Abstract
Purpose
Gene expression in human ART cumulus cell (CC) has been related to oocyte maturity and competence but requires further validation. Expression dynamics were investigated in CC of oocytes at different maturational stages and with different developmental competence in a standard in vivo mouse superovulation model.
Methods
Quantitative PCR analysis of Has2, Vcan, Sdc4, Alcam, Grem1, Ptgs1 and Ptgs2 in CC collected at regular time intervals from 0 to 24 h post hCG injection.
Results
Three expression patterns were observed each with strong regulation (4–230× differences). Immediately prior to ovulation CC of GVBD oocytes have 5× less Sdc4 and Ptgs1 and 5× more Ptgs2 when compared to the CC of freshly ovulated PB oocytes. When compared to the latter, the post-ovulatory aged PB oocytes had a 2× reduced blastocyst forming capacity and their CC expressed 2× more Sdc4 and 6× less Alcam.
Conclusions
Morphologically identical cumulus oocyte complexes with different developmental competence can be differentiated by CC gene expression.
Keywords: Cumulus cells, Gene expression, Oocyte maturation, Oocyte quality
Introduction
Oocyte maturation is induced by the pre-ovulatory gonadotrophin surge and requires a strictly timed coordination of events in nuclear and cytoplasmic compartments [1]. The time course of successive nuclear stages of in vivo and in vitro matured oocytes from stimulated adult mice has been well-documented [2, 3]. In stimulated cycles in adult mice, chromatin condensation, dissolution of the Germinal Vesicle (GV) and reorganization of the oocyte’s cytoskeleton are first seen 2 h after stimulation and most oocytes resume maturation by 4 h post hCG. The formation of the first metaphase spindle is completed by 8 h post stimulation. Approximately 12 h after hCG, the first polar body (PB) is extruded and a MII oocyte is ovulated [2, 3]. Within 6 h after ovulation, oocytes are expected to be fertilized [4]. Outside this fertilization window, post-ovulatory oocyte ageing sets in as demonstrated in in vivo [4, 5] and in vitro maturation settings [6].
Also in the cumulus cells (CC), a timed cascade of events occurs [7–9]. Post hCG, the CC transform and start producing a specific extracellular environment for the oocyte. The presence and contact with the CC were found to be essential to obtain good blastocyst rates in several species [10–12].
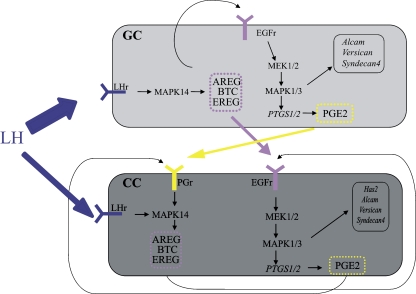
The genes studied here, hyaluronic acid synthetase-2 (Has2), Versican (Vcan), Syndecan 4 (Sdc4), activated leukocyte cell adhesion molecule (Alcam), Gremlin 1 (Grem1) and prostaglandin-endoperoxide synthase 1 and 2 (Ptgs1 and 2), are involved in the signaling and the extracellular matrix formation during the ovulatory response (Fig. 1) and were found differentially expressed in human CC in relation to oocyte developmental quality and patient and treatment characteristics [13–15].
Fig. 1.
The interactions occuring in the ovarian mural granulosa cells (GC) and cumulus cells (CC) after the administration of hCG for ovulation induction. The hypothetical figure is based on earlier literature [16, 56, 57]. LH will mainly act on the mural granulosa cells where it will induce the production of Egf like family members: Amphireguline (Areg), Betacelluline (Btc), Epireguline (Ereg). All can bind to the Egf receptor (Egfr) present in both GC and CC. Egfr stimulation involves the MEK1/2 and MAPK1/3 pathway and will induce Ptgs expression and therefore the production of Prostaglandin E2 (Pge2). Both prostaglandin and the Egf like family members are secreted by GC and can transduce the ovulatory response in the CC and also have an autocrine function in the CC. The ovulatory response will trigger the production of Hyaluronic acid synthetase 2 (Has2), Versican (Vcan) and activated leukocyte cell adhesion molecule (Alcam), genes occuring predominantly in the CC. The expression of Ptgs1 and Syndecan 4 (Sdc4) in GC or CC was not earlier documented so its involvement as represented here needs to be further documented
The ovulation cascade in CC depends on a strong autocrine regulation involving the EGF-like family members and the prostaglandins [16]. EGF-like factors bind to CC and induce Ptgs2 [17]. Ptgs2 is the rate limiting enzyme in Prostaglandin E production, and prostaglandin receptor Ep2 null mice do not ovulate and have a defective COC expansion [18–20]. This indicates that Ptgs2 expression is crucial for obtaining competent oocytes. The expression of Ptgs1 is less well-documented and is described as constitutive, whereas the induction of Ptgs2 seems to be rate limiting [21]. The relation between Ptgs1 and Ptgs2 in the ovary or CC is currently unknown.
During the first hours following the ovulatory hCG, CC start to express Has2, which produces hyaluronic acid (HA), the principal glycosaminoglycan attached to the CC [22]. The degree of expansion caused by HA is positively related to developmental competence [23, 24]. The CC derived matrix also contains the proteoglycans brevican (Bcan) and versican (Vcan) also known as chondroitin sulfate proteoglycan 2. Versican is a proteoglycan described in mouse mural granulosa cells after the ovulatory stimulus [25] and can bind HA via its N-terminal link protein domain, while the C-terminal domain interacts with cell surface proteins like the EGF receptor [26] and integrins [27]. Versican is cleaved by a disintegrin-like and metallopeptidase with thrombospondin type 1 motif 1 (Adamts1), the main proteolytic enzyme in the COC [28]. Adamts1 is also induced by the ovulatory dose of LH and cleaved Versican is detected in ovulated COCs [28]. Since deficient Adamts1 reduces fertilization [29] and since Versican binds important growth factor receptors, an altered Versican expression might also alter the functional properties of the COC matrix during oocyte maturation, ovulation and fertilization.
