Abstract
Purpose
To evaluate levels of DNA fragmentation and chromosomal abnormalities in ejaculated sperm of males with isolated teratozoospermia and to determine if specific sperm morphological types occur simultaneously with these nuclear defects.
Methods
Sperm obtained from isolated teratozoospermic men (n = 70) and fertile men (n = 30) were analysed using fluorescence in situ hybridization and TUNEL assay.
Results
Teratozoospermic men, compared to fertile men, showed significantly higher rates of sex chromosomes disomy, and diploidy. Significant correlations were found between amorphous head, microcephalic head, short tail, and sex chromosomes disomy. Level of sperm DNA fragmentation was significantly higher in teratozoospermic men than in controls and positively correlated to the incidence of macrocephalic heads, amorphous heads, and short flagella.
Conclusions
Patients with isolated teratozoospermia have increased levels of DNA fragmentation and chromosomal aneuploidy. Some specific morphological abnormalities were shown to be predictive of chromosomal abnormalities and DNA alteration.
Keywords: Aneuploidy, DNA fragmentation, Infertility, Teratozoospermia
Introduction
Teratozoospermia is characterized by the presence of spermatozoa with abnormal morphology in sperm. This condition is frequently associated with infertility and intracytoplasmic sperm injection (ICSI) is frequently used as the treatment of choice. Selection of sperm for ICSI involves the pickup of a motile and morphologically normal-appearing sperm. In cases of severe teratozoospermia, spermatozoa with least abnormalities are used. However, the use of ICSI has created consequential debate concerning the genetic risk for the offspring [1, 2]. Fluorescence in situ hybridization technique (FISH), allowing the specific identification of human chromosomes in sperm nuclei, has been widely used to study chromosomes abnormalities in sperm from infertile men. Some authors have reported a higher frequency of sperm aneuploidy rate in patients with abnormal semen parameters compared to normal controls, at least for some chromosomes [3–5]. Conversely, other studies have failed to show any significant difference in sperm aneuploidy rate between fertile and infertile patients [6, 7]. It is quite likely that some subsets of infertility have an elevated risk of sperm chromosomal abnormalities whereas others do not. Most of the studies have concentrated on infertile men with oligoasthenoteratozoospermia (OAT) and fewer studies have analyzed infertile men with isolated teratozoospermia and the correlation between specific sperm morphology and chromosomal abnormality is still relatively limited.
Sperm may also be genetically defective at the proportion of spermatozoa with DNA fragmentation. Over the last years, the integrity of sperm DNA is being recognized as a new parameter of semen quality and a potential fertility predictor [8, 9]. Sperm DNA fragmentation consists of single and double stranded DNA breaks, frequently occurring in semen of subfertile patients, and now is more and more clearly designated as obvious parameters in early or late failures of assisted reproductive technologies (ART) [10–12]. Taken together, sperm morphology may serve more as a barrier to reaching and penetrating the oocyte zona, and the sperm DNA fragmentation may affect cycle outcome, embryonic development, and blastocyst formation [11, 12]. Therefore, determination of correlations between specific forms of sperm morphological abnormalities, elevated degrees of DNA fragmentation and chromosomal abnormalities in sperm could be of great importance for successful outcome of the ART, especially ICSI. Thus, the aim of the present study was to evaluate levels of DNA fragmentation and to analyze the meiotic segregation in ejaculated sperm of males with isolated teratozoospermia. We then aimed to determine if specific sperm morphological types occur simultaneously with levels of DNA fragmentation and chromosomal abnormalities.
Materials and methods
Study design and inclusion criteria
A prospective controlled study was employed, involving 70 patients presenting for infertility evaluation at our department of Cytogenetic and Reproductive Biology, Farhat Hached University teaching hospital, Sousse, Tunisia. This study group consists of isolated teratozoospermic men whose sub-infertility is determined mainly by less than normal levels of morphologically normal sperm (<20 %), with normal values of sperm concentration (>20 × 106 sperm/ml) and motility (>50% of sperm are progressively motile). In addition 30 healthy men with normal semen profiles and proven fertility were recruited as controls. Semen samples were collected by masturbation in to sterile cups following 3 days of sexual abstinence. After liquefaction of the sperm, standard semen parameters were evaluated according to the World Health Organization [13] guidelines. Detailed morphological assessment according to the David classification [14] was performed on each semen sample to provide a breakdown of the specific types of morphological abnormalities found in the sperm.
All subjects in either group had a normal 46, XY karyotype and testicular volume within the normal range, and they had no history of radiotherapy, chemotherapy, chronic illness, medication or varicocele.
This protocol was approved by the local ethics committee and all patients and controls had previously given informed consent for the study.
