Abstract
Purpose
To evaluate the meiotic spindle and chromosomal distribution of in vitro-matured oocytes from infertile nonobese women with PCOS and male or tubal causes of infertility (controls), and to compare in vitro maturation (IVM) rates between groups.
Methods
Seventy four patients (26 with PCOS and 48 controls) undergoing stimulated cycles of oocyte retrieval for ICSI were selected prospectively. Thirteen PCOS patients and 27 controls had immature oocytes retrieved submitted to IVM. After IVM, oocytes showing extrusion of the first polar body were fixed and processed for evaluation of the meiotic spindle and chromosome distribution by immunofluorescence microscopy.
Results
There were no differences between PCOS and control groups with respect to IVM rates (50.0% and 42.9%, respectively) nor the percentage of meiotic abnormalities in metaphase II oocytes (35.3% and 25%, respectively).
Conclusions
In vitro-matured oocytes obtained from stimulated cycles of nonobese PCOS did not have an increased ratio of meiotic abnormalities.
Keywords: Infertility, In vitro maturation, Meiotic spindle, Polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous syndrome characterized by ovarian hiperandrogenism, luteinizing hormone (LH) hypersecretion, polycystic ovaries (PCO), hyperinsulinemia from insulin resistance, and reduced fecundity [1]. The variable phenotypic expression of reproductive and metabolic abnormalities in PCOS patients leads to differences in oocyte developmental competence [2–5] defined as the ability of the oocyte to complete meiosis and undergo fertilization, embryogenesis, and term development [6].
Despite the increasingly frequent use of assisted reproduction techniques (ART) for the treatment of infertility related to polycystic ovary syndrome (PCOS), it is still uncertain whether the success of these procedures differs between patients with and without this syndrome. Some authors have reported lower fertilization rates after ART in PCOS patients [3, 7]. The role of compromised endometrial receptivity and oocyte quality in contributing to the deleterious effects of PCOS on female fertility is being questioned [4, 8]. It has been postulated that high concentrations of luteinizing hormone (LH) during the follicular phase may result in poor quality oocytes by interfering with folliculogenesis [9]. Other endocrine and metabolic changes associated with this syndrome may also be related to the impairment of oocyte quality, a possibility that needs to be elucidated. Impaired oocyte quality, in turn, may contribute to the reduction of fertilization rates observed after ART.
Studies that have evaluated oocyte quality in patients with PCOS in a noninvasive manner have used the analysis of parameters such as the number of metaphase II oocytes [5, 10] and morphological analysis of the cumulus-oocyte complex [11, 12]. However, in view of the weak correlation of these parameters with subsequent embryo development and consequently with the prognosis of pregnancy, these criteria are assumed not to be adequate predictors of the real oocyte quality.
Oocyte quality depends on proper cytoplasmic and nuclear maturation [6], with the latter requiring the presence of normal cell spindles that guide chromosome segregation during meiosis [13–15] Thus, for a mature oocyte to be prepared for fertilization, the meiotic spindle must maintain its integrity and functionality. No studies are currently available that have compared the cell spindle and chromosome distribution in the oocytes of women with PCOS to those of infertile women without this syndrome. We emphasize that the assessment of oocyte quality by means of a morphological study of the organization and structural distribution of the meiotic spindle is a simple method with good reproducibility that would help characterize one of the potential variables related to the suggested impaired oocyte quality in women with PCOS.
Thus, in the present study, we compared the meiotic spindle and the chromosome distribution of in vitro-matured oocytes obtained from stimulated cycles of infertile nonobese women with PCOS to data from oocytes obtained from a control group consisting of women with infertility resulting from male and/or tubal factors. A secondary objective was the comparison of rates of in vitro maturation (IVM) of immature oocytes obtained in cycles stimulated with gonadotropins between PCOS and control women.