The next two genes, Alcam and Sdc4, code for transmembranal ECM related proteins. Alcam may be involved in promoting cell-cell adhesion and cell to matrix adhesion. In female reproductive tissue, Alcam was described in endometrial epithelium and blastocysts where it could contribute to implantation [30], and in cumulus and granulosa during the ovulatory response [7]. Sdc4 is a heparan sulfate proteoglycan that appears to act as receptor or co-receptor [31, 32] and is involved in intracellular communication by binding anticoagulant heparan sulfate chains (aHSPG) on the cell surface and in the extracellular matrix. The aHSPG expression in the ovarian follicle is hormonally induced to culminate in preovulatory follicles and controls proteolysis and fibrin formation at ovulation, possibly protecting the oocyte [33].
Growth differentiation factor 9 (Gdf9), i.e. an oocyte-secreted factor required for normal ovarian follicle development and female fertility, acts using transforming growth factor-beta signaling as a paracrine factor on granulosa cell [34]. Gdf9 is a key mediator of cumulus expansion and induces the expression of Has2 and Ptgs2 in the presence of FSH [35]. Gremlin is secreted and favors the GDF9 pathway (and mucification) in cumulus/granulosa over the BMP pathway [36, 37].
Studying the expression dynamics in a mouse model can provide insight into a) how these genes are regulated and what the magnitude is of the changes observed during the ovulatory response, b) whether up or down regulation can be related to oocyte maturity, c) how stable the expression is in CC of PB oocytes (the target population in human ART-cycles), and d) whether a known population of COC with identical morphological characteristics, but different oocyte quality, could be distinguished (as a proof of concept).
Materials and methods
Mouse cumulus cells
Mice used for retrieval of COCs were 7-to-9-week old F1 hybrids (C57Bl/6J x CBA/ca, Charles River, Brussels, Belgium), housed and bred according to the national legislation on animal care and with the consent of the “Commissie voor Ethisch Proefdiergebruik VUB” (project n° 05-395-2). The adult anoestrous mice were primed for 48 h with a single injection of 5IU eCG (Folligon, Intervet, Mechelen, Belgium), and CC were collected before (0 h) and 2, 4, 8, 12, 16 and 24 h after administration of the ovulatory stimulus of 5IU hCG (Chorulon, Intervet, Mechelen, Belgium). Collection of the in vivo grown COCs was repeated 3 times with two mice for each of the 7 time points. The CC of individual COCs were analyzed and per time point the CC of at least 2 COCs from each biological replicate were used in the current study. Because of the low detection levels for Ptgs1 and Ptgs2, the number of samples was tripled for these 2 genes.
The pre ovulatory COCs were punctured from the large antral follicles in the ovary. The post-ovulatory COCs (from 12 h post hCG onwards) were flushed from the ampulla using L15 Leibovitz-GlutaMAXI medium supplemented with 10% heat inactivated Fetal Bovine Serum (HIA FBS), 100 IU/ml penicillin and 100 μg/ml streptomycin (all from Life Technologies, Merelbeke, Belgium). At 0 and 2 h, the collected oocytes were in germinal vesicle (GV) stage; at 4 and 8 h, all oocytes were undergoing germinal vesicle breakdown (GVBD), and from 12 h onwards all oocytes extruded a polar body (PB). All manipulations were performed under a stereomicroscope SZX9 (Olympus, Aartselaar, Belgium) on heated stages. COCs with 2–5 cumulus cell (CC) layers were washed and transferred using the mouth pipette to an individual 10 μl droplet containing 40 IU/ml of Hyaluronidase (Sigma-Aldrich, Bornem, Belgium) where oocytes were freed. The CC from individual COCs were snap-frozen in liquid nitrogen and stored at −80°C for further use.
RNA extraction & RT reaction
Total-RNA of CC of individual COCs was extracted using the RNeasy Micro kit (Qiagen, Westburg, Venlo, The Netherlands) and reverse transcribed using the Taqman Reverse Transcription Reagents (Applied Biosystems, Lennik, Belgium) as described earlier [38]. Negative controls were generated by omitting the RNA or the reverse transcription enzyme and proved to be blank. All resulting cDNA was frozen at −80°C and used for PCR within 2 weeks.
Quantitative PCR (QPCR)
PCR primer design was performed using the Universal Probe Library (UPL) software (https://www.roche-applied-science.com/sis/rtpcr/upl/index.jsp). Primers were selected to be complementary to the sequence of Has2, Ptgs1, Ptgs2, Sdc4, Alcam, Grem1, Vcan (Table 1). Ribosomal Rn18s was used as endogenous control (primer sequences were kindly provided by Prof. Dr. R. Einspanier, HU Berlin, Germany). Quantitative PCR was performed on the LightCycler 480 (Roche Diagnostics, Vilvoorde, Belgium) with the LC480 SYBR Green I Master (Roche Diagnostics, Vilvoorde, Belgium) containing 2 μl of cDNA and 0.6 μM of primers in a total volume of 15 μl. After 10 min activation at 95°C, cycling conditions were 10 s at 95°C, 30 s at 60°C for 45 cycles. A log6 dilution series of a synthetic oligonucleotide, corresponding to the amplicon, and no template controls were simultaneously run. The specificity of the PCR products was confirmed by melting curve analysis performed at the end of the amplification and by gel electrophoresis. Each sample was tested in triplicate and the standard curve was used to calculate the absolute quantity, taking into account the actual PCR efficiency prior to the normalization to his Rn18S. Results are depicted in graphs with relative ratios, using the median of the condition with the lowest expression level different from 0 as the calibrator.
Table 1.