Semen preparation for FISH analysis and TUNEL assay
An aliquot of the fresh semen was washed twice in Phosphate Buffered Saline (PBS, pH 7.4) and centrifuged at 400 g for 5 minutes. The sediment was then fixed in methanol/acetic acid (3: 1) for at least 30 min at 4°C. The fixed specimens were smeared on slides and stored at −20°C until further processing.
Aneuploidy analysis
Fluorescence in situ hybridization, which employs sequence-specific DNA probes incorporated with fluorescently labeled nucleotides, was carried out on each patient and control, using alpha centromeric probes for chromosomes 8, X, and Y. The probes were provided by the University of Bari (Bari, Italy).
Sperm head decondensation
In order to render the sperm chromatin accessible to DNA probes, slides were incubated in NaOH 1 N, at room temperature for 2 min. The slides were distilled water washed. Then, they were dehydrated through an ethanol series (70-90-100%) and air-dried.
DNA probes
The probe mixture for triple FISH consisted of a repetitive DNA sequence of centromeric probes for chromosome X (pDMX1) labeled fluorescein isothiocyanate (FITC), for chromosome Y (pLAY5.5) labeled Rhodamine and for chromosome 8 (pZ8.4) labeled FITC and Rhodamine. The use of an autosomal probe, in addition to X and Y probes, allowed the distinction between disomy and diploidy.
Hybridization procedure
Slides were incubated in a denaturation solution of 70% formamide, 20X standard saline citrate (SSC) (pH 5,3) and distilled water at 72°C for 2 min. Slides were snap-cooled in 70% ethanol at −20°C for 2 min and then dehydrated through an ethanol series (90–100%) at room temperature. The probes, precipitated and denatured at 72°C for 8 min, were applied directly to the slides which were then covered with a cover slip and sealed with rubber cement. Slides were hybridized for 2 hours in a dark humidified container at 37°C. Finally slides were washed in 1x SSC, counterstained with 4′,6-diamidino-2-pheneylindole (DAPI) and stored in the dark at 4°C prior to carrying out microscopic observation.
Scoring criteria
The slides were observed using an Axioplan epifluorescence microscope (Leica, Wetzlar, Germany) with the appropriate set of filters: single band DAPI, FITC, and Rhodamine. For each probe a minimum of 500 spermatozoa were counted per patient. Only intact spermatozoa bearing a similar degree of decondensation and clear hybridization signals were scored; disrupted or overlapping spermatozoa were excluded from analysis.
Measurement of DNA fragmentation
The presence of apoptosis-related DNA strand breaks in spermatozoa was evaluated by the terminal desoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick-end labeling (TUNEL) assay, using the ApopTag® Apoptosis Detection Kits (QBiogene, Paris, France) in controls and patients. For cell permeabilization, slides were incubated in phosphate buffer saline (PBS) with a solution of 1% Triton X100 (Sigma). The rest of the procedure was carried out according to the manufacturer’s instruction. Briefly, the specimens were washed twice in PBS 1X, equilibrated with the equilibration buffer at room temperature for 10 seconds and incubated in a dark moist chamber at 37°C, for 1 h, with the Terminal Desoxynucleotidyl Transferase (TdT) solution in order to allow DNA elongation. After stopping the enzyme reaction, the slides were washed twice in PBS and the DNA elongation was revealed by incubation of the cells with anti-digoxigenin antibody coupled to peroxidase, during 30 min in a dark moist chamber. The peroxidase was revealed with DiAminoBenzidine (DAB). Slides were then counterstained with Harris' haematoxylin (RAL, Martillac, France) and finally mounted using Faramount mounting (Dako, Carpinteria, CA, USA).
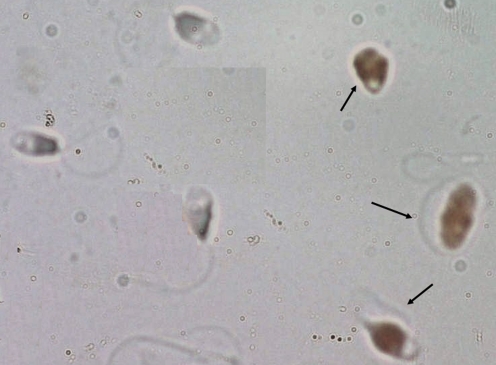
Slides were observed under a microscope (Zeiss, Oberkochen, Germany) equipped with a 100 magnification lens. Spermatozoa with fragmented DNA had brown-colored nuclei, whereas the other cells were blue-gray (counter coloration with Harris’s haematoxylin) (Fig. 1). On each slide, approximately 500 cells were counted, and the DNA fragmentation index (DFI) was calculated.
Fig. 1.
