Abstract
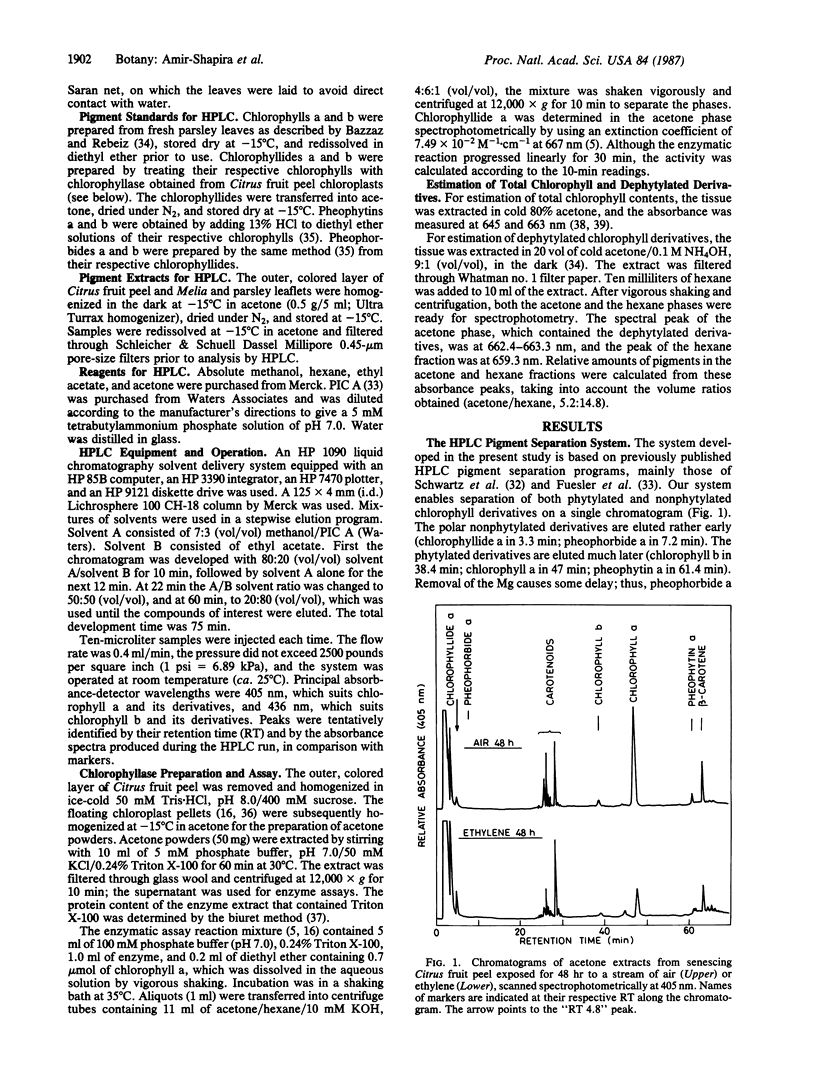
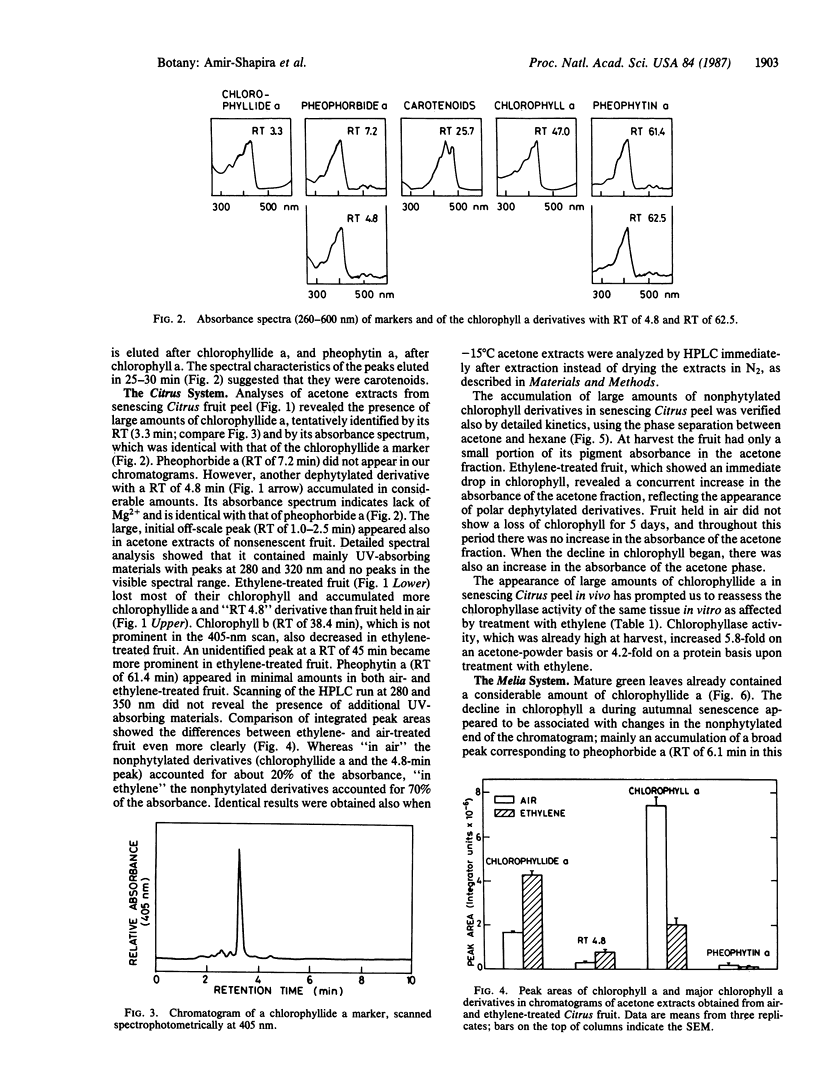
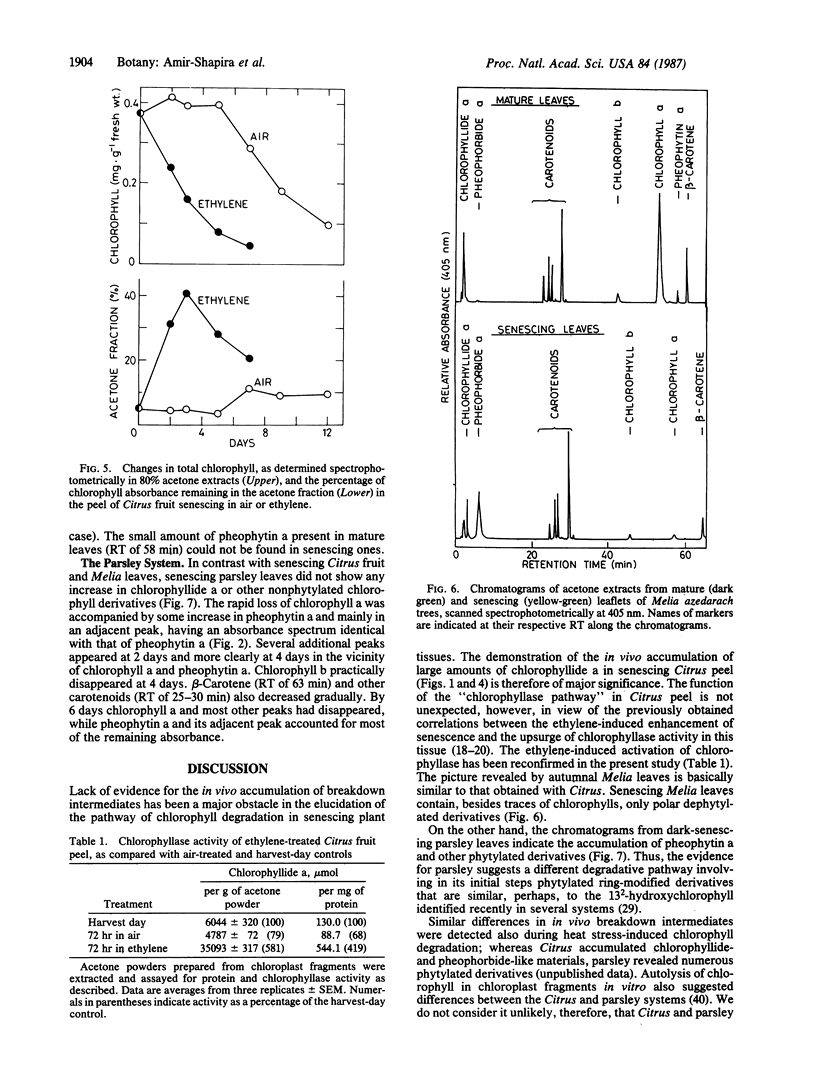
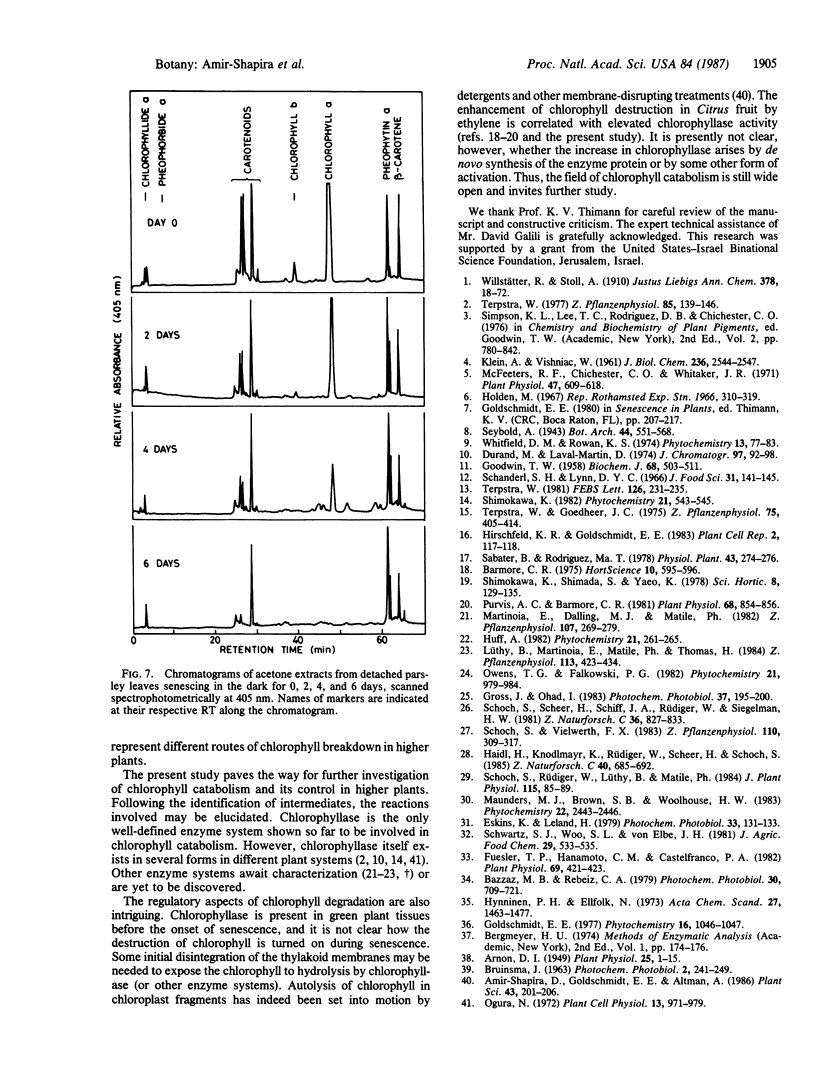
High-pressure liquid chromatography was used to separate chlorophyll derivatives in acetone extracts from senescing Citrus fruit peel, autumnal Melia azedarach L. leaves, and dark-held detached parsley (Petroselinum sativum L.) leaves. Chlorophyllide a and another polar, dephytylated derivative accumulated in large amounts in senescing Citrus peel, particularly in fruit treated with ethylene. Ethylene also induced a 4-fold increase in the specific activity of Citrus chlorophyllase (chlorophyll chlorophyllidohydrolase, EC 3.1.1.14). Detailed kinetics based on a hexane/acetone solvent partition system showed that the in vivo increase in dephytylated derivatives coincided with the decrease in total chlorophyll. Polar, dephytylated