) | Rate (μL min−1) | Dopant | Diameter (nm) |
|---|---|---|---|---|
| Tecoflex | 8 | 15 | - | b |
| 8 | 15 | 0.4% proline | 548 ± 237 | |
| 8 | 15 | 0.8% proline | 399 ± 125 | |
| 8 | 15 | 1.6% proline | b | |
| 8 | 15 | 0.4% PROLI/NO | 308 ± 85 | |
| 8 | 15 | 0.8% PROLI/NO | 313 ± 103 | |
| 8 | 15 | 1.6% PROLI/NO | 353 ± 140 | |
| 12 | 50 | - | 947 ± 283 | |
| 12 | 50 | 0.6% proline | 769 ± 235 | |
| 12 | 50 | 1.2% proline | 742 ± 246 | |
| 12 | 50 | 2.4% proline | 687 ± 173 | |
| 12 | 50 | 0.6% PROLI/NO | 549 ± 124 | |
| 12 | 50 | 1.2% PROLI/NO | 453 ± 170 | |
| 12 | 50 | 2.4% PROLI/NO | 790 ± 296 | |
| 16 | 15 | - | 2025 ± 527 | |
| 16 | 15 | 0.8% proline | 818 ± 197 | |
| 16 | 15 | 1.6% proline | 924 ± 241 | |
| 16 | 15 | 3.2% proline | c | |
| 16 | 15 | 0.8% PROLI/NO | 1047 ± 188 | |
| 16 | 15 | 1.6% PROLI/NO | 938 ± 232 | |
| 16 | 15 | 3.2% PROLI/NO | c | |
| Tecophilic | 8 | 15 | - | 334 ± 77 |
| 8 | 15 | 0.4% proline | 416 ± 135 | |
| 8 | 15 | 0.8% proline | 503 ± 180 | |
| 8 | 15 | 1.6% proline | 558 ± 132 | |
| 8 | 15 | 0.4% PROLI/NO | 330 ± 103 | |
| 8 | 15 | 0.8% PROLI/NO | 373 ± 104 | |
| 8 | 15 | 1.6% PROLI/NO | 308 ± 81 | |
| 12 | 15 | - | 621 ± 185 | |
| 12 | 15 | 0.6% proline | 804 ± 223 | |
| 12 | 15 | 1.2% proline | 743 ± 246 | |
| 12 | 15 | 2.4% proline | 795 ± 220 | |
| 12 | 15 | 0.6% PROLI/NO | 972 ± 200 | |
| 12 | 15 | 1.2% PROLI/NO | 870 ± 214 | |
| 12 | 15 | 2.4% PROLI/NO | 754 ± 197 | |
| 16 | 15 | - | 719 ± 168 | |
| 16 | 15 | 0.8% proline | 1408 ± 243 | |
| 16 | 15 | 1.6% proline | c | |
| 16 | 15 | 3.2% proline | c | |
| 16 | 15 | 0.8% PROLI/NO | 1857 ± 524 | |
| 16 | 15 | 1.6% PROLI/NO | c | |
| 16 | 15 | 3.2% PROLI/NO | c | |
| Poly(vinyl chloride) | 8 | 15 | - | 125 ± 47 |
| 8 | 15 | 0.4% proline | 195 ± 69 | |
| 8 | 15 | 0.8% proline | 226 ± 75 | |
| 8 | 15 | 1.6% proline | 192 ± 84 | |
| 8 | 15 | 0.4% PROLI/NO | 128 ± 67 | |
| 8 | 15 | 0.8% PROLI/NO | 135 ± 51 | |
| 8 | 15 | 1.6% PROLI/NO | 144 ± 64 | |
| 12 | 15 | - | 418 ± 210 | |
| 12 | 15 | 0.6% proline | 432 ± 202 | |
| 12 | 15 | 1.2% proline | 500 ± 256 | |
| 12 | 15 | 2.4% proline | 576 ± 214 | |
| 12 | 15 | 0.6% PROLI/NO | 302 ± 134 | |
| 12 | 15 | 1.2% PROLI/NO | 254 ± 136 | |
| 12 | 15 | 2.4% PROLI/NO | 232 ± 115 | |
| 16 | 15 | - | 524 ± 174 | |
| 16 | 15 | 0.8% proline | 226 ± 98 | |
| 16 | 15 | 1.6% proline | c | |
| 16 | 15 | 3.2% proline | c | |
| 16 | 15 | 0.8% PROLI/NO | 565 ± 242 | |
| 16 | 15 | 1.6% PROLI/NO | c | |
| 16 | 15 | 3.2% PROLI/NO | c | |
-electrospinning resulted in the formation of beaded fibers.