Detection of DNA fragmentation with TUNEL assay in patients with isolated teratozoospermia: Spermatozoa with DNA fragmentation were brown (arrow), and spermatozoa with intact DNA were gray blue
Statistical analysis
Statistical analysis was performed using SPSS.13 (SPSS, Chicago, IL, USA). All variables were initially tested in order to determine variance homogeneity and data normality. Data are represented as Mean ± standard deviation (SD). The student's t-test was used to compare the levels of DNA fragmentation and chromosomal abnormalities between controls and teratozoospermic subjects. Pearson’s correlation was performed to determine if specific sperm morphological types occur simultaneously with levels of DNA fragmentation and chromosomal abnormalities. All hypothesis testing was two-sided with a probability value of 0.05 deemed as significant.
Results
Semen characteristics and detailed morphology assessment
The average age of the teratozoospermic men was not significantly different from that of the controls (37.02 ± 6.63 vs 36.76 ± 5.62, respectively, P > 0.05). The seminal parameters and the results of the detailed morphological assessment for these two groups were reported in Table 1. All 70 patients had isolated teratozoospermia with mean volume of 3 ± 1.12 ml, mean concentration of 65.07 ± 43.83 X 106/ml, mean of total progressive motility of 51.52 ± 2.32 % and mean percentage fraction of sperm with abnormal forms of 90.86 ± 5.70%. Sperm head abnormalities were significantly more frequent in the teratozoospermic patients compared with the controls (p < 0.05), with predominance of the amorphous heads (12.72 ± 8.50), tapered heads (22.00 ± 13.60) and microcephalic heads (29.74 ± 16.94). For the tails abnormalities also, there are a significant difference between the two groups (p < 0.05); especially for short tail (1.86 ± 2.17 vs 7.28 ± 5.95), bent tail (9.00 ± 3.34 vs 15.68 ± 6.1), and coiled tail (4.76 ± 4.06 vs 7.24 ± 6.59).
Table 1.
Semen analysis results for men with normal semen analysis (control group) and men with teratozoospermia (study group)
| Control (n = 30) | Teratozoospermic men (n = 70) | P value | |
|---|---|---|---|
| Volume (ml) | 3.2 ± 1.43 | 3.00 ± 1.12 | 0.518 |
| pH | 7.74 ± 0.24 | 7.76 ± 0.14 | 0.55 |
| Necrozoospermia (%) | 16.67 ± 4.40 | 22.32 ± 6.08 | 0.01 |
| Concentration (X 106/ml) | 139.72 ± 59.97 | 65.07 ± 43.83 | 0.001 |
| Sperm motility (%) | 55.51 ± 5.31 | 51.52 ± 2.32 | 0.510 |
| Atypical forms (%) | 54.33 ± 10.65 | 90.86 ± 5.70 | 0.001 |
| Macrocephalic (%) | 2.62 ± 2.63 | 6.08 ± 6.11 | 0.015 |
| Microcephalic (%) | 14.86 ± 12.37 | 29.74 ± 16.94 | 0.001 |
| Amorphous head (%) | 8.00 ± 5.71 | 12.72 ± 8.50 | 0.023 |
| Tapered head (%) | 11.19 ± 11.94 | 22.00 ± 13.60 | 0.002 |
| Double head (%) | 0.43 ± 0.74 | 0.70 ± 1.05 | 0.28 |
| Acrosomal abnormalities (%) | 15.67 ± 9.57 | 31.78 ± 12.87 | 0.001 |
| Cytoplasmic droplet (%) | 6.19 ± 3.38 | 4.32 ± 3.52 | 0.043 |
| Two tailed (%) | 0.19 ± 0.402 | 0.4 ± 0.88 | 0.301 |
| Coiled tail (%) | 4.76 ± 4.06 | 7.24 ± 6.59 | 0.011 |
| Bent tail (%) | 9.00 ± 3.34 | 15.68 ± 6.1 | 0.001 |
| Short tail (%) | 1.86 ± 2.17 | 7.28 ± 5.95 | 0.001 |
| Multiple anomalies index | 1.41 ± 0.147 | 1.56 ± 0.19 | 0.003 |
Analysis of meiotic segregation
The results of triple color FISH were reported in Table 2 and illustrated in Fig. 2. In comparison with the aneuploidy rates, between controls and teratozoospermic subjects, the total aneuploidy (combined disomy and nullisomy of all chromosomes analyzed) of the teratozoospermic men was significantly higher from that of the controls (5.74 ± 1.36% vs 1.48 ± 0.22%, P < 0.01). Similarly, the diploidy rate of the teratozoospermic group was significantly higher compared to the one in the controls (0.56 ± 0.42 % vs 0.13 ± 0.08; p < 0.001). The total rate of disomy of sex chromosome was 3.99 ± 1.21% in the teratozoospermic group, significantly higher (p < 0.001) than in the controls (1.08 ± 0.24%). Ours results also showed that the Meiosis I (MI) derived XY disomy was significantly higher than the Meiosis II (MII) derived XX/YY forms. Another observation was that the ratio of XX:YY bearing sperm in the normal population is comparable (0.24 ± 0.07% vs 0.28 ± 0.08%), but in infertile groups, the YY bearing sperm were nearly 3 times greater than those in the XX group (1.35 ± 0.51% vs 0.46 ± 0.21%). The incidence of disomy for chromosome 8 was significantly higher in the teratozoospermic men compared to the one in the controls (0.60 ± 0.35 vs 0.15 ± 0.07).