Overview of the primersequences 5′ → 3′
| Gene symbol | Accession n° | Forward primer | Reversed primer | Amplicon length |
|---|---|---|---|---|
| Alcam | NM_009655.1 | CTGATTGTGGGAATTGTCGTT | TCCTTGTTTACATGTTTTGATGC | 110bp |
| Grem1 | NM_011824.3 | GAGGACCCACGGAAGTGA | CCTCAGCTGTTGGCAGTAGG | 92bp |
| Has2 | NM_008216.2 | TGAGCAGGAGCTGAACAAGA | GCCAACAATATAAGCAGCTGTG | 118bp |
| Ptgs1 | NM_008969.2 | CCTCTTTCCAGGAGCTCACA | TCGATGTCACCGTACAGCTC | 70bp |
| Ptgs2 | NM_011198.3 | GGGAGTCTGGAACATTGTGAA | GCACATTGTAAGTAGGTGGACTGT | 110bp |
| Sdc4 | NM_011521.1 | CCCTTCCCTGAAGTGATTGA | AGTTCCTTGGGCTCTGAGG | 98bp |
| Vcan | NM_001081249.1 | CTGTGTGGATGGTTTTAATACATTTAG | CAGCCATAGTCACATGTCTCAGT | 96bp |
| Rn18s | NR_003278.1 | TCAAGAACGAAAGTCGGAGG | GGACATCTAAGGGCATCACA | 489bp |
In vitro fertilization
Mucified COCs of F1 mice stimulated as described above were collected at 12, 16 and 24 h post hCG for in vitro fertilization and embryo culture. Sperm was collected from two adult F1 mice and sperm capacitation and IVF were performed in M16 (Sigma-Aldrich, Bornem, Belgium) supplemented with 2.6% bovine serum albumin fraction V (BSA; Calbiochem, Merck, Leuven, Belgium) and 10 μl/ml non-essential amino acids (100× NEAA; Life Technologies, Merelbeke, Belgium). Embryos were cultured in M16 supplemented with NEAA (10 μl/ml) and essential amino acids (20 μl/ml; 50× EAA; Life Technologies, Merelbeke, Belgium). Embryo and blastocyst evaluation was performed as described earlier [39]. Three biological replicates were performed, each time with 2 female mice /time point, and each time point was considered an experimental unit and contained on average 34 ± 13 COCs.
Statistical analysis
To ensure Gaussian distribution, the expression ratios were log transformed and the fertilization and embryo culture percentages were arcsine transformed. All data were analyzed using the two-tailed ANOVA and Tukey post test using Graphpad Prism V4.0 (Graphpad software, CA, USA).
Results
Developmental competence of the ovulated COCs
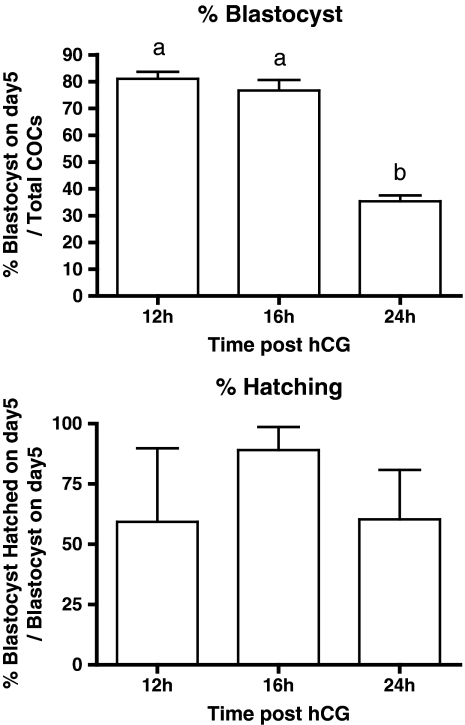
Ovulated COCs collected at 12–24 h post hCG are all morphologically identical with expanded CC and oocytes at the PB stage. The developmental competence of the oocytes collected at 12, 16 and 24 h was analyzed. The 3 groups had a comparable fertilization capacity with 2cell rates of (Mean ± SD) 97% ± 6, 82% ± 11, and 81% ± 10 for respectively 12, 16 and 24 h, but 24 h COCs had a 2× reduced blastocyst forming capacity (p < 0.001) (Fig. 2). The capacity to hatch was comparable in the 3 conditions (Fig. 2).
Fig. 2.
Blastocyst forming and hatching capacity of ageing PB oocytes. Cumulus oocyte complexes (COCs) of eCG primed mice collected at 12, 16 and 24 h post hCG injection were in vitro fertilized and grown to blastocyst. The % (Mean ± SEM) of blastocysts formed and blastocysts hatched of three biological replicates are depicted. For blastocyst forming capacity the time points marked with different letters were statistically different after two-tailed ANOVA and Tukey post test (P < 0.0001). Hatching capacity was comparable in the three conditions. Based on the differences in developmental capacity the 12 and 16 h CC samples are further referred to as “Optimal PB CC” and the 24 h CC samples are further called “Aged PB CC”
The different COC classes
At 0 h the CC have not yet been exposed to hCG and are referred to as “GV CC”. At 2 h no morphologic distinction with 0 h COCs could be found, but expression changes are already initiated in the CC = “Activated GV CC”. At 4 and 8 h oocytes undergo the germinal vesicle breackdown = “GVBD CC”. Based on the differences in developmental capacity described above, polar body oocyte CC were subdivided into 12 and 16 h CC samples = “Optimal PB CC” and 24 h CC samples = “Aged PB CC”. (Figure 3).
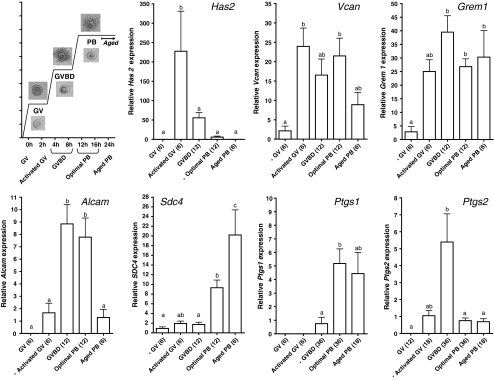
Fig. 3.