Methods
A prospective study was conducted on infertile patients consecutively treated at the Marital Infertility outpatient clinic of the University Hospital, Faculty of Medicine of Ribeirão Preto, University of São Paulo, from April 2006 to August 2007, and submitted to ovarian stimulation for intracytoplasmic sperm injection (ICSI). The study was approved by the Research Ethics Committee of the institution and all patients evaluated gave written informed consent to participate.
Seventy-four infertile patients (26 PCOS patients with infertility secondary to the exclusive presence of chronic anovulation and 48 patients with infertility secondary to male and/or tubal factors—control group) fulfilled the inclusion criteria during the study period. Thirteen PCOS patients and 27 controls had immature (germinal vesicle or metaphase I) oocytes retrieved and submitted to in vitro maturation (IVM). After IVM, only oocytes showing extrusion of the first polar body were fixed and processed for evaluation of the meiotic spindle and chromosome distribution by immunofluorescence microscopy.
The inclusion criteria for patients with PCOS were confirmation of this diagnosis according to Rotterdam Criteria [1], and absence of associated clinical or gynecologic diseases. The patients in the control group had no pelvic disease associated with infertility when submitted to diagnostic laparoscopy used as part of the procedures for the investigation of infertility. Age ≤ 38 years, body mass index (BMI) < 30 kg/m2, and basal follicle stimulating hormone levels (FSH) < 10 mIU/ml were inclusion criteria for both groups. The presence diabetes mellitus, cardiovascular disease, dyslipidemia, systemic lupus erythematosus and other rheumatologic diseases, HIV infection, any active infection, smoking habit or use of hormonal medications and hormonal or non-hormonal anti-inflammatory medications in the last 6 months were exclusion criteria for both groups.
There were no differences between PCOS and control groups with respect to median age (33 and 35 years, respectively), body mass index (24.5 ± 3.9 kg/m² and 23.6 ± 3.1 kg/m², respectively), and basal FSH levels (5.4 ± 2.5 mIU/ml and 4.8 ± 2.0 mIU/ml, respectively).
Controlled ovarian stimulation (COH) followed the protocol of the sector, which consists of pituitary desensitization with gonadotropin releasing hormone (GnRH, Lupron®, Abott, Brazil) using the long protocol, controlled ovarian stimulation with recombinant follicle stimulating hormone (FSH, Gonal F, Serono, Geneva, Switzerland; Puregon, Organon, The Netherlands) and administration of human urinary chorionic gonadotropin (hCG, Ovidrel®, Serono, São Paulo, Brazil) in order to promote ovulation induction, followed by oocyte retrieval 34 to 36 h later.
Each patient received a daily subcutaneous injection of 0.5 mg leuprolide acetate (Lupron®, Abott, Brazil) starting 10 days before the basal ultrasound exam when ultrasonographic evaluation was performed before the beginning of ovarian stimulation. Two hundred to 300 units of recombinant FSH were used daily during the first 6 days of ovarian stimulation. Ultrasonographic monitoring of the cycle was started on the seventh day of stimulation and performed daily or every 2 days, and the gonadotropin dose was adjusted according to the follicular growth observed. The gonadotropins and the GnRH agonist were discontinued when at least two follicles had a mean diameter of 18 mm, and 250 μg of recombinant hCG was administered at that time at 22:00 h. About 34 to 36 h after hCG administration, each patient underwent oocyte retrieval under intravenous sedation. The aspirated material was analyzed for the identification and isolation of oocyte-cumulus complexes (OCCs). After careful washing, the identified OCCs were cultured for 2 to 4 h. Oocytes were denuded enzymatically with hyaluronidase (H4272 type IV-S, Sigma; 80 IU/mL) for 30 s and, after that, mechanically with the aid of a stripper pipette (130 μm denuding pipette, Cook, Melbourne, Australia).