resulted in capillary clogging, no fiber formation.
Figure 5.
SEM images of Tecoflex fibers electrospun at 15 kV using a 15 μL min−1 flow rate, 22 gauge needle, and 8 wt% polymer solution with A) no additives B) 0.4 wt% proline, and C) 0.4 wt% PROLI/NO.
Although Tecoflex and Tecophilic are synthesized from similar components, the influence of additives on fibers electrospun from these polyurethanes was vastly different. In general, the size of doped Tecophilic fibers was less than that observed using Tecoflex (Table 1). Proline-containing Tecoflex fibers electrospun from low polymer concentration solutions (8 wt%) exhibited higher average fiber diameters than their undoped counterparts. The addition of PROLI/NO to low concentration Tecophilic solutions resulted in fiber diameters similar to those of undoped Tecophilic fibers electrospun from the same concentration solution. As the Tecophilic concentration was increased, the incorporation of either proline or PROLI/NO resulted in increased fiber diameters relative to undoped fibers, in contrast to the behavior of Tecoflex. Despite higher conductivities for solutions containing PROLI/NO, the increased size of fibers containing additives was actually smaller in magnitude for fibers containing proline versus PROLI/NO. This trend remained consistent at both 12 and 16 wt% polymer. Furthermore, the incidence of fiber branching, which was prevalent for doped Tecoflex fibers, was greatly diminished for doped Tecophilic polyurethane fibers.
Doping of additives also altered diameters of polymeric microfibers composed of PVC. Low concentration PVC solutions containing proline formed slightly larger fibers compared to undoped counterparts (125 ± 47 nm vs. 195 ± 69 for undoped and 0.4 wt% proline solutions of 8% PVC). However, PROLI/NO inclusion did not appreciably change the size of the fibers (Table 1). As polymer concentration was increased to 12 wt%, the fiber diameter increased slightly for compositions containing proline, while significant decreases were observed for PROLI/NO-doped materials. High concentrations (16 wt%) of PVC resulted in substantial capillary clogging with dopant concentrations ≥ 0.8 wt%. Of note, 16 wt% PVC solutions containing 0.8 wt% proline resulted in smaller diameter fibers compared to those prepared with PROLI/NO. Contrary to what was observed for the Tecoflex and Tecophilic polyurethanes, the incorporation of PROLI/NO did not alter fiber size relative to undoped materials.
Nitric Oxide Release
The preparation of scaffolds capable of prolonging NO release from low molecular weight NO donors is an important aspect for the development of NO-based therapeutics. Additionally, the ability to control NO release is also essential. Several strategies may be employed to generate well-defined structural features that control water uptake by a material, allowing for exploitation of the proton-induced dissociation mechanism of diazeniumdiolates. The ability to easily control fiber diameter makes electrospinning a useful technique for preparing materials with well-defined structural features for controlling NO release.
We thus investigated the NO release properties of electrospun polymers containing PROLI/NO as a function of polymer composition and fiber size. PROLI/NO-doped Tecophilic fibers (the most hydrophilic polymer investigated) were characterized with NO release half-lives approximately double that of PROLI/NO alone (Table 2). Surprisingly, the NO-release kinetics were not altered as a function of fiber diameter with NO release half-life ranging from 75–85 s regardless of fiber size. However, total NO release was tunable based on the incorporation of different wt% NO donor in the electrospinning solution. The inability to regulate the NO release kinetics with Tecophilic fibers is attributed to the high rates of water uptake associated with the hydrophilic fibers.