Table 2.
Results of chromosomal abnormalities in controls and teratozoospermic men
| Fertile controls % Mean±SD | Teratozoospermia % Mean±SD | P-Value | |
|---|---|---|---|
| Disomy X | 0.24 ± 0.07 | 0.46 ± 0.21 | 0.001 |
| Disomy Y | 0.28 ± 0.08 | 1.35 ± 0.51 | 0.001 |
| Disomy XY | 0.55 ± 0.17 | 2.16 ± 0.73 | 0.001 |
| Total Disomy of sex chromosomes | 1.08 ± 0.24 | 3.99 ± 1.21 | 0.001 |
| Disomy 8 | 0.15 ± 0.07 | 0.60 ± 0.35 | 0.001 |
| Nullisomy of sex chromosomes | 0.13 ± 0.04 | 0.58 ± 0.30 | 0.001 |
| Nullisomy 8 | 0.10 ± 0.04 | 0.55 ± 0.16 | 0.001 |
| Total aneuploidy rate | 1.48 ± 0.22 | 5.74 ± 1.36 | 0.001 |
| Diploidy | 0.13 ± 0.08 | 0.56 ± 0.49 | 0.001 |
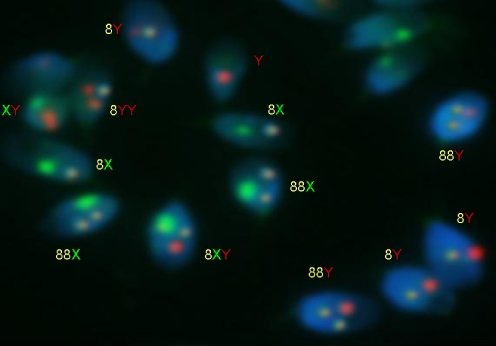
Fig. 2.
Fluorescence in situ hybridisation with probes for chromosomes 8 (yellow), X (green) and Y (red) on sperm of an infertile patient with isolated teratozoospermia. Haploid spermatozoa were 18X or 18Y, and the rest of spermatozoa were aneuploid (Y, XY, 18XY, 18YY, 1818X, 1818Y)
Correlation between aneuploidy and semen parameters
No significant relationship was established between the age, the total motility, the sperm density and the mean rate of aneuploidy or disomy in the group of patients with severely teratozoospermia (p > 0.05). Nevertheless, significant and strong correlations were found between the morphological abnormalities, and the total aneuploidy rate (r = 0.767, p < 0.01), and the total rate of sex chromosomes aneuploidy (r = 0.690, p < 0.01). The correlation analyses of the chromosomal abnormalities were compared with the specific types of morphological abnormalities of the controls and teratozoospermic subjects. In the sperm of the controls, none of the morphological abnormalities correlated significantly to the total aneuploidy rate or mean rate of disomy. However in the teratozoospermic subjects many more statistically significant correlations were found. The incidence of amorphous shaped sperm was positively correlated to the incidence of disomy X (r = 0.377, p = 0.01), and total sex chromosomes aneuploidy (r = 0.310, p < 0.05). Significant positive correlations were also found between total flagellum morphology and chromosome XY disomy (r = 0.406, p = 0.003), and total aneuploidy rate (r = 0.356, p < 0.05). Especially, the incidence of short tails was significantly correlated to the incidence of disomy X (r = 0.293, p < 0.05), and disomy XY (r = 0.287, p < 0.05). Abnormally large cytoplasmic droplets were significantly correlated with the disomy X (r = 0.326, p p < 0.05). Finally the multiple anomalies index correlated significantly to the incidence of total sex chromosomes aneuploidy (r = 0.335, p = 0.017) and total aneuploidy rate (r = 0.349, p < 0.05).
Analysis of DNA fragmentation
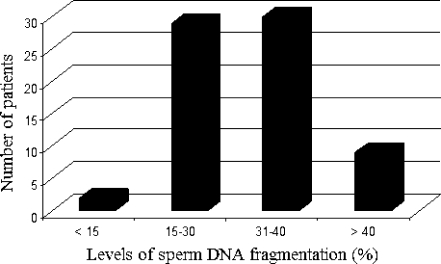
The level of sperm DNA fragmentation was significantly higher in the teratozoospermic men compared to the controls (respectively, 32.54 ± 10.61% and 10.14 ± 3.77%, p < 0.01). For our patients with severely teratozoospermia, two patients showed a TUNEL-positive rate of <15%, 29 patients, of between 15% and 30% and the rest of the patients [39] had a TUNEL rate of >30%. Of these patients, 9 had a rate of >40% (Fig. 3).