Immediately after oocyte denudation, we performed morphological analysis for the identification of the degree of oocyte maturity under a light microscope. The oocytes that had reached metaphase II (MII) were considered to be mature and were injected for ICSI. The oocytes that showed signs of mechanical damage or atresia were discarded. The oocytes that were in the germinal vesicle stage (GV, absence of the extruded polar body and presence of the germinal vesicle) or in metaphase I (MI, absence a polar body and of the germinal vesicle) were considered to be immature and were submitted to IVM.
In vitro maturation
Immature denuded oocytes were individually cultured in 25 μL droplets of HTF + 10% SSS medium (previously equilibrated for a period of ≥ 4 h) under mineral oil at 37°C, 95% humidity, and 5% CO2 for a previously defined period of time.
Before the beginning of the present study we constructed a standard curve for IVM in order to determine the mean time of culture necessary for most immature (GV and MI) oocytes to present first polar body (PB) extrusion. Briefly, twenty GV oocytes were cultured for 16 h and then evaluated at 1 h intervals until first PB extrusion (median time of IVM = 19 h, 25th percentile = 18 h, 75th percentile = 20 h, minimum = 17 h, and maximum = 24 h). Twenty-nine MI oocytes were cultured for 3 h and then evaluated at 30 min intervals until first PB extrusion (median time = 4 h, 25th percentile = 3.5 h, 75th percentile = 4.5 h, minimum = 3 h, and maximum = 7 h).
Thus, GV oocytes were cultured for 19 ± 1 h and MI oocytes were cultured for 4 h ± 30 min. After this pre-established culture period, the degree of oocyte maturation was analyzed again, and oocytes showing extrusion of the first PB were fixed for analysis by immunofluorescence microscopy in order to identify the cell spindle and chromosome distribution. We discarded all oocytes that did not show first PB extrusion after the incubation period together with any degenerated oocytes.
The IVM rate was calculated for both groups by dividing the number of oocytes showing extrusion of the first PB × 100 by the total number of immature oocytes submitted to IVM.
Immunofluorescence microscopy of tubulin and chromatin
Denuded oocytes were fixed and extracted for 30 min at 37°C in a microtubule-stabilizing buffer as previously described [16, 17]. Oocytes were washed extensively and blocked overnight at 4°C in the wash medium (PBS supplemented with 0.02% NaN3, 0.01% Triton X-100, 0.2% nonfat dry milk, 2% goat serum, 2% BSA and 0.1 M of glycine). They were then incubated with β-tubulin mouse monoclonal antibodies (1:150), washed again and incubated with fluorescein isothiocyanate-conjugated anti-mouse IgG (1:200; Molecular Probes, OR) at 37°C for 2 h. After being washed, the samples were stained for DNA with Hoechst 33342 (10 μg/ml) in Vectashield mounting medium (H-1000, Vector, Burlingame, CA) on a glass slide and sealed. The samples were observed under a Zeiss Axiovert 100TV inverted fluorescence microscope.
Oocyte classification based on meiotic spindle morphology and chromosome configuration
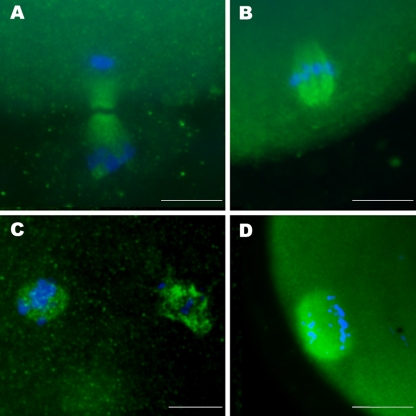
Oocytes were considered to be in telophase I when they had an elongated cell spindle perpendicular to the oocyte membrane with chromosomes distributed at the ends (Fig. 1a). For better accuracy, oocytes in MII were subdivided into analyzable and non-analyzable structures. Oocytes were termed analyzable when they presented their spindles in a lateral or sagittal view and non-analyzable when they presented spindles in a polar view, preventing an overall evaluation of the spindle and only permitting the view of chromosome arrangement. The spindle configuration of MII oocytes was regarded as morphologically normal when a barrel-shaped structure with slightly pointed poles linked by organized microtubules could be observed under fluorescent microscopy (Fig. 1b). Chromosome configuration was regarded as normal when chromosomes were arranged on a compact metaphase plate at the equator of the structure (Fig. 1b). Abnormal chromosome organization included chromosomal misalignment (Fig. 1c) and dispersal of chromosomes (Fig. 1d). Thus, meiotic abnormalities consisted of spindle disruption or chromosomal misalignment, fragmentation, or dispersal. Parthenogenetic activation was considered to be present when oocytes were at anaphase II or telophase II (with or without extrusion of the second polar body).