Table 2.
Nitric oxide-release characteristics of PROLI/NO-doped electrospun polymer microfibers
| Polymer | Weight Percent | Dopant (NO Donor) (wt% in solution) | Fiber Diameter (nm) | t[NO] (μmol mg−1) | [NO]m (ppb mg−1) | t1/2 (s) |
|---|---|---|---|---|---|---|
| Tecophilic Polyurethane | 8 | 0.4 | 330 ± 103 | 0.16 ± 0.03 | 11800 ± 3800 | 81 ± 8 |
| 8 | 0.8 | 373 ± 104 | 0.42 ± 0.05 | 29100 ± 3600 | 85 ± 9 | |
| 8 | 1.6 | 308 ± 81 | 0.85 ± 0.06 | 67000 ± 10500 | 75 ± 5 | |
| 12 | 0.6 | 972 ± 200 | 0.23 ± 0.03 | 19000 ± 5600 | 77 ± 12 | |
| 12 | 1.2 | 870 ± 214 | 0.44 ± 0.04 | 31500 ± 4000 | 85 ± 15 | |
| 12 | 2.4 | 754 ± 197 | 0.99 ± 0.14 | 83700 ± 5300 | 75 ± 13 | |
| 16 | 0.8 | c | - | - | - | |
| 16 | 1.6 | c | - | - | - | |
| 16 | 3.2 | c | - | - | - | |
| Tecoflex Polyurethane | 8 | 0.4 | 308 ± 85 | 0.20 ± 0.01 | 7600 ± 4100 | 173 ± 79 |
| 8 | 0.8 | 313 ± 103 | 0.42 ± 0.06 | 11000 ± 3000 | 168 ± 52 | |
| 8 | 1.6 | 353 ± 140 | 0.79 ± 0.15 | 44100 ± 800 | 105 ± 25 | |
| 12 | 0.6 | 549 ± 124 | 0.18 ± 0.06 | 4000 ± 2600 | 229 ± 77 | |
| 12 | 1.2 | 453 ± 170 | 0.50 ± 0.02 | 11700 ± 3800 | 260 ± 126 | |
| 12 | 2.4 | 790 ± 296 | 0.95 ± 0.03 | 39400 ± 4900 | 143 ± 27 | |
| 16 | 0.8 | 1047 ± 188 | 0.24 ± 0.06 | 7100 ± 3100 | 275 ± 152 | |
| 16 | 1.6 | 938 ± 232 | 0.49 ± 0.04 | 6800 ± 2800 | 734 ± 329 | |
| 16 | 3.2 | c | - | - | - | |
| Poly(vinyl chloride) | 8 | 0.4 | 128 ± 67 | 0.09 ± 0.01 | 1500 ± 200 | 1288 ± 110 |
| 8 | 0.8 | 135 ± 51 | 0.35 ± 0.13 | 3800 ± 2500 | 2600 ± 3700 | |
| 8 | 1.6 | 144 ± 64 | 0.78 ± 0.07 | 25500 ± 10600 | 209 ± 73 | |
| 12 | 0.6 | 302 ± 134 | 0.15 ± 0.05 | 900 ± 300 | 5241 ± 3271 | |
| 12 | 1.2 | 254 ± 136 | 0.38 ± 0.10 | 2200 ± 900 | 5055 ± 1078 | |
| 12 | 2.4 | 232 ± 115 | 0.77 ± 0.11 | 28500 ± 3500 | 198 ± 110 | |
| 16 | 0.8 | 565 ± 242 | 0.20 ± 0.02 | 1200 ± 500 | 5613 ± 4568 | |
| 16 | 1.6 | c | - | - | - | |
| 16 | 3.2 | c | - | - | - | |
resulted in capillary clogging, no fiber formation.
