Abstract
The mammalian ovary consists of a large number of dormant immature follicles, each containing a single oocyte and located on the periphery of the ovary. With each reproductive cycle, a group of immature follicles is sequentially activated to resume growth, and pituitary gonadotropins and ovarian steroid and peptide hormones cooperate to ensure further growth and development. A single dominant follicle eventually emerges, ovulates, and then involutes to allow the selection of the next group of follicles. While hormones are known to control the later stages of folliculogenesis, little is known about the pathways that activate individual immature primordial follicles in the dormant follicle pool. We advance a new hypothesis: that follicle activation is dependent on the physical environment of the ovary in addition to well-established hormonal cues. This novel perspective on ovarian function may provide new avenues to study follicle dynamics and identify therapeutic targets for ovarian dysfunction.
Keywords: Ovary, Follicle, Endocrine, Alginate, Biomaterials
The role of hormones, architecture and a predicted role for the physical environment in the control of follicle development
Hormone- and growth factor-driven follicle growth The initial activation and development of primordial follicles to the primary and early secondary stages is generally considered to occur independent of pituitary gonadotropin [follicle-stimulating hormone (FSH) and luteinizing hormone (LH)] control [1, 2]. These follicles lack gonadotropin receptors and are distal to ovarian blood supply, and are thus not influenced by the fluctuation of hormone levels that occurs with each reproductive cycle. Rather, early-stage follicle somatic cell (i.e., granulosa cell) proliferation and oocyte growth depend on complex and sequential paracrine communication by various oocyte-, granulosa cell-, and theca cell-derived factors between and within individual follicles [1, 2]. Platelet-derived growth factor (PDGF), bone morphogenic protein-6 (BMP6), anti-Müllerian hormone (AMH), BMP15, kit ligand, basic fibroblast growth factor (bFGF), BMP4/7, GDF-9, activin, and inhibin are produced by and act on the various early-stage follicle cells to stimulate growth and differentiation. Terminal oocyte maturation is also dependent on growth factors, particularly those in the EGF-like family [3–5].Once follicles have reached the secondary stage and contain sufficient levels of FSH receptor, they are ‘recruited’ and begin a rapid phase of granulosa cell proliferation and hormone production in response to pituitary gonadotropins. While we know many of the steps that occur in the hormone-responsive phase of follicle maturation, and we are learning more about the paracrine signals required for early-stage follicle growth, little is known about what initially activates an individual primordial follicle at a specific time during the reproductive life of an animal. In humans, whether a follicle remains quiescent, is selected into the growing pool of follicles, becomes the dominant follicle, or is lost to atresia, is determined over a period of decades, so some underlying non-stochastic spatio-temporal mechanism must be operational. Likewise, the mechanisms underlying dysfunctional follicle development are only partially explained by deficiencies in growth factor or hormone action. In polycystic ovary syndrome (PCOS), for example, follicles begin, but do not complete, development despite the presence of the necessary paracrine and endocrine signals. Thus, hormonal influences are necessary but not sufficient to control follicle development and oocyte maturation.
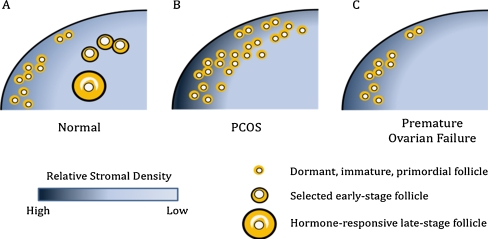
The physical environment in normal ovarian function In vitro culture of immature mouse follicles or granulosa cell-oocyte complexes on tissue culture plates or membranes has been a mainstay of research efforts investigating the hormonal environment of the ovary and its impact on follicle function [6]. These two-dimensional (2D) cultures have produced mature (MII stage) oocytes; however, the efficiency of embryo production is low. The disruption of follicular architecture in 2D cultures may limit the ability to culture immature follicles, which, as described above, depend on paracrine communication between cell types, and thus may be insufficient for culturing follicles from species such as humans, which require a long development period.three-dimensional (3D) matrices with the objective of maintaining the follicular architecture and cell–cell communication present in the ovary. Our basal 3D culture system employs the biomaterial alginate, which has been used successfully in the culture of murine, monkey, and human immature follicles [7–11]. Compared with 2D culture systems, follicles cultured in the alginate hydrogel have similar growth trends in follicle diameter and steroid production, yet produce a significantly greater percentage of mature oocytes. One of the primary architectural features preserved in the alginate culture system is the network of transzonal projections (TZPs) between the oocyte and granulosa cells [12]. Maintenance of these TZPs and other somatic cell–cell connections preserves the bi-directional communication between multiple follicular compartments that is necessary for early follicle development and oocyte maturation.An unexpected finding from 3D follicle cultures was that the physical environment regulates follicle function when hormonal stimulation is held constant [13–15]. Follicle growth, antrum formation, theca cell development, steroid production, and oocyte quality are influenced by the solids content and mechanical properties of the alginate hydrogel. Evidence supporting a role for the physical environment may be inferred from histological analyses, which found that the majority of immature ovarian