Fig. 3.
Distribution of sperm DNA fragmentation in patients with isolated teratozoospermia
Correlation between DNA fragmentation and semen characteristics
No significant correlation was observed between the TUNEL rate and the following variables in the teratozoospermic men: age of the patients, semen volume, semen pH, sperm concentration, total motility, progressive motility and rapid progression. However statistically significant and positive correlations were observed between the levels of sperm DNA fragmentation, and necrozoospermia (r = 0.387, p < 0.01), and the percentage of atypical forms (r = 0.436, p < 0.01). In order to determine if specific sperm morphological types occur simultaneously with levels of DNA fragmentation, sperm DNA fragmentation was correlated to the detailed morphological assessment. In the fertile control samples any correlation was found. However in the teratozoospermic group, the incidence of macrocephalic head (r = 0.402, p < 0.01), amorphous head (r = 0.386, p < 0.01) and shorten flagella (r = 0.388, p < 0.01) were positively correlated to the levels of DNA fragmentation. Also the multiple anomalies index was positively correlated to the DNA fragmentation index (r = 0.389, p <0.01).
Correlation between DNA fragmentation and aneuploidy rate
A significant correlation was observed between DNA fragmentation and total aneuploidy rate in the teratozoospermic men (r = 0.377, p = 0.007).
Discussion
Despite a normal blood karyotype, our patients with isolated teratozoospermia have a significantly increased frequency of chromosomal abnormalities in their sperm compared to normozoospermics men. The total aneuploidy rate seen in the sperm of our teratozoospermic men was comparable with rates found in polymorphic teratozoospermic men in others studies with preponderance of sex chromosomes aneuploidy over the other chromosomes studies [15–17] but not with the study of Tang et al. who found similar rates of aneuploidy among the different chromosomes studied [18]. The rate of XY bearing sperm was significantly higher in the teratozoospermic group, suggesting that nondisjunction at the first meiotic division is far greater than at the second meiotic division. Our results are in agreement with those of Gole et al. [16] and Templado et al. [19] but not with Calegero et al. [15] and Tang et al. [18], who showed that in cases of teratozoospermia chromosomal nondisjunction occurs mainly during the second meiotic division.
Another observation was that the ratio of XX: YY bearing sperm in the normal population is similar, but in the teratozoospermic men, the YY bearing sperm were nearly 3 times greater than those in the XX group. This would suggest that the Y chromosome was more prone to nondisjunction in the second meiotic division than chromosome X. Similar results were observed in the study of Rives et al. [20]. This is, however, in contrast to others studies [4, 16, 21], in which XX disomy was increased as opposed to YY disomy. Moreover, for these patients with severely teratozoospermia a significant correlation between aneuploidy rates and sperm morphology was found. Such a correlation was present for all the investigated chromosomes (X, Y and 8). On the contrary, we did not find a significant correlation between chromosomal abnormalities and other semen parameters (volume, concentration, motility, necrozoospermia). In the sperm of the controls, none of the morphological abnormalities correlated significantly to the total aneuploidy rate or mean rate of disomy. However in the teratozoospermic subjects many more statistically significant correlations were found, suggesting that abnormal sperm morphology of teratozoospermic patients is a much better indicator for chromosomal abnormality than that of fertile men. This association was proved to be especially robust between sex chromosomes aneuploidy and abnormal head morphology. The incidence of amorphous shaped heads correlated positively to the incidence of sperm with sex chromosomes disomy. Similarly, increases in chromosomal abnormalities have been found in amorphous sperm when investigated by FISH [18] or by human sperm injection into mouse oocytes [22], and by two different imaging software assisted strategies of simultaneously investigating morphology and aneuploidy in the same sperm [23, 24]. Additionally, a significant positive correlation was also found between flagellum morphology (especially short tails), chromosome X disomy and total aneuploidy rate. In a recent study, Tang et al. found that the incidence of two tails were correlated to the incidence of chromosome 13 disomy and supernumerary chromosomal abnormalities in the teratozoospermic subjects [18]. Abnormally large cytoplasmic droplets were significantly correlated with the disomy X. In studies with the combined methods of sperm Creatine Kinase immunocytochemistry and FISH, diminished sperm maturity, as detected by cytoplasmic retention, was related to increased frequencies of chromosomal disomy [25].