Although fibers composed of Tecoflex polyurethane exhibited similar fiber diameter ranges as the Tecophilic materials, more tunable NO release was expected based on the differences in hydrophilicty. For instance, the reduced water uptake for Tecoflex fibers should prolong NO release half-lives compared to Tecophilic fibers. The NO-release half-lives of PROLI/NO-doped Tecoflex fibers were more than twice as long as fibers composed of the more hydrophilic Tecophilic (Table 2). Greater NO release durations were also observed with increasing NO donor concentrations. Tecoflex fibers containing 10 wt% PROLI/NO showed similar half-lives to those containing 5 wt% PROLI/NO. However, fibers generated from 12 and 16 wt% polymer solutions showed increased NO-release half-lives (e.g., 30 to 500 s) upon additional PROLI/NO incorporation, attributed to larger fibers. As PROLI/NO concentrations were increased further to 20 wt%, the NO-release half-lives decreased from that observed at lower NO donor concentrations. Such behavior may be attributed to concomitant decreases in the relative polymer wt%, thus reducing the water uptake-mediated effects on diazeniumdiolate decomposition.
As PVC was the most hydrophobic polymer investigated, we expected that PROLI/NO-doped PVC fibers would possess the longest NO-release half-lives. Indeed, electrospun PVC fibers containing 5 wt% PROLI/NO exhibited half-lives that were significantly longer than any of the polyurethane compositions (Table 2). Of note, fibers electrospun from more dilute PVC solutions (i.e., 8 and 12 wt%) had less than theoretical NO release. This disparity may be attributed to decreased water uptake by the hydrophobic PVC fibers compared to the polyurethane compositions resulting in incomplete diazeniumdiolate decomposition. As the viscosity of 16 wt% polymer solutions inhibited efficient mixing of PROLI/NO within the solution, the resulting fibers lacked homogenous PROLI/NO distribution. Water uptake was thus concentrated around the more hydrophilic PROLI/NO-containing domains, resulting in more efficient diazeniumdiolate breakdown.
By increasing the NO donor concentration to 10 wt% (in fibers), theoretical NO release was achieved for fibers formed from both 8 and 12 wt% PVC solutions. Such NO release may be attributed to the increased water uptake associated with more hydrophilic NO donor in the fibers. Unfortunately, additional NO donor (3.2 wt% in solution, 20 wt% in fibers) circumvented the electrospinning of fibers from 16 wt% solutions. Similar to Tecoflex fibers, PVC fibers electrospun with 20 wt% PROLI/NO resulted in fibers with reduced NO-release half-lives compared to fibers containing lower NO donor concentrations. This general pattern likely arises from the increased concentrations of water-soluble additives in the fibers. As fibers with high concentrations of these dopants are exposed to solution, the additives dissolve generating an uninhibited path for increased water uptake into the fiber and accelerated diazeniumdiolate decomposition and NO release.
Conclusions
The preparation of electrospun polymer microfibers containing a low molecular weight NO donor (PROLI/NO) was demonstrated as a unique NO-releasing platform. Polymer composition, fiber diameter, and NO donor concentration mediated both the fiber size and NO release, but to varying extents. The ability to tune NO release kinetics by varying specific electrospinning parameters supports further investigation into their use as biomedical scaffolds that release NO. Future studies aim to investigate the antibacterial and antithrombotic capabilities of these materials as potential medical device coatings.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH EB000708).