The relative expression observed in cumulus cells from eCG primed mice at different time points post hCG injection. The first graph depicts the source of the material and the type of oocyte it surrounded. Magnification of the pictures is 300× for cumulus oocyte complexes and 400× for the oocytes. The succeeding graphs depicts the relative expression level (Mean + SEM) of each of the genes with the condition used as calibrator marked with an *. Conditions marked with different letters are statistically different after two-tailed ANOVA and the Tukey post test (P < 0.05)
Expression in pre and post hCG cumulus cells
Prior to the induction of final oocyte maturation, mRNA for Has2, Alcam, Ptgs1 and 2 was undetectable. For Vcan, Grem1 and Sdc4, at least 50% of the samples analyzed contained quantifiable amounts of mRNA prior to hCG. Post hCG, all genes demonstrated distinct patterns (Fig. 3). In the activated GV CC, the first time point after hCG administration, mRNA was detectable in all samples for all investigated genes except for Ptgs1; with a significant induction for Has2 and Vcan (Fig. 3).
When considering the physiologically most relevant timespan (0–16 h), 3 expression patterns could be distinguished 1) the expression is significantly induced prior to ovulation (12 h) and fades away at ovulation, the “early response genes” (Has2, Ptgs2; Fig. 3), or 2) the expression is significantly induced prior to ovulation and is equally expressed in the optimal COC after ovulation, the “early remaining genes” (Vcan, Grem1 and Alcam; Fig. 3) or 3) the expression is only induced post ovulation, the “late response genes” (Sdc4 and Ptgs1; Fig. 3).
Has2 was the gene with the strongest induction (230-fold in activated GVs); it then gradually decreased until absent in all post-ovulatory aged PB CC samples. Ptgs2 reached a maximal induction (5×) in GVBD CC and decreased to a baseline expression level in PB CC.
Vcan expression showed a strong induction immediately after hCG exposure (23×), which was maintained until the optimal PB CC stage. Grem1 was rapidly upregulated (25×) by the ovulatory dose of hCG, reaching a maximal response (40×) in GVBD CC, after which a steady state was maintained in the PB oocyte CC. Alcam mRNA was most highly expressed (5-fold) around the time of ovulation in CC of GVBD and optimal PB.
Finally, for the two late response genes, expression levels did not vary much until the PB stage. Sdc4 expression was 10 and 20 times induced in competent and post-ovulatory aged PB CC respectively, and Ptgs1 was mainly detected in the CC of ovulated COCs. The patterns for Ptgs1 and 2 are complementary. It should however be noted that Ptgs1 expression was not present in all COCs, but was only detectable in 8, 67 and 44% of the COCs of respectively GVBD CC, optimal PB CC and post-ovulatory aged PB CC. An overview of the % positive samples per gene and condition is shown in Table 2.
Table 2.
Fraction of samples containing the specific gene of the total number of samples analyzed (n), when analyzing the cumulus cells of single cumulus oocyte complexes
| 18S | Has2 | Sdc4 | Alcam | Vcan | Grem1 | Ptgs1 | Ptgs2 | |
|---|---|---|---|---|---|---|---|---|
| GV | 100% (12) | 0% (6) | 67% (6) | 0% (6) | 50% (6) | 50% (6) | 0% (6) | 0% (12) |
| Activated GV | 100% (18) | 100% (6) | 100% (6) | 100% (6) | 100% (6) | 100% (6) | 0% (6) | 72% (18) |
| GVBD | 100% (36) | 100% (12) | 67% (12) | 100% (12) | 100% (12) | 100% (12) | 8% (36) | 100% (36) |
| Optimal PB | 100% (36) | 67% (12) | 100% (12) | 100% (12) | 100% (12) | 100% (12) | 67% (36) | 100% (36) |
| Aged PB | 100% (18) | 0% (6) | 100% (6) | 67% (6) | 100% (6) | 100% (6) | 44% (18) | 100% (18) |
The ratio of Ptgs2/1 expression
Ptgs1 and 2 were both found to be expressed in CC, but seemed to be important at different moments of maturation. Since an absolute quantification to the copy number using the standard curve was performed prior to the relative quantification, a ratio of mean copy numbers could also be made. In COCs expressing both Ptgs1 and Ptgs2 (from 12 h post hCG onwards), the Ptgs2/Ptgs1 ratio was more than 700. This indicated that the amount of Ptgs2 mRNA copies is much greater in the CC.
CC expression differences in oocytes with different nuclear maturity
When comparing the expression in CC of GV (0 h), GVBD and optimal PB, the three patterns distinguished earlier correspond to: 1) the expression is only higher in the CC of the GVBD oocytes (Ptgs2), 2) expression is upregulated from the GVBD stage and is unchanged in the CC of competent PB oocytes (Vcan, Grem1 and Alcam), 3) expression is only increased in the CC of PB oocytes (Sdc4 and Ptgs1). It is remarkable that expression differences in each pattern are at least five-fold and can rise to 14-fold. As such, the ovulated competent PB oocytes were surrounded with CC with, on average, a 9× decreased level of Has2 (P > 0.05), a 5× increased Sdc4 (P < 0.05), a 5× increased Ptgs1 (P < 0.05) and a 5× decreased Ptgs2 (P < 0.05) when compared to the CC of not ovulated GVBD oocytes.
Comparing CC of competent or post-ovulatory aged oocytes
Post-ovulatory aged COCs collected 24 h after ovulation induction had a morphology undistinguishable from the COCs collected at 12 or 16 h, but on average, had 2× more Sdc4 (P < 0.05), and respectively 2 and 6× less Vcan (ANOVA P > 0.05, t-test P < 0.05) and Alcam (P < 0.05) in their CC. CC of post-ovulatory aged PB oocytes did not contain Has2, whereas it was still detectable in 8/12 CC samples of competent PB oocytes.
Discussion
The corona-cumulus cells were examined for their expression of factors related to extracellular matrix formation and signaling triggered by an ovulatory dose of hCG in in vivo grown and matured follicles. As expected, all genes were expressed at one or more time points during the ovulatory response. The fact that 30–50% of the in vivo grown COCs from superovulated mice expressed Vcan, Sdc4 or Grem1 prior to hCG administration might seem unexpected. However, experiments by others have indicated the presence of limited amounts of Grem1, Sdc4 mRNA and Vcan mRNA and protein in granulosa cells of growing follicles [25, 37, 40].