Fig. 1.
Fluorescence microscopy images from cell spindle and chromosomes. a Oocyte in normal telophase I showing an elongated cell spindle perpendicular to the oocyte membrane with chromosomes distributed at the ends. b Chromosomes properly aligned in the median portion of a normal barrel-shaped spindle of a metaphase II oocyte. c Chromosome misalignments over a normal barrel-shaped spindle of a metaphase II oocyte. d Chromosome spreading over a normal spindle of a metaphase II oocyte. Green: microtubules forming the cell spindle; Blue: chromosomes. Scale bar = 10 μm
Statistical analysis
Statistical analysis was performed using GraphPad 5.0 for Windows (GraphPad Software Inc., San Diego, CA, USA). We compared the observed proportions between the two groups by using Fisher’s exact test. P-value <0.05 was considered as statistically significant.
Results
Of the 34 immature oocytes obtained from the PCOS group, 17 showed extrusion of the first polar body after IVM; this resulted in a 50.0% IVM rate. From the 56 oocytes obtained from the control group, 24 presented extrusion of the first polar body resulting in a 42.9% IVM rate. No significant difference in IVM rates was observed between the two groups
Four oocytes from the control group were not analyzed owing to staining failure or loss of the cells during processing. Thus, 20 in vitro-matured oocytes from the control group (11 in GV and 9 in MI) and 17 oocytes from the PCOS group (7 in GV and 10 in MI) were analyzed by immunofluorescence microscopy.
First, in vitro-matured oocytes in the GV and MI stages were pooled together for analysis. In the PCOS group, 3 (17.6%) of 17 oocytes analyzed by fluorescence microscopy were in normal telophase I and 14 (82.4%) were in metaphase II (29.4% of normal MII). In the control group, 2 (10.0%) of 20 oocytes analyzed by fluorescence microscopy were in normal telophase I, 15 (75.0%) were in metaphase II (30.0% of normal MII), and 3 (15.0%) expressed signs of parthenogenetic activation. Three and 4 MII oocytes, respectively from the PCOS and control groups, were fixed with their spindles in a polar view, being considered non-analyzable in the study. The incidence of oocyte meiotic abnormalities, characterized by spindle disruption and/or chromosomal misalignment visualized by fluorescence microscopy after immunostaining, did not differ significantly between groups (35.3% and 25%, respectively for PCOS and control oocytes; Table 1).
Table 1.