In addition to the high levels of chromosomal abnormalities, our patients with isolated teratozoospermia showed a significantly higher level of DNA fragmentation compared to the controls. This observation is in agreement with the reports of several recent studies; witch indicated an increased percentage of spermatozoa with damaged DNA in the ejaculates of infertile men with abnormal semen parameters compared with normozoospermics controls [26–28]. A significant association between sperm morphology and sperm DNA damage was identified in our study. Moreover, DNA fragmentation was correlated to the incidence of sperm head abnormalities especially with macrocephalic and amorphous heads. These correlations suggest that sperm head defects may be in part due to a reduction of sperm DNA integrity. This is in agreement with the data of others studies [28, 29]. We also reported a significant correlation between DNA fragmentation and total abnormal tails especially short tails. Similarly using a TUNEL coupled flow cytometry method, Muratori et al. showed that the extent of sperm DNA fragmentation in unselected spermatozoa was positively related to abnormal morphology and associated with defects of the sperm tail [30]. Concerning the vitality, a very strong correlation between necrozoospermia and sperm DNA fragmentation has been described [31, 32]. This is in agreement with our inverse correlation between TUNEL assay and vitality. The origin of DNA fragmentation in spermatozoa is still a matter of debate and many factors could be responsible for it. Three major hypotheses have been postulated to explain the source of DNA damage in sperm: improper packaging and ligation during sperm maturation [33, 34], oxidative stress [35, 36] and defective apoptosis before ejaculation [36, 37]. These three mechanisms might independently or co-dependently be responsible for sperm DNA damage. However the impact of age on sperm DNA fragmentation is still an unsolved question. In the present study, no correlation was found between the age of our patients and the levels of DNA fragmentation. This is in agreement with the results of Dakouane et al. [38] who found no difference in DNA fragmentation rate according to age among 212 men aged between 25 and 70 years, but in disagreement with the results of others studies [39, 40] who had found a significant correlation between DNA fragmentation and age of the patients. In addition, a positive correlation was found between the rate of sperm DNA fragmentation and the rate of sperm chromosomal aberration. Using linear regression analysis, Morel et al. [41] have show that disomy frequency are correlated with the degree of nuclear maturity as determined by the percentage of nuclei that stained with aniline blue.
In conclusion, our results indicate that spermatozoa from patients with isolated teratozoospermia have an increased level of DNA fragmentation and chromosomal aneuploidy with an increased frequency of sex numerical chromosomes abnormalities, mainly XY disomy. The transmission of this kind of gamete by ICSI may lead to the birth of an infant with sex chromosomes abnormalities. Moreover, significant correlations were found between morphology, DNA fragmentation and aneuploidy. Some specific morphological abnormalities were shown to be predictive of chromosomal abnormalities and DNA alteration: amorphous head and short tails. Hence, in the absence of normal sperm, the choice of spermatozoa with theses abnormalities should not be recommended.
Footnotes
Capsule
Patients with isolated teratozoospermia have increased levels of DNA fragmentation and chromosomal aneuploidy.
References
- 1.Rudak E, Jacobs PA, Yanagimachi R. Direct analysis of the chromosome constitution of human spermatozoa. Nature. 1978;274:911–913. doi: 10.1038/274911a0. [DOI] [PubMed] [Google Scholar]
- 2.Meschede D, Horst J. Genetic counselling for infertile male patients. Int J Androl. 1997;20:20–30. [PubMed] [Google Scholar]
- 3.Moosani N, Pattinson H, Carter M, Cox D, Rademaker A, Martin R. Chromosomal analysis of sperm from men with idiopathic infertility using sperm karyotyping and fluorescence in situ hybridization. Fertil Steril. 1995;64:811–817. doi: 10.1016/s0015-0282(16)57859-5. [DOI] [PubMed] [Google Scholar]
