Abstract
Rationale
Early life stress is a risk factor for the development of psychopathology in later life. Consequences of adverse life events, however, may depend on the genetic makeup of an individual. Reduced serotonin1A receptor function may predispose to the development of anxiety disorders.
Objective
Determine susceptibility of serotonin1A receptor knockout (1AKO) mice on different background strains to the effects of maternal separation (MS) by assessing startle plasticity in adulthood.
Methods
1AKO mice on a 129S6 and a Swiss Webster (SW) background were used. MS groups were separated daily from their mother for 180 min/day from postnatal days 2 to 14. Control groups underwent normal animal facility rearing. In adulthood, effects on acoustic startle response, habituation, prepulse inhibition (PPI), and foot shock sensitization were determined.
Results
MS increased startle reactivity and reduced PPI in 129S6 mice. These effects of MS were independent of genotype. MS had no effect on the other readouts. In SW mice, MS had no consistent effect on startle reactivity and did not alter startle plasticity in wild type or in 1AKO mice. 1AKO mice did not differ from wild-type mice in startle plasticity.
Conclusion
Serotonin1A receptor deletion does not enhance vulnerability to the effects of MS on startle plasticity. The life-long increase in startle reactivity and PPI deficit induced by MS are strain-dependent. Further, the use of startle reactivity and plasticity may have added value in translational studies relating to early life stress.
Keywords: Early life stress, 5-HT1A, Knockout, PPI, Acoustic startle response, Foot shock sensitization, Context conditioning, Gene–environment interaction, Mouse, Strain differences
Introduction
Adverse life events during early childhood, such as parental loss, physical or sexual abuse, are an important risk factor in the development of stress-related psychiatric disorders. Early life stress is thought to alter brain systems involved in stress-related processes permanently, resulting in increased vulnerability to develop psychopathology later in life (Green et al. 2010). Consequences of adverse life events, however, also depend on the genetic makeup of an individual. Several lines of evidence suggest that gene–environment interactions may confer susceptibility to disease. Although the finding of Caspi and co-workers (2003) that serotonin transporter polymorphisms may moderate the influence of life stress on depression is not unequivocal (Caspi et al. 2003; for meta-analyses, see Munafo et al. 2009; Risch et al. 2009), it prompted a search for gene–environment interactions potentially relevant for psychiatric disorders (Rutter 2010). As such, recent studies suggest that polymorphisms in the 5-hydroxytryptamine serotonin1A (5-HT1A) receptor may also interact with negative life events to turn vulnerability into disorder (Zhang et al. 2009).
The 5-HT1A receptor has been implicated in the etiology of anxiety disorders (De Vry 1995; Holmes 2008). The distribution of 5-HT1A receptors in the brain correlates well with a function in emotional processes and is comparable between species including man. 5-HT1A receptors are present in high densities in limbic areas such as hippocampus, the septum, some of the amygdaloid nuclei, and entorhinal cortex (Pazos and Palacios 1985). High densities of 5-HT1A receptors are also found in the raphe nuclei, where serotonergic cell bodies are located. Activation of these somatodendritic autoreceptors reduces the firing rate of 5-HT neurons, which results in the suppression of 5-HT synthesis, turnover, and 5-HT release in the projection areas (Boess and Martin 1994). Together with the serotonin transporter and the 5-HT1B receptor, which are both located presynaptically in the synaptic cleft, 5-HT1A receptors thus regulate the fine-tuning of the activity of 5-HT neurons. Disturbances in this regulation may underlie various psychiatric disorders including anxiety disorders (Pineyro and Blier 1999).
Imaging studies indicate that both presynaptic and postsynaptic 5-HT1A receptor binding may be reduced in patients with anxiety disorders (Lanzenberger et al. 2007; Nash et al. 2008; Neumeister et al. 2004). The finding that 5-HT1A receptor deletion in mice results in an anxiety-related phenotype supports this notion (Heisler et al. 1998; Parks et al. 1998; Ramboz et al. 1998). The association of a functional 5-HT1A receptor polymorphism with anxiety disorder and related traits (Rothe et al. 2004; Strobel et al. 2003; Zhang et al. 2009) further suggests that allelic variations in 5-HT1A receptor expression may predispose to the development of anxiety disorders, although absence of such associations has also been reported (Chipman et al. 2010; Hettema et al. 2008).