follicles are present within the ovarian cortex, a collagen-rich zone of the ovary [1, 2]. By contrast, the perimedullar zone of the ovary has an extracellular matrix (ECM) density lower than that of the cortex. During follicle growth, follicles move from the cortex toward the medulla, and then complete their development along the perimedullar zone prior to ovulation at the ovarian surface. Thus, a gradient in the physical environment of the ovary may direct immature follicles to move from a dense matrix to a less dense matrix to initiate the growth process.We hypothesize that the more rigid ovarian cortex is relatively non-permissive for follicle growth (and the perimedullar zone relatively permissive), and that the biomechanical environment within each zone modulates the follicular response to hormones (Fig. 1a). Follicular cells would sense a dynamic mechanical environment as the growing follicle expands outward and moves through the ovarian stroma. Mechanical stimuli would be communicated rapidly throughout the follicle, as the various cell types are physically connected (e.g., via TZPs or connexins) and are actively involved in paracrine signaling. Mechanical stimuli can be powerful regulators of cellular differentiation, including the response of ovarian somatic cells to hormones [16]. As follicles transition from the non-permissive to the permissive zone of the ovary, their response to the hormonal milieu would change and initiate a cascade of cellular processes leading to activation of follicle growth and development. We found that for follicles cultured in a non-degradable alginate matrix, follicle growth leads to an increase in mechanical stress [17]. Inclusion of a degradable component within the matrix reduced the accumulation of mechanical stress and the solids content, which significantly enhanced oocyte quality [17]. Within the intact ovary, changes in follicular versus stromal production of matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs) may also be necessary for follicle growth and migration through the ovary [18, 19]. Taken together, these findings support our hypothesis that the physical properties of the ovarian environment, through modulation of follicle responsiveness to paracrine factors and hormones, regulate follicle development. The idea that biomechanically activated signaling pathways determine the effect of ovarian rigidity on follicle development opens up many new lines of investigation.
Fig. 1.
Schematic representation of proposed hypothesis for mechanical regulation of ovarian function and disease. a Immature follicles reside in the cortex in the human ovary. Unknown signals stimulate immature primordial follicle activation to enter the growing follicle pool. We hypothesize that the biomechanics of the ovarian environment contribute to primordial follicle activation and that the relatively dense cortex maintains follicle quiescence while the perimedullar region represents an environment more permissive to growth. b The follicles found in a PCOS ovary are usually small, accumulate in the cortex, and secrete high levels of androgen and relatively low levels of estrogen. The underlying etiology of this disease is unknown. Based on our work, we hypothesize that the relatively dense cortex creates a biomechanically non-permissive environment and is one of the contributing factors in the disease. c If this hypothesis is correct, one would predict that premature loss of ovarian function would be associated with a physically less rigid ovarian cortex
Implications for ovarian disorders The hypothesis that the physical environment of the ovary dictates normal follicle development suggests that disruption of the normal physical environment may underlie ovarian disorders (Fig. 1b). In 3D in vitro follicle cultures, non-permissive environments produce follicles with a number of abnormal traits: (1) they are unable to make appropriate fate decisions (die or progress to mature follicles); (2) they produce higher than normal ratios of androgen to estrogen, which suggests inadequate steroid conversion; and (3) they produce poor quality oocytes [13–15]. This follicle phenotype is similar to that observed in the ovaries of women with PCOS. Interestingly, the PCOS ovarian cortex is thicker and contains more collagen than the cortex of normal ovaries [20]. PCOS is also associated with obesity, and we have observed that the ovaries of obese mice are more rigid than ovaries from aged-matched control mice (unpublished observation). In a proteomic comparison of normal ovaries and ovaries from women with PCOS, alterations in protein expression that support accumulation of fibrin and collagen in the polycystic ovaries were observed [21]. Others have described increased collagen precursor, as well as the presence of a chaperone protein (HSP47), which has been implicated in the pathogenesis of fibrotic diseases, cyst formation, and/or a thick ovarian capsule in PCOS ovaries [22].PCOS was once treated by wedge resection and ovarian drilling, a controversial technique that was abandoned in favor of laparoscopic techniques or hormonal management with ovulation-inducing medications (e.g., metformin, clomiphene, gonadotropins) [23]. The long-held belief was that the removal of a part of the ovary would be sufficient to provide temporary relief of hormonal negative feedback between the ovary and the pituitary. Our hypothesis provides a contrasting view: wedge resection or drilling may relieve inhibition of follicle growth by changing the physical environment of the ovary to create a more permissive environment for follicle growth. Our interpretation would suggest that biochemical methods aimed at altering the physical environment of the ovary, or that target biomechanical signaling pathways within the ovary, may provide new avenues for therapeutic development.