Analysis of the cell spindle and chromosomal configuration of in vitro matured oocytes obtained from stimulated cycles of women with PCOS and from women with infertility due to male and/or tubal factors (control group)
| PCOS | Control | p | |
|---|---|---|---|
| Germinal vesicle oocytes | |||
| Analyzed oocytes | 17 | 20 | |
| Normal Telophase I—N (%) | 3 (17.6) | 2 (10.0) | 0.64 |
| Normal Metaphase II—N (%) | 5 (29.4) | 6 (30.0) | 1.00 |
| Abnormal Metaphase II—N (%) | 6 (35.3) | 5 (25.0) | 0.72 |
| Non-analyzable Metaphase II—N (%) | 3 (17.6) | 4 (20.0) | 1.00 |
| Parthenogenetic activation—N (%) | 0 (0) | 3 (15.0) | 0.23 |
N number, PB polar body, IVM in vitro maturation; analyzed oocytes: oocytes obtained from gonadotropin-stimulated cycles that were analyzed by fluorescence microscopy after observation of the first PB extrusion after IVM; non-analyzable Metaphase II: oocytes fixed with spindles in a polar view; p value obtained by Fisher’s exact test
We also analyzed in vitro-matured oocytes in GV and MI separately in each group. Considering oocytes originating from GV, in the PCOS group, 2 (28.6%) of 7 oocytes analyzed were in normal telophase I and 5 (71.4%) were in metaphase II (2 normal, 2 abnormal, and 1 non-analyzable). In the control group, 2 (18.2%) of 11 oocytes were in normal telophase I, 7 (63.6%) were in metaphase II (2 normal, 3 abnormal, and 2 non-analyzable), and 2 (18.2%) expressed signs of parthenogenetic activation. Considering oocytes originating from MI, in the PCOS group, 1 oocyte (10%) was in normal telophase I and 9 (90%) were in metaphase II (3 normal, 4 abnormal, and 2 non-analyzable). In the control group, all 9 oocytes originating from MI were in metaphase II (4 normal, 2 abnormal, and 2 non-analyzable). The incidence of meiotic abnormalities of oocytes also did not differ significantly between groups when oocytes originated either from GV (28.6% and 27.3% of abnormal MII, respectively, in the PCOS and control groups) or MI (40% and 20% of abnormal MII, respectively, in the PCOS and control groups) were analyzed separately.
Discussion
To our knowledge the present study was the first to assess the proportions of meiotic anomalies in in vitro-matured oocytes from stimulated cycles of patients with PCOS compared to those of women with infertility due to tubal and/or male factors (control group). There is evidence in the literature supporting the fact that obesity [2, 18] may affect oocyte quality and consequently influence the results of ART, reducing fertilization rates and increasing spontaneous abortion rates in patients with PCOS [3, 7]. Thus, in the present study, we only evaluated oocytes from non-obese PCOS patients and controls to avoid this confounding factor. The preliminary data obtained in the present study did not demonstrate a significant difference in the frequency of meiotic abnormalities, characterized by spindle disruption and/or chromosomal misalignment, between the two groups studied. As GV and MI oocytes might have a different ability to progress to MII and support the constitution of a normal MII spindle, the frequency of spindle and chromosome normality in the PCOS and control groups was also analyzed separately in in vitro-matured oocytes originating from GV and MI. Despite the small sample size evaluated, the incidence of meiotic abnormalities of oocytes also did not differ significantly between groups when oocytes originating from GV or MI were analyzed separately. The present data suggest that meiotic abnormalities are not responsible for compromised oocyte quality in non-obese infertile PCOS patients submitted to ovarian stimulation for ART. However, further evaluation is needed in studies with larger sample sizes analyzing in vivo-matured oocytes.
We found only one published report evaluating the incidence of meiotic anomalies of in vitro matured human oocytes from patients with PCOS, which detected a 43.7% frequency of cell spindle anomalies and a 33.3% frequency of chromosome abnormalities [19]. However, the study was designed to test the specific hypothesis that human oocyte IVM results in a higher percentage of meiotic abnormalities compared with oocytes matured in vivo and all oocytes were obtained from PCOS women. Although the in vitro matured oocytes analyzed in the cited study were obtained in unstimulated cycles, the proportion of meiotic anomalies observed (43.7% in the cell spindle) was similar to that found in the present study (35.3%) where only oocytes that presented their spindles in a lateral or sagittal view were considered analyzable by allowing an overall evaluation of the spindle and chromosome configuration.