The relation between the complex processes leading to cumulus expansion downstream of the ovulatory response and normal fertilization and development [41] generated expectations that some of the tested genes could prospectively be used as prognostic markers. These genes were found differentially expressed in human CC in relation to oocyte developmental quality and patient and treatment characteristics [13]. Studying the dynamics of gene expression in an animal model may provide useful information for evaluating the predictive potential of this approach in human ART.
Which expression changes occur in an in vivo ovulation response?
Three categories of genes could be distinguished: early (Has2 and Ptgs2), early and persistent (Vcan, Grem1 and Alcam) and late response genes (Sdc4 and Ptgs1).
The early response and fading away pattern as observed for Has2 and Ptgs2 is in line with earlier reports by others [42–46]. One paper reports a second upregulation for Ptgs2 from 8 to 12 h [47], which we did not observe in our QPCR setting. The early occurrence and disappearance of these genes might indicate that their main role is prior to ovulation. This is in line with the fact that the HA synthesis by mice COCs is first detected 2–3 h post hCG and increases to a maximum between 4–10 h [48].
The Vcan and Grem1 pattern reported here is to our knowledge the first quantitative expression pattern in CC. The Alcam expression pattern is in line with an earlier study in mice [7]. The observed expression pattern fits in with the fact that Alcam protein was detectable at the CC surface from 8 to 16 h post hCG [7]. Since recombinant Gremlin inhibits BMP4 activity—a theca cell factor involved in androgen production—it was hypothesized that Gremlin blocks steroidogenesis and luteinisation in CC [49]. In this perspective, its persistence in CC would make sense.
Finally, there are the 2 last wave genes (Sdc4 and Ptgs1), which are only induced post ovulation, and whose expression pattern in CC is also poorly documented in current literature. In mouse ovary, Sdc4 was expressed by GC from type 4 follicles (large preantral follicle) [50] onwards. In situ hybridization also indicated that the GC lining the antrum in type 6 and 7 follicles and the Corpus Luteum were positive. It was also suggested that increased Sdc4 expression was related to increased apoptosis [40], but the main increase in Sdc4 occurred from ovulated GVBD to optimal PB CC (5-fold) where apoptosis in CC is mainly observed from 5 h post ovulation onwards [51], which corresponds most with the transition from optimal PB to post-ovulatory aged PB oocyte CC, which only had a 2-fold increase in the current study. Sdc4 was found in follicle fluid aspirates of ART patients and was 10-fold increased at 36 h post hCG compared to pre hCG (patients undergoing follicle reduction prior to hCG). The protein levels also seemed to be negatively correlated to follicle fluid estradiol and progesterone concentrations [52].
The pattern observed for Ptgs1 suggests that Ptgs1 is complementary to Ptgs2. These findings confirm the compensatory upregulation of Ptgs1 described in CD1 Ptgs2 null mice, where it resulted in the partial rescue of ovulation, fertilization, and implantation capacity [53]. Although the expression level of Ptgs1 was much lower than that for Ptgs2, the occurrence of Ptgs1 around the time of ovulation could make it a useful marker at pick up in an ART-setting.
Can an up or down regulation be related to oocyte maturity or intrinsic oocyte quality?
There is a within patient heterogeneity in the COCs/oocytes collected at pick up in human ART. This heterogeneity is reflected in the COC morphology, the oocyte maturity, and the oocyte developmental competence. The underlying cause of this heterogeneity might be related to a difference in the vascularisation of the follicles and the maturity of the follicle/oocyte at the start of stimulation. Both might result in a speeding up or slowing down of the ovulatory response in the CC of the respective COCs and the heterogeneity observed at the fixed time point of pick up. In this perspective, more knowledge as to whether one specific gene is a fast response gene like Has2 or hardly undergoes any change in the CC of PB oocytes like Grem1 is useful information when we compare expression levels in human CC.
Completion of nuclear maturation (PB) coincided with an increase in expression for all genes studied here except for Ptgs2. Vcan, Grem1 and Alcam were high from GVBD on Ptgs1 and Sdc4 from PB on.
Care should be taken when extrapolating these results to expression differences found in human ART CC. One argument is the fact that all COCs in the clinic are collected 36 h post hCG injection from large antral follicles (usually >17 mm) and are thus most likely to be exposed to some hCG. Bearing this in mind, we should consider the expression levels in the activated GV population of the current study. Compared to activated GV CC, PB oocyte CC have an increased Alcam, Sdc4, Ptgs1 and decreased Has2 expression.
In human oocytes, nuclear maturity correlated positively to PTGS2 [14, 54], GREM1 [14] and negatively to VCAN [13, 14] expression in CC. Overall, the expression differences found in human in relation to oocyte nuclear maturity (≤2-fold) are smaller than those observed in the current study (5-fold and more), which also suggests the different nature of the samples compared. The results from the current setting are therefore of limited use to validate the observations made in relation to oocyte maturity CC of human oocytes after ART.
Post-ovulatory ageing as in the current setting up to 12 h (the 24 h time point) does not occur in a clinical setting, but allowed to make a clear distinction between two populations of morphologically identical COCs. The dynamics observed might give an indication as to how expression evolves and might underlie the subtle differences observed in human CC at pick up. In the mouse model, post-ovulatory ageing resulted in the disappearance of Has2 expression (below the detection limit), a decreased expression of Vcan and Alcam, and an increased Sdc4.
In human ICSI samples, increased HAS2, ALCAM, PTGS2, SDC4 and decreased VCAN were reported in PB oocyte CC resulting in higher grade embryos or blastocysts after ICSI [13, 23, 55]. The Has2 and Alcam observations seem to be in line with our findings since post-ovulatory aged oocytes proved to have less Has2 and Alcam.