derivatives accumulated also in senescing Melia leaves. Senescing parsley leaves revealed a very different picture. The gradual disappearance of chlorophyll a was accompanied by an increase in pheophytin a and by the transient appearance of several phytylated derivatives. Only pheophytin a and an adjacent peak were left when all the chlorophyll a had disappeared. The pathways for breakdown of chlorophyll in the Citrus and parsley senescence systems are discussed.
Keywords: chlorophyllide, pheophorbide, pheophytin, ethylene, chlorophyllase
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuesler T. P., Hanamoto C. M., Castelfranco P. A. Separation of Mg-Protoporphyrin IX and Mg-Protoporphyrin IX Monomethyl Ester Synthesized de novo by Developing Cucumber Etioplasts. Plant Physiol. 1982 Feb;69(2):421–423. doi: 10.1104/pp.69.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODWIN T. W. Studies in carotenogenesis. 24. The changes in carotenoid and chlorophyll pigments in the leaves of deciduous trees during autumn necrosis. Biochem J. 1958 Mar;68(3):503–511. doi: 10.1042/bj0680503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN A. O., VISHNIAC W. Activity and partial purification of chlorophyllase in aqueous systems. J Biol Chem. 1961 Sep;236:2544–2547. [PubMed] [Google Scholar]
- McFeeters R. F., Chichester C. O., Whitaker J. R. Purification and Properties of Chlorophyllase from Ailanthus altissima (Tree-of-Heaven). Plant Physiol. 1971 May;47(5):609–618. doi: 10.1104/pp.47.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis A. C., Barmore C. R. Involvement of ethylene in chlorophyll degradation in peel of citrus fruits. Plant Physiol. 1981 Oct;68(4):854–856. doi: 10.1104/pp.68.4.854. [DOI] [PMC free article] [PubMed] [Google Scholar]