Cell spindles and chromosome distribution from in vivo-matured instead of in vitro matured oocytes should be evaluated to properly associate the presence of meiotic oocyte anomalies with ART results [20–22]. However, the scarcity of available mature human oocytes for invasive research that prevent the clinical use of this material, as is the case for immunofluorescence microscopy used in the present study, justified the use of immature oocytes obtained from gonadotropin-stimulated cycles for ICSI which presented extrusion of the first polar body after IVM. However, the methodology used has limitations. First, the oocytes used for the study may have failed to mature in vivo despite ovarian stimulation due to intrinsic incompetence to support a normal meiotic maturation, a fact that might be potentially associated with a higher frequency of meiotic anomalies. Second, immature oocytes (GV and MI) were matured in the absence of companion cumulus cells , a fact possibly related to a higher frequency of meiotic and spindle anomalies [23], irrespective of the original oocyte quality. Third, IVM itself is known to cause a significant increase in the occurrence of meiotic abnormalities, as previously described [24]. Last, the low proportion of immature oocytes obtained in stimulated cycles and the need to subject them to IVM before fixation for immunofluorescence limited the supply of in vitro-matured oocytes for the current study, justifying the very low number of samples analyzed. Therefore we cannot extrapolate the proportion of meiotic abnormalities detected here in in vitro-matured oocytes obtained from stimulated cycles to that occurring in in vivo-matured oocytes. However, we emphasize that the same methodology was also used for the control group in order to minimize these potential confounding factors.
The cell spindle of the oocyte is a highly dynamic structure that is extremely sensitive to the action of various factors [25–27]. These factors may affect the spindle, causing tubulin depolymerization and chromosome dislocation from the metaphase equator and generating meiotic anomalies. Some investigators have demonstrated that the time of culture after the completion of nuclear maturation may promote metaphase aging of the oocyte, which may also be one of the factors related to the increased incidence of meiotic abnormalities [28]. This justified the construction of a standard IVM curve in order to establish the mean culture time under the conditions and with the culture medium used in the present study at which most oocytes would reach metaphase II. Thus, only immature oocytes that presented extrusion of first PB after the predefined culture time were used and analyzed in the present study.
In vitro maturation rates were found to not differ significantly between groups. The relatively high yield of this strategy could thus be of practical utility, especially in cases where few mature oocytes are retrieved and immature oocytes are available. Futures studies will be important for determining viability and competence for the subsequent development of embryos from in vitro-matured oocytes obtained from stimulated cycles.
Conclusions
In summary, the present pilot study did not demonstrate a significant difference neither in IVM rates nor in the frequency of meiotic anomalies between in vitro-matured oocytes from stimulated cycles of nonobese patients with PCOS and those from women with infertility due to tubal and/or male factors. The small series, study design, and the possibility that adverse follicular conditions (such as altered levels of androgen, insulin, and growth factors, among others) in PCOS patients could occur during the process of meiotic resumption and progression to MII may compromise the extrapolation of our results to in vivo-matured oocytes. However, if the present findings can be confirmed in studies on larger series, they will support the hypothesis that damage to the meiotic spindle and the chromosome distribution of MII oocytes are not responsible for the suggested worsening of oocyte quality in infertile nonobese women with PCOS. Future studies conducted on larger series and, ideally, evaluating in vivo-matured oocytes are still necessary before further conclusions.
Acknowledgments
Financial support Rodolpho Cruz Vieira was supported by a scholarship granted by National Council of Research—CNPq—Brazil. CNPq 478396/2007-4
Footnotes
Capsule
Preliminary data from a prospective controlled study did not demonstrate an increased ratio of meiotic abnormalities in in vitro-matured oocytes from nonobese PCOS women.