The CC analyzed and compared for developmental competence in human ART are CC of PB oocytes collected prior to ovulation. In the mice setting, all CC of PB oocytes were collected post ovulation. In this perspective, the expression level in the CC of mice GVBD oocytes might be relevant. Considering also the GVBD expression levels suggests that a decrease in Ptgs2 would be detrimental to the oocyte competence, which is in line with reports in human CC. The Vcan and Sdc4 regulation could not be confirmed.
Higher GREM1 expression in the CC of ICSI patients has been described as a marker of better blastocyst forming capacity [23, 55]. Grem1 seems to be on steady state level from ovulation on; a higher expression of Grem1 might result from the intrinsic oocyte factors and/or might be determined by follicular development stage at the time of ovulation induction.
On the other hand, stimulating patients with a gonadotrophin preparation containing an hCG action resulted in increased SDC4 expression in the CC [13]. Given the specific pattern of Sdc4 expression, with its gradual increase, it could be hypothesized that pre-exposing follicle to a low hCG/LH concentration during the follicular phase results in a more advanced ovulatory response, which would be characterised by an increased Sdc4.
Conclusion
In the current study, genes reported to be differentially expressed in relation to oocyte competence in human CC were investigated during the ovulatory response. Overall expression differences were quite large compared to the differences currently described in human CC at time of pick up. While all genes proved to be regulated, Vcan, Grem1 and Alcam expression were quite stable around the time of ovulation (the time of pick up in human). This makes them interesting markers since expression changes observed for these genes in human CC might than primarily originate from intrinsic oocyte factors. Immediately prior to ovulation CC of GVBD oocytes have 5× less Sdc4 and Ptgs1 and 5× more Ptgs2 when compared to the CC of freshly ovulated PB oocytes. The post-ovulatory aged PB oocytes had a 2× reduced blastocyst forming capacity and their CC expressed 2× more Sdc4 and 6× less Alcam when compared to the freshly ovulated PB oocytes. Morphologically identical mouse cumulus oocyte complexes with different developmental competence could thus be differentiated by CC gene expression. We cannot exclude that the dynamics in CC gene expression would differ between human and mice, but decreased HAS2, ALCAM and PTGS2 seems to coincide with reduced oocyte competence in both species.
Acknowledgements
The authors wish to thank J.-C. Arce and L. Helmgaard, Clinical Research & Development, Ferring Pharmaceuticals, for their contribution to the study and the manuscript, and M. Whitburn, ITO, Vrije Universiteit Brussel, for his editorial assistance.
This research has been supported by funds from the FWO (N° FWOAL389), Ferring Pharmaceuticals and the Vrije Universiteit Brussel (VUB) OZR (N° OZR627).
Footnotes
Capsule
Gene expression patterns in cumulus cell are different when surrounding an immature, mature or postovulatory aged oocyte
References
- 1.Albertini DF, Sanfins A, Combelles CM. Origins and manifestations of oocyte maturation competencies. Reprod Biomed Online. 2003;6:410–5. doi: 10.1016/S1472-6483(10)62159-1. [DOI] [PubMed] [Google Scholar]
- 2.Edwards RG, Gates AH. Timing of the stages of the maturation divisions, ovulation, fertilization and the first cleavage of eggs of adult mice treated with gonadotrophins. J Endocrinol. 1959;18:292–304. doi: 10.1677/joe.0.0180292. [DOI] [PubMed] [Google Scholar]
- 3.Sanfins A, Lee GY, Plancha CE, Overstrom EW, Albertini DF. Distinctions in meiotic spindle structure and assembly during in vitro and in vivo maturation of mouse oocytes. Biol Reprod. 2003;69:2059–67. doi: 10.1095/biolreprod.103.020537. [DOI] [PubMed] [Google Scholar]
- 4.Braden AW, Austin CR. The fertile life of mouse and rat eggs. Science. 1954;120:610–1. doi: 10.1126/science.120.3120.610. [DOI] [PubMed] [Google Scholar]
- 5.Eichenlaub-Ritter U, Chandley AC, Gosden RG. Alterations to the microtubular cytoskeleton and increased disorder of chromosome alignment in spontaneously ovulated mouse oocytes aged in vivo: an immunofluorescence study. Chromosoma. 1986;94:337–45. doi: 10.1007/BF00328633. [DOI] [PubMed] [Google Scholar]
- 6.Segers I, Adriaenssens T, Coucke W, Cortvrindt R, Smitz J. Timing of nuclear maturation and post-ovulatory aging in oocytes of in vitro grown mouse follicles with or without oil-overlay. Biol Reprod 2008;78:859–68. [DOI] [PubMed]
- 7.Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L, et al. Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol Endocrinol. 2006;20:1300–21. doi: 10.1210/me.2005-0420. [DOI] [PubMed] [Google Scholar]
- 8.Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13:289–312. doi: 10.1093/humupd/dml062. [DOI] [PubMed] [Google Scholar]
- 9.Espey LL, Richards JS. Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol Reprod. 2002;67:1662–70. doi: 10.1095/biolreprod.102.005173. [DOI] [PubMed] [Google Scholar]
- 10.Luciano AM, Lodde V, Beretta MS, Colleoni S, Lauria A, Modina S. Developmental capability of denuded bovine oocyte in a co-culture system with intact cumulus-oocyte complexes: role of cumulus cells, cyclic adenosine 3′, 5′-monophosphate, and glutathione. Mol Reprod Dev. 2005;71:389–97. doi: 10.1002/mrd.20304. [DOI] [PubMed] [Google Scholar]