- 4.Bernardini L, Martini L, Geraedts JP, Hopman AH, Lanteri S, Conte N, Capitanio GL. Comparison of gonosomal aneuploidy in spermatozoa of normal fertile men and those with severe male factor detected by in- situ hybridization. Mol Hum Reprod. 1997;3:431–439. doi: 10.1093/molehr/3.5.431. [DOI] [PubMed] [Google Scholar]
- 5.Ushijima C, Kumasako Y, Kihaile PE, Hirotsuru K, Utsunomiya T. Analysis of chromosomal abnormalities in human spermatozoa using multi- colour fluorescence in-situ hybridization. Hum Reprod. 2000;15:1107–1111. doi: 10.1093/humrep/15.5.1107. [DOI] [PubMed] [Google Scholar]
- 6.Miharu N, Best RG, Young SR. Numerical chromosome abnormalities in spermatozoa of fertile and infertile men detected by fluorescence in situ hybridization. Hum Genet. 1994;93:502–506. doi: 10.1007/BF00202812. [DOI] [PubMed] [Google Scholar]
- 7.Guttenbach M. Martinez-Exposito MJ, Michelmann HW, Engel W, Schmid M: Incidence of diploid and disomic sperm nuclei in 45 infertile men. Hum Reprod. 1997;12:468–473. doi: 10.1093/humrep/12.3.468. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–843. doi: 10.1016/S0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 9.Sakkas D, Seli E, Bizzaro D, Tarozzi N, Manicardi GC. Abnormal spermatozoa in the ejaculate: abortive apoptosis and faulty nuclear remodelling during spermatogenesis. Reprod Biomed Online. 2003;7:428–432. doi: 10.1016/S1472-6483(10)61886-X. [DOI] [PubMed] [Google Scholar]
- 10.Seli E, Sakkas D. Spermatozoal nuclear determinants of reproductive outcome: implications for ART. Hum Reprod Updat. 2005;11:337–349. doi: 10.1093/humupd/dmi011. [DOI] [PubMed] [Google Scholar]
- 11.Benchaib M, Lornage J, Mazoyer C, Lejeune H, Salle B, Guérin JF. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil Steril. 2007;87:93–100. doi: 10.1016/j.fertnstert.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 12.Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erenpreiss J, Giwercman A. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22:174–179. doi: 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 13.Laboratory manual for the examination of human semen and semen-cervical mucus interaction. New York: Cambridge University Press; 1992. [Google Scholar]
- 14.Auger J, Eustache F. standardisation de la classification morphologique des spermatozoïdes humains selon la méthode de David modifiée. Andrologie. 2000;10:358–373. doi: 10.1007/BF03034491. [DOI] [Google Scholar]
- 15.Calogero AE, Palma A, Grazioso C, Barone N, Romeo R, Rappazzo G, D’Agata R. Aneuploidy rate in spermatozoa of selected men with abnormal semen parameters. Hum Reprod. 2001;16:1172–1179. doi: 10.1093/humrep/16.6.1172. [DOI] [PubMed] [Google Scholar]
- 16.Gole LA, Wong PF, Ng PL, Wang XQ, Ng SC, Bongso A. Does sperm morphology play a significant role in increased sex chromosomal disomy? A comparison between patients with teratozoospermia and OAT by FISH. J Androl. 2001;22:759–763. [PubMed] [Google Scholar]
- 17.Härkönen K, Suominen J, Lähdetie J. Aneuploidy in spermatozoa of infertile men with teratozoospermia. Int J Androl. 2001;24:197–205. doi: 10.1046/j.1365-2605.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 18.Tang SS, Gao H, Zhao Y, Ma S. Aneuploidy and DNA fragmentation in morphologically abnormal sperm. Int J Androl. 2010;33:e163–e179. doi: 10.1111/j.1365-2605.2009.00982.x. [DOI] [PubMed] [Google Scholar]
- 19.Templado C, Hoang T, Greene C, Rademaker A, Chernos J, Martin R. Aneuploid spermatozoa in infertile men: teratozoospermia. Mol Reprod Dev. 2002;61:200–204. doi: 10.1002/mrd.1148. [DOI] [PubMed] [Google Scholar]
- 20.Rives N, Joly G, Machy A, Simeon N, Leclerc P, Macé B. Assessment of sex chromosome aneuploidy in sperm nuclei from 47, XXY and 46, XY/47, XXY males: comparison with normal karyotype. Mol Hum Reprod. 2000;6:107–112. doi: 10.1093/molehr/6.2.107. [DOI] [PubMed] [Google Scholar]
- 21.Strassburger D, Reichart M, Kaufman S, Kasterstein E, Komarovsky D, Bern O, Friedler S, Schachter M, Ron-El R, Raziel A. A morphology assessment and fluorescence in situ hybridization of the same spermatozoon using a computerized cell-scanning system. Hum Reprod. 2007;22:201–209. doi: 10.1093/humrep/del357. [DOI] [PubMed] [Google Scholar]
- 22.Lee JD, Kamiguchi Y, Yanagimachi R. Analysis of chromosome constitution of human spermatozoa with normal and aberrant head morphologies after injection into mouse oocytes. Hum Reprod. 1996;11:1942–1946. doi: 10.1093/oxfordjournals.humrep.a019521. [DOI] [PubMed] [Google Scholar]