References
- 1.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25. [DOI] [PubMed]
- 2.Fedorcsak P, Dale PO, Storeng R, Tanbo T, Abyholm T. The impact of obesity and insulin resistance on the outcome of IVF or ICSI in women with polycystic ovarian syndrome. Hum Reprod. 2001;16:1086–1091. doi: 10.1093/humrep/16.6.1086. [DOI] [PubMed] [Google Scholar]
- 3.Heijnen EM, Eijkemans MJ, Hughes EG, Laven JS, Macklon NS, Fauser BC. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:13–21. doi: 10.1093/humupd/dmi036. [DOI] [PubMed] [Google Scholar]
- 4.Patel SS, Carr BR. Oocyte quality in adult polycystic ovary syndrome. Semin Reprod Med. 2008;26:196–203. doi: 10.1055/s-2008-1042958. [DOI] [PubMed] [Google Scholar]
- 5.Sahu B, Ozturk O, Ranierri M, Serhal P. Comparison of oocyte quality and intracytoplasmic sperm injection outcome in women with isolated polycystic ovaries or polycystic ovarian syndrome. Arch Gynecol Obstet. 2008;277:239–244. doi: 10.1007/s00404-007-0462-x. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira EM, Vireque AA, Adona PR, Meirelles FV, Ferriani RA, Navarro PA. Cytoplasmic maturation of bovine oocytes: structural and biochemical modifications and acquisition of developmental competence. Theriogenology. 2009;71:836–848. doi: 10.1016/j.theriogenology.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Homburg R. Pregnancy complications in PCOS. Best Pract Res Clin Endocrinol Metab. 2006;20:281–292. doi: 10.1016/j.beem.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Giudice LC. Endometrium in PCOS: Implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab. 2006;20:235–244. doi: 10.1016/j.beem.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Regan L, Owen EJ, Jacobs HS. Hypersecretion of luteinising hormone, infertility, and miscarriage. Lancet. 1990;336:1141–1144. doi: 10.1016/0140-6736(90)92765-A. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig M, Finas DF, al-Hasani S, Diedrich K, Ortmann O. Oocyte quality and treatment outcome in intracytoplasmic sperm injection cycles of polycystic ovarian syndrome patients. Hum Reprod. 1999;14:354–358. doi: 10.1093/humrep/14.2.354. [DOI] [PubMed] [Google Scholar]
- 11.Kodama H, Fukuda J, Karube H, Matsui T, Shimizu Y, Tanaka T. High incidence of embryo transfer cancellations in patients with polycystic ovarian syndrome. Hum Reprod. 1995;10:1962–1967. doi: 10.1093/oxfordjournals.humrep.a136217. [DOI] [PubMed] [Google Scholar]
- 12.Tarlatzis BC, Grimbizis G, Pournaropoulos F, Bontis J, Lagos S, Spanos E, et al. The prognostic value of basal luteinizing hormone:follicle-stimulating hormone ratio in the treatment of patients with polycystic ovarian syndrome by assisted reproduction techniques. Hum Reprod. 1995;10:2545–2549. doi: 10.1093/oxfordjournals.humrep.a135742. [DOI] [PubMed] [Google Scholar]
- 13.Santis L, Cino I, Rabellotti E, Calzi F, Persico P, Borini A, et al. Polar body morphology and spindle imaging as predictors of oocyte quality. Reprod Biomed Online. 2005;11:36–42. doi: 10.1016/S1472-6483(10)61296-5. [DOI] [PubMed] [Google Scholar]
- 14.Volarcik K, Sheean L, Goldfarb J, Woods L, Abdul-Karim FW, Hunt P. The meiotic competence of in-vitro matured human oocytes is influenced by donor age: evidence that folliculogenesis is compromised in the reproductively aged ovary. Hum Reprod. 1998;13:154–160. doi: 10.1093/humrep/13.1.154. [DOI] [PubMed] [Google Scholar]
- 15.Blerkom J, Davis P. Differential effects of repeated ovarian stimulation on cytoplasmic and spindle organization in metaphase II mouse oocytes matured in vivo and in vitro. Hum Reprod. 2001;16:757–764. doi: 10.1093/humrep/16.4.757. [DOI] [PubMed] [Google Scholar]