Significance and the next five years The intersection of three kinds of signals to the ovary—hormone and growth factor signaling, the structural architecture and relationship between cells in the follicle, and the physical environment of the ovary—opens a new perspective to the study of an old problem—how individual follicles are led to their various fates over a woman’s reproductive lifetime. In vitro follicle culture systems provide an invaluable model in which to study folliculogenesis, and will enable many key mysteries of normal ovarian function to be unraveled. These culture systems may also identify the mechanisms underlying disorders of follicle growth, such as PCOS or premature ovarian insufficiency (Fig. 1c).A consequence of this model is the need to develop tools that measure the physical properties of the ovary in vivo, which could ultimately be employed for patient diagnostics. This tool would ideally measure the local environment around individual follicles, on length scales of 10 to 100 μm. The inability to non-invasively measure ovarian physical properties presents a significant obstacle to testing our hypothesis in vivo. In the meantime, 3D follicle culture offers a unique opportunity to study the physical regulation of follicle growth and perhaps identify the signaling pathways that are engaged in permissive and non-permissive environments within the ovary.
Conclusion
We present here a new hypothesis that has supporting evidence but to date no proof that the physical environment of the ovary in addition to hormonal cues contributes to follicle health and disease. To test the hypothesis requires the development of new tools and new approaches to the ovarian environment. The study of biomechanical signaling, which may exist as a gradient, may contribute to the solution of the most interesting unsolved question in reproductive biology—what causes a single primordial follicle to exit dormancy and begin the hormonally-dependent process of oocyte growth, follicle development and terminal oocyte maturation.
Acknowledgements
The authors are grateful for technical input from Dr. Jenny Hirshfield-Cytron, Dr. Frank Miller, Dr. Ariella Shikanov, and Rachel Smith. This research was supported by the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NIH grant numbers: U54HD041857) and the National Institutes of Health (Grant Numbers: UL1DE019587, PL1EB008542). Editorial assistance was provided by Stacey C. Tobin, PhD.
Footnotes
Capsule
Follicle development is controlled by hormones, the 3D relationship between oocyte and somatic cells and the physical environment of the ovary.
References
- 1.Tingen C, Kim A, Woodruff TK. The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol Hum Reprod. 2009;15:795–803. doi: 10.1093/molehr/gap073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 4.Freimann S, Ben-Ami I, Dantes A, Ron-El R, Amsterdam A. EGF-like factor epiregulin and amphiregulin expression is regulated by gonadotropins/cAMP in human ovarian follicular cells. Biochem Biophys Res Commun. 2004;324:829–834. doi: 10.1016/j.bbrc.2004.09.129. [DOI] [PubMed] [Google Scholar]
- 5.Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130:791–799. doi: 10.1530/rep.1.00793. [DOI] [PubMed] [Google Scholar]
- 6.Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, Picton HM, Plancha C, Shea LD, Stouffer RL, Telfer EE, Woodruff TK, Zelinski MB. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell-oocyte complexes. Tissue Eng. 2003;9:1013–1021. doi: 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- 8.Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714–723. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu M, Barrett SL, West-Farrell E, et al. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009;24:2531–2540. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587–594. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett SL, Shea LD, Woodruff TK. Noninvasive index of cryorecovery and growth potential for human follicles in vitro. Biol Reprod. 2010;82:1180–1189. doi: 10.1095/biolreprod.109.082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28:4439–4448. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West-Farrell ER, Xu M, Gomberg MA, Chow YH, Woodruff TK, Shea LD. The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol Reprod. 2009;80:432–439. doi: 10.1095/biolreprod.108.071142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75:916–923. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- 16.Kreeger PK, Woodruff TK, Shea LD. Murine granulosa cell morphology and function are regulated by a synthetic Arg-Gly-Asp matrix. Mol Cell Endocrinol. 2003;205:1–10. doi: 10.1016/S0303-7207(03)00209-0. [DOI] [PubMed] [Google Scholar]
- 17.Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476–5485. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith MF, Ricke WA, Bakke LJ, Dow MP, Smith GW. Ovarian tissue remodeling: role of matrix metalloproteinases and their inhibitors. Mol Cell Endocrinol. 2002;191:45–56. doi: 10.1016/S0303-7207(02)00054-0. [DOI] [PubMed] [Google Scholar]
- 19.Berkholtz CB, Shea LD, Woodruff TK. Extracellular matrix functions in follicle maturation. Semin Reprod Med. 2006;24:262–269. doi: 10.1055/s-2006-948555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis”. Obstet Gynecol Surv. 1982;37:59–77. doi: 10.1097/00006254-198202000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, Fan L, Meng Y, et al. Proteomic analysis of human ovaries from normal and polycystic ovarian syndrome. Mol Hum Reprod. 2007;30:527–535. doi: 10.1093/molehr/gam036. [DOI] [PubMed] [Google Scholar]
- 22.Tasab M, Batten MR, Buleid NJ. Hsp47: a molecular chaperone that interacts with and stabilizes correctly-folded procollagen. EMBO J. 2000;19:2204–2211. doi: 10.1093/emboj/19.10.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farquhar C, Lilford RJ, Marjoribanks J, Vandekerckhove P. Laparoscopic ‘drilling’ by diathermy or laser for ovulation induction in anovulatory polycystic ovary syndrome. Cochrane Database Syst Rev. 2007:CD001122. [DOI] [PubMed]