- 23.Celik-Ozenci C, Jakab A, Kovacs T, Catalanotti J, Demir R, Bray-Ward P, Ward D, Huszar G. Sperm selection for ICSI: shape properties do not predict the absence or presence of numerical chromosomal aberrations. Hum Reprod. 2004;19:2052–2059. doi: 10.1093/humrep/deh361. [DOI] [PubMed] [Google Scholar]
- 24.Strassburger D, Reichart M, Kaufman S, Kasterstein E, Komarovsky D, Bern O, Friedler S, Schachter M, Ron-El R, Raziel A. A morphology assessment and fluorescence in situ hybridization of the same spermatozoon using a computerized cell-scanning system. Hum Reprod. 2007;22:201–209. doi: 10.1093/humrep/del357. [DOI] [PubMed] [Google Scholar]
- 25.Kovanci E, Kovacs T, Moretti E, Vigue L, Bray-Ward P, Ward DC, Huszar G. FISH assessment of aneuploidy frequencies in mature and immature human spermatozoa classified by the absence or presence of cytoplasmic retention. Hum Reprod. 2001;16:1209–1217. doi: 10.1093/humrep/16.6.1209. [DOI] [PubMed] [Google Scholar]
- 26.Aitken RJ, Iuliis GN. Origins and consequences of DNA damage in male germ cells. Reprod Biomed Online. 2007;14:727–733. doi: 10.1016/S1472-6483(10)60676-1. [DOI] [PubMed] [Google Scholar]
- 27.Huang C, Lin D, Tsao H, Cheng TC, Liu CH, Lee MS. Sperm DNA fragmentation negatively correlated with velocity and fertilization rate but might not affect pregnancy rates. Fertil Steril. 2005;84:130–140. doi: 10.1016/j.fertnstert.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 28.Mehdi M, Khantouche L, Ajina M, Saad A. Detection of DNA fragmentation in human spermatozoa: correlation with semen parameters. Andrologia. 2009;41:383–386. doi: 10.1111/j.1439-0272.2009.00953.x. [DOI] [PubMed] [Google Scholar]
- 29.Zini A, Bielecki R, Phang D, Zenzes MT. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil Steril. 2001;75:674–677. doi: 10.1016/S0015-0282(00)01796-9. [DOI] [PubMed] [Google Scholar]
- 30.Muratori M, Piomboni P, Baldi E, Filimberti E, Pecchioli P, Moretti E, Gambera L, Baccetti B, Biagiotti R, Forti G, Maggi M. Functional and ultrastructural features of DNA-fragmented human sperm. J Androl. 2000;21:903–912. [PubMed] [Google Scholar]
- 31.Guerin P, Matillon C, Bleau G, Levy R, Menezo Y. Impact of sperm DNA fragmentation on ART outcome. Gynecol Obstet Fertil. 2005;33:665–668. doi: 10.1016/j.gyobfe.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Cohen-Bacrie P, Belloc S, Ménézo YJ, Clement P, Hamidi J, Benkhalifa M. Correlation between DNA damage and sperm parameters: a prospective study of 1, 633 patients. Fertil Steril. 2008;91:1801–1805. doi: 10.1016/j.fertnstert.2008.01.086. [DOI] [PubMed] [Google Scholar]
- 33.Sailer BL, Jost LK, Evenson DP. Mammalian sperm DNA susceptibility to in situ denaturation associated with the presence of DNA strand breaks as measured by the terminal desoxynucleotidyl transferase assay. J Androl. 1995;16:80–87. [PubMed] [Google Scholar]
- 34.Zini A, Libman J. Sperm DNA damage: clinical significance in the era of assisted reproduction. CMAJ. 2006;175:495–500. doi: 10.1503/cmaj.060218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal A, Saleh RA. Utility of oxidative stress test in the male infertility clinic. Zhonghua Nan Ke Xue. 2002;8:1–9. [PubMed] [Google Scholar]
- 36.O’Brien J, Zini A. Sperm DNA integrity and male infertility. Urology. 2005;65:16–22. doi: 10.1016/j.urology.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Sakkas D, Mariethoz E, Manicardi G, Bizzaro D, Bianchi PG, Bianchi U. Origin of DNA damage in ejaculated human spermatozoa. Rev Reprod. 1999;4:31–37. doi: 10.1530/ror.0.0040031. [DOI] [PubMed] [Google Scholar]
- 38.Dakouane M, Albert M, Bergere M, Sabbagh C, Brayotel F, Vialard F, Lombroso R, Bicchieray L, Selva J. Aging and spermatogenesis: an histologic, cytogenetic and apoptosis study. Gynecol Obstet Fertil. 2005;33:659–664. doi: 10.1016/j.gyobfe.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril. 2003;80:1420–1430. doi: 10.1016/j.fertnstert.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, Glaser RL, Pearson FS, Evenson D. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci U S A. 2006;103:9601–9606. doi: 10.1073/pnas.0506468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morel F, Mercier S, Roux C, Elmrini T, Clavequin MC, Bresson, JL: Interindividual variations in the disomy frequencies of human spermatozoa and their correlation with nuclear maturity as evaluated by aniline blue staining. Fertil Steril 1998;69 [DOI] [PubMed]