- 16.Allworth AE, Albertini DF. Meiotic maturation in cultured bovine oocytes is accompanied by remodeling of the cumulus cell cytoskeleton. Dev Biol. 1993;158:101–112. doi: 10.1006/dbio.1993.1171. [DOI] [PubMed] [Google Scholar]
- 17.Navarro PA, Liu L, Ferriani RA, Keefe DL. Arsenite induces aberrations in meiosis that can be prevented by coadministration of N-acetylcysteine in mice. Fertil Steril. 2006;85(Suppl 1):1187–1194. doi: 10.1016/j.fertnstert.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 18.Wang JX, Davies MJ, Norman RJ. Polycystic ovarian syndrome and the risk of spontaneous abortion following assisted reproductive technology treatment. Hum Reprod. 2001;16:2606–2609. doi: 10.1093/humrep/16.12.2606. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Feng HL, Cao YJ, Zheng GJ, Yang Y, Mullen S, et al. Confocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitro. Fertil Steril. 2006;85:827–832. doi: 10.1016/j.fertnstert.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 20.Lee ST, Han HJ, Oh SJ, Lee EJ, Han JY, Lim JM. Influence of ovarian hyperstimulation and ovulation induction on the cytoskeletal dynamics and developmental competence of oocytes. Mol Reprod Dev. 2006;73:1022–1033. doi: 10.1002/mrd.20500. [DOI] [PubMed] [Google Scholar]
- 21.Baart EB, Martini E, Eijkemans MJ, Opstal D, Beckers NG, Verhoeff A, et al. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod. 2007;22:980–988. doi: 10.1093/humrep/del484. [DOI] [PubMed] [Google Scholar]
- 22.Grupen CG, Gilchrist RB, Nayudu PL, Barry MF, Schulz SJ, Ritter LJ, et al. Effects of ovarian stimulation, with and without human chorionic gonadotrophin, on oocyte meiotic and developmental competence in the marmoset monkey (Callithrix jacchus) Theriogenology. 2007;68:861–872. doi: 10.1016/j.theriogenology.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Barrett SL, Albertini DF. Cumulus cell contact during oocyte maturation in mice regulates meiotic spindle positioning and enhances developmental competence. J Assist Reprod Genet. 2010;27:29–39. doi: 10.1007/s10815-009-9376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barcelos ID, Vieira RC, Ferreira EM, Martins WP, Ferriani RA, Navarro PA. Comparative analysis of the spindle and chromosome configurations of in vitro-matured oocytes from patients with endometriosis and from control subjects: a pilot study. Fertil Steril. 2009;92:1749–1752. doi: 10.1016/j.fertnstert.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Hu Y, Betzendahl I, Cortvrindt R, Smitz J, Eichenlaub-Ritter U. Effects of low O2 and ageing on spindles and chromosomes in mouse oocytes from pre-antral follicle culture. Hum Reprod. 2001;16:737–748. doi: 10.1093/humrep/16.4.737. [DOI] [PubMed] [Google Scholar]
- 26.Mullen SF, Agca Y, Broermann DC, Jenkins CL, Johnson CA, Critser JK. The effect of osmotic stress on the metaphase II spindle of human oocytes, and the relevance to cryopreservation. Hum Reprod. 2004;19:1148–1154. doi: 10.1093/humrep/deh201. [DOI] [PubMed] [Google Scholar]
- 27.Navarro PA, Liu L, Keefe DL. In vivo effects of arsenite on meiosis, preimplantation development, and apoptosis in the mouse. Biol Reprod. 2004;70:980–985. doi: 10.1095/biolreprod.103.020586. [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Keefe DL. Ageing-associated aberration in meiosis of oocytes from senescence-accelerated mice. Hum Reprod. 2002;17:2678–2685. doi: 10.1093/humrep/17.10.2678. [DOI] [PubMed] [Google Scholar]