- 11.Ebner T, Moser M, Sommergruber M, Shebl O, Tews G. Incomplete denudation of oocytes prior to ICSI enhances embryo quality and blastocyst development. Hum Reprod. 2006;21:2972–7. doi: 10.1093/humrep/del272. [DOI] [PubMed] [Google Scholar]
- 12.Amano T, Mori T, Matsumoto K, Iritani A, Watanabe T. Role of cumulus cells during maturation of porcine oocytes in the rise in intracellular Ca2+ induced by inositol 1, 4, 5-trisphosphate. Theriogenology. 2005;64:261–74. doi: 10.1016/j.theriogenology.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Adriaenssens T, Wathlet S, Segers I, Verheyen G, Vos A, Elst J, et al. Cumulus cell gene expression is associated with oocyte developmental quality and influenced by patient and treatment characteristics. Hum Reprod. 2010;25:1259–70. doi: 10.1093/humrep/deq049. [DOI] [PubMed] [Google Scholar]
- 14.Wathlet S, Adriaenssens T, Segers I, Verheyen G, Van de Velde H, Coucke W, et al. Cumulus cell gene expression in ICSI patients predicts better cleavage-stage embryo or blastocyst development or pregnancy. Human Reprod 2010. [DOI] [PubMed]
- 15.Adriaenssens T, White R, Segers I, Kastertein E, Bern O, Ron-El R, et al. Effect of LH activity in Menopur on the expression of ovulaton response genes in human cumulus corona complexes at the time of oocyte retrieval. Hum Reprod. 2007;22:i15–6. [Google Scholar]
- 16.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20:1352–65. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- 17.Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–4. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 18.Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–82. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 19.Hizaki H, Segi E, Sugimoto Y, Hirose M, Saji T, Ushikubi F, et al. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2) Proc Natl Acad Sci U S A. 1999;96:10501–6. doi: 10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis BJ, Lennard DE, Lee CA, Tiano HF, Morham SG, Wetsel WC, et al. Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1beta. Endocrinology. 1999;140:2685–95. doi: 10.1210/en.140.6.2685. [DOI] [PubMed] [Google Scholar]
- 21.Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, et al. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–9. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- 22.Salustri A, Camaioni A, Giacomo M, Fulop C, Hascall VC. Hyaluronan and proteoglycans in ovarian follicles. Hum Reprod Update. 1999;5:293–301. doi: 10.1093/humupd/5.4.293. [DOI] [PubMed] [Google Scholar]
- 23.McKenzie LJ, Pangas SA, Carson SA, Kovanci E, Cisneros P, Buster JE, et al. Human cumulus granulosa cell gene expression: a predictor of fertilization and embryo selection in women undergoing IVF. Hum Reprod. 2004;19:2869–74. doi: 10.1093/humrep/deh535. [DOI] [PubMed] [Google Scholar]
- 24.Ball GD, Leibfried ML, Lenz RW, Ax RL, Bavister BD, First NL. Factors affecting successful in vitro fertilization of bovine follicular oocytes. Biol Reprod. 1983;28:717–25. doi: 10.1095/biolreprod28.3.717. [DOI] [PubMed] [Google Scholar]
- 25.Russell DL, Ochsner SA, Hsieh M, Mulders S, Richards JS. Hormone-regulated expression and localization of versican in the rodent ovary. Endocrinology. 2003;144:1020–31. doi: 10.1210/en.2002-220434. [DOI] [PubMed] [Google Scholar]
- 26.Xiang YY, Dong H, Wan Y, Li J, Yee A, Yang BB, et al. Versican G3 domain regulates neurite growth and synaptic transmission of hippocampal neurons by activation of epidermal growth factor receptor. J Biol Chem. 2006;281:19358–68. doi: 10.1074/jbc.M512980200. [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Chen L, Zheng PS, Yang BB. beta 1-Integrin-mediated glioma cell adhesion and free radical-induced apoptosis are regulated by binding to a C-terminal domain of PG-M/versican. J Biol Chem. 2002;277:12294–301. doi: 10.1074/jbc.M110748200. [DOI] [PubMed] [Google Scholar]
- 28.Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem. 2003;278:42330–9. doi: 10.1074/jbc.M300519200. [DOI] [PubMed] [Google Scholar]
- 29.Shindo T, Kurihara H, Kuno K, Yokoyama H, Wada T, Kurihara Y, et al. ADAMTS-1: a metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J Clin Invest. 2000;105:1345–52. doi: 10.1172/JCI8635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujiwara H, Tatsumi K, Kosaka K, Sato Y, Higuchi T, Yoshioka S, et al. Human blastocysts and endometrial epithelial cells express activated leukocyte cell adhesion molecule (ALCAM/CD166) J Clin Endocrinol Metab. 2003;88:3437–43. doi: 10.1210/jc.2002-021888. [DOI] [PubMed] [Google Scholar]
- 31.Kojima T, Katsumi A, Yamazaki T, Muramatsu T, Nagasaka T, Ohsumi K, et al. Human ryudocan from endothelium-like cells binds basic fibroblast growth factor, midkine, and tissue factor pathway inhibitor. J Biol Chem. 1996;271:5914–20. doi: 10.1074/jbc.271.10.5914. [DOI] [PubMed] [Google Scholar]
- 32.Beauvais DM, Rapraeger AC. Syndecans in tumor cell adhesion and signaling. Reprod Biol Endocrinol. 2004;2:3. doi: 10.1186/1477-7827-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Princivalle M, Hasan S, Hosseini G, Agostini AI. Anticoagulant heparan sulfate proteoglycans expression in the rat ovary peaks in preovulatory granulosa cells. Glycobiology. 2001;11:183–94. doi: 10.1093/glycob/11.3.183. [DOI] [PubMed] [Google Scholar]
- 34.Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–66. doi: 10.1210/me.15.6.854. [DOI] [PubMed] [Google Scholar]
- 35.Gui LM, Joyce IM. RNA interference evidence that growth differentiation factor-9 mediates oocyte regulation of cumulus expansion in mice. Biol Reprod. 2005;72:195–9. doi: 10.1095/biolreprod.104.033357. [DOI] [PubMed] [Google Scholar]