Footnotes
Supporting Information Available: Flow rate and needle gauge dependence on electrospun fiber diameters, and conductivity measurements of electrospinning solutions. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Witte MB, Barbul A. Am J Surg. 2002;183:406–412. doi: 10.1016/s0002-9610(02)00815-2. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ, Buga GM, Wood KS, Byrns SE, Chaudhuri G. Proc Natl Acad Sci. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke JP. Atheroscler Suppl. 2003;4:53–60. doi: 10.1016/s1567-5688(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 4.Miller MR, Megson IL. Br J Pharmacol. 2007;151:305–321. doi: 10.1038/sj.bjp.0707224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, Colton CA, Harris CC, Roberts DD, Wink DA. Free Radical Biol Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollenberg SM, Cinel I. Crit Care. 2009;13:218–226. doi: 10.1186/cc7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang PG, Xian M, Tang X, Wu X, Wen Z, Cai T, Janczuk AJ. Chem Rev. 2002;102:1091–1134. doi: 10.1021/cr000040l. [DOI] [PubMed] [Google Scholar]
- 8.Frost MC, Batchelor MM, Lee Y, Zhang H, Kang Y, Oh B, Wilson GS, Gifford R, Rudich SM, Meyerhoff ME. Microchem J. 2003;74:277–288. [Google Scholar]
- 9.Reynolds MM, Frost MC, Meyerhoff ME. Free Radical Biol Med. 2004;37:926–936. doi: 10.1016/j.freeradbiomed.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Meyerhoff ME. Talanta. 2008;75:642–650. doi: 10.1016/j.talanta.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parzuchowski PG, Frost MC, Meyerhoff ME. J Am Chem Soc. 2002;124:12182–12191. doi: 10.1021/ja020268l. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds MM, Hrabie JA, Oh BK, Politis JK, Citro ML, Keefer LK, Meyerhoff ME. Biomacromolecules. 2006;7:987–994. doi: 10.1021/bm060028o. [DOI] [PubMed] [Google Scholar]
- 13.Teo WE, Ramakrishna S. Nanotechnology. 2006;17:R89–R106. doi: 10.1088/0957-4484/17/14/R01. [DOI] [PubMed] [Google Scholar]
- 14.Reneker DH, Yarin AL. Polymer. 2008;49:2387–2425. [Google Scholar]
- 15.Wang H, Liu Q, Yang Q, Li Y, Wang W, Sun L, Zhang C, Li Y. J Mater Sci. 2010;45:1032–1038. [Google Scholar]
- 16.Agarwal S, Wendorff JH, Greiner A. Polymer. 2008;49:5603–5621. [Google Scholar]
- 17.McCullen SD, Zhu Y, Bernacki SH, Narayan RJ, Pourdeyhimi B, Gorga RE, Loboa EG. Biomed Mater. 2009;4:035002/1–035002/9. doi: 10.1088/1748-6041/4/3/035002. [DOI] [PubMed] [Google Scholar]
- 18.Sell SA, McClure MJ, Garg K, Wolfe PS, Bowlin GL. Adv Drug Delivery Rev. 2009;61:1007–1019. doi: 10.1016/j.addr.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Jang JH, Castano O, Kim HW. Adv Drug Delivery Rev. 2009;61:1065–1083. doi: 10.1016/j.addr.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Wang B, Wang Y, Yin T, Yu Q. Chem Eng Commun. 2010;197:1315–1338. [Google Scholar]
- 21.Zahedi P, Rezaeian I, Ranaei-Siadat SO, Jafari SH, Supaphol P. Polym Adv Technol. 2009;21:77–95. [Google Scholar]
- 22.Liu HA, Balkus KJ. Chem Mater. 2009;21:5032–5041. [Google Scholar]
- 23.Saavedra JA, Southan GJ, Davies KM, Lundell A, Markou C, Hanson SR, Adrie C, Hurford WE, Zapol WM, Keefer LK. J Med Chem. 1996;39:4361–4365. doi: 10.1021/jm960616s. [DOI] [PubMed] [Google Scholar]
- 24.Theron SA, Zussman E, Yarin AL. Polymer. 2004;45:2017–2030. [Google Scholar]
- 25.Tan SH, Inai R, Kotaki M, Ramakrishna S. Polymer. 2005;46:6128–6134. [Google Scholar]
- 26.Shawon J, Sung C. J Mater Sci. 2004;39:4605–4613. [Google Scholar]
- 27.Taylor G. Proc R Soc A. 1964;280:383–397. [Google Scholar]
- 28.Taylor G. Proc R Soc A. 1969;313:453–475. [Google Scholar]
- 29.Arumugam GK, Khan S, Heiden PA. Macromol Mater Eng. 2009;294:45–53. [Google Scholar]
- 30.Barakat NAM, Abadir MF, Sheikh FA, Kanjwal MA, Park SJ, Kim HY. Chem Eng J. 2010;156:487–495. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.