- 36.Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell. 1998;1:673–83. doi: 10.1016/S1097-2765(00)80067-2. [DOI] [PubMed] [Google Scholar]
- 37.Pangas SA, Jorgez CJ, Matzuk MM. Growth differentiation factor 9 regulates expression of the bone morphogenetic protein antagonist gremlin. J Biol Chem. 2004;279:32281–6. doi: 10.1074/jbc.M403212200. [DOI] [PubMed] [Google Scholar]
- 38.Adriaenssens T, Mazoyer C, Segers I, Wathlet S, Smitz J. Differences in collagen expression in cumulus cells after exposure to highly purified menotropin or recombinant follicle-stimulating hormone in a mouse follicle culture model. Biol Reprod. 2009;80:1015–25. doi: 10.1095/biolreprod.107.067462. [DOI] [PubMed] [Google Scholar]
- 39.Segers I, Adriaenssens T, Ozturk E, Smitz J. Acquisition and loss of oocyte meiotic and developmental competence during in vitro antral follicle growth in mouse. Fertil Steril 2010;93:2695–700. [DOI] [PubMed]
- 40.Ishiguro K, Kojima T, Taguchi O, Saito H, Muramatsu T, Kadomatsu K. Syndecan-4 expression is associated with follicular atresia in mouse ovary. Histochem Cell Biol. 1999;112:25–33. doi: 10.1007/s004180050388. [DOI] [PubMed] [Google Scholar]
- 41.Richards JS. Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol. 2005;234:75–9. doi: 10.1016/j.mce.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Morris JK, Richards JS. Hormone induction of luteinization and prostaglandin endoperoxide synthase-2 involves multiple cellular signaling pathways. Endocrinology. 1993;133:770–9. doi: 10.1210/en.133.2.770. [DOI] [PubMed] [Google Scholar]
- 43.Sirois J, Richards JS. Transcriptional regulation of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Evidence for the role of a cis-acting C/EBP beta promoter element. J Biol Chem. 1993;268:21931–8. [PubMed] [Google Scholar]
- 44.Morris JK, Richards JS. Luteinizing hormone induces prostaglandin endoperoxide synthase-2 and luteinization in vitro by A-kinase and C-kinase pathways. Endocrinology. 1995;136:1549–58. doi: 10.1210/en.136.4.1549. [DOI] [PubMed] [Google Scholar]
- 45.Fulop C, Salustri A, Hascall VC. Coding sequence of a hyaluronan synthase homologue expressed during expansion of the mouse cumulus-oocyte complex. Arch Biochem Biophys. 1997;337:261–6. doi: 10.1006/abbi.1996.9793. [DOI] [PubMed] [Google Scholar]
- 46.Salustri A, Yanagishita M, Hascall VC. Synthesis and accumulation of hyaluronic acid and proteoglycans in the mouse cumulus cell-oocyte complex during follicle-stimulating hormone-induced mucification. J Biol Chem. 1989;264:13840–7. [PubMed] [Google Scholar]
- 47.Joyce IM, Pendola FL, O’Brien M, Eppig JJ. Regulation of prostaglandin-endoperoxide synthase 2 messenger ribonucleic acid expression in mouse granulosa cells during ovulation. Endocrinology. 2001;142:3187–97. doi: 10.1210/en.142.7.3187. [DOI] [PubMed] [Google Scholar]
- 48.Tirone E, D’Alessandris C, Hascall VC, Siracusa G, Salustri A. Hyaluronan synthesis by mouse cumulus cells is regulated by interactions between follicle-stimulating hormone (or epidermal growth factor) and a soluble oocyte factor (or transforming growth factor beta1) J Biol Chem. 1997;272:4787–94. doi: 10.1074/jbc.272.8.4787. [DOI] [PubMed] [Google Scholar]
- 49.Glister C, Richards SL, Knight PG. Bone morphogenetic proteins (BMP) -4, -6, and -7 potently suppress basal and luteinizing hormone-induced androgen production by bovine theca interna cells in primary culture: could ovarian hyperandrogenic dysfunction be caused by a defect in thecal BMP signaling? Endocrinology. 2005;146:1883–92. doi: 10.1210/en.2004-1303. [DOI] [PubMed] [Google Scholar]
- 50.Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17:555–7. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- 51.Szoltys M, Tabarowski Z, Pawlik A. Apoptosis of postovulatory cumulus granulosa cells of the rat. Anat Embryol (Berl) 2000;202:523–9. doi: 10.1007/s004290000122. [DOI] [PubMed] [Google Scholar]
- 52.Sakata M, Kobayashi H, Sun GW, Mochizuki O, Takagi A, Kojima T. Ryudocan expression by luteinized granulosa cells is associated with the process of follicle atresia. Fertil Steril. 2000;74:1208–14. doi: 10.1016/S0015-0282(00)01587-9. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, Ma WG, Tejada L, Zhang H, Morrow JD, Das SK, et al. Rescue of female infertility from the loss of cyclooxygenase-2 by compensatory up-regulation of cyclooxygenase-1 is a function of genetic makeup. J Biol Chem. 2004;279:10649–58. doi: 10.1074/jbc.M312203200. [DOI] [PubMed] [Google Scholar]
- 54.Feuerstein P, Cadoret V, Dalbies-Tran R, Guerif F, Bidault R, Royere D. Gene expression in human cumulus cells: one approach to oocyte competence. Hum Reprod. 2007;22:3069–77. doi: 10.1093/humrep/dem336. [DOI] [PubMed] [Google Scholar]
- 55.Cillo F, Brevini TA, Antonini S, Paffoni A, Ragni G, Gandolfi F. Association between human oocyte developmental competence and expression levels of some cumulus genes. Reproduction. 2007;134:645–50. doi: 10.1530/REP-07-0182. [DOI] [PubMed] [Google Scholar]
- 56.Richards JS, Hernandez-Gonzalez I, Gonzalez-Robayna I, Teuling E, Lo Y, Boerboom D, et al. Regulated expression of ADAMTS family members in follicles and cumulus oocyte complexes: evidence for specific and redundant patterns during ovulation. Biol Reprod. 2005;72:1241–55. doi: 10.1095/biolreprod.104.038083. [DOI] [PubMed] [Google Scholar]
- 57.Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci. 2007;120:1330–40. doi: 10.1242/jcs.000968. [DOI] [PubMed] [Google Scholar]