Preclinical research may help to better understand the neurobiological mechanisms underlying the increased vulnerability to psychiatric disease. In rodents, the effects of early adverse experience have been modelled in a neonatal maternal separation (MS) paradigm. A large body of neurobiological evidence has been presented in favor of the repeated neonatal MS as an early life stress model, including neuroendocrine and neurochemical changes associated with anxiety (Holmes et al. 2005). Several studies, but certainly not all, further indicate that neonatal MS also increases anxiety-like behavior in later life (Millstein and Holmes 2007; Pryce et al. 2005).
As genetic vulnerability may enhance the impact of early life stress, we studied the behavior of adult serotonin1A receptor knockout (1AKO) male mice on two different background strains that had been exposed to MS from postnatal days 2 through 14 for 180 min/day. 1AKO mice show increased anxiety-like behavior as well as enhanced autonomic responses (Groenink et al. 2003b; Gross et al. 2000; Pattij et al. 2002a). Interestingly, expression of 5-HT1A receptors in the forebrain can restore the anxiety behavior in 1AKO mice (Gross et al. 2002). Furthermore, absence or blockade of 5-HT1A receptors during the postnatal period results in increased anxiety in adult mice (Gross et al. 2002; Vinkers et al. 2010). Together, these findings imply that abnormal functioning of 5-HT1A forebrain receptors during postnatal development may result in life-long exaggerated anxiety.
1AKO mice have been created on three different genetic background strains: 129S6, C57BL/6J, and Swiss Webster (SW). The fact that increased anxiety-like behavior is observed in all 1AKO mice independent of background strain supports the involvement of 5-HT1A receptors in anxiety. However, differences between 1AKO mice on different backgrounds were also observed. Changes in baseline serotonin levels were observed in 1AKO mice on a C57BL/6J background (Parsons et al. 2001), but not in the other strains (He et al. 2001; Knobelman et al. 2001a, b). Also, only 1AKO-SW mice show alterations in GABAA subunit composition and benzodiazepine sensitivity (Pattij et al. 2002b; Sibille et al. 2000). These findings indicate that the effects of gene deletion can greatly depend on the genetic background strain. As not only the effect of receptor deletion may depend on genetic background but also the effect of early life stress and the interaction of early life stress with genetic alterations (Gillespie et al. 2009; Joober et al. 2002), we determined the effect of MS on 1AKO mice on two different genetic background strains.
To determine whether MS of 1AKO mice had long-lasting effects on the neural control of behavior, startle reactivity and plasticity were measured. The acoustic startle response is a defensive reaction to a sudden unexpected loud noise and involves fast involuntary contraction of facial and body muscles. The startle response is a cross-species phenomenon that is mediated by a simple neuronal circuit in the lower brainstem but can be modulated by forebrain structures (Koch 1999). The test battery used consisted of acoustic startle reactivity, habituation, prepulse inhibition (PPI), and foot shock sensitization. Habituation is regarded as a form of nonassociative learning, in which an animal learns to differentiate meaningful from irrelevant stimuli (Geyer and Braff 1987). PPI of the acoustic startle is the normal suppression of the startle response that occurs if the loud startling stimulus is preceded by a weak prepulse stimulus. PPI is a measure of the early preattentive stages of information processing and is used as an operational measure of sensorimotor gating. In the foot shock sensitization paradigm, context conditioning can be studied by measuring the increase in the startle response after foot shock (Richardson 2000).
Methods
Subjects
Mice (male 1AKO and WT mice on 129S6 (formerly known as 129SvEvTac) and SW background strain) used within this study were bred from parents that were bred as homozygotes at the local breeding facility of the Central Laboratory Animals Institute, Utrecht, The Netherlands. The breeding founders of the 129S6 strain were originally obtained from Dr. Hen (Columbia University, New York, USA) and of the SW strain from Dr. M. Toth (Cornell University, New York, USA). The breeding founders were initially crossbred with commercially available mice (Taconic, M&B, Denmark) from the same background (ten generations). These crossbreds resulted in heterozygous F1 generations, which were used to breed homozygous 1AKO and WT generations (F2). These homozygous F2 generations were used to breed the experimental animals used in this study. After birth, pups were housed with both parents in Macrolon-III cages. On PND 21, male pups were weaned and socially housed in same genotype, same strain groups with 3–5 animals/cage. Animals were housed at constant room temperature (22±2°C) and relative humidity (40–60%) in standard Macrolon-II cages (22 × 16 × 14 cm) with a piece of PVC-tubing as cage enrichment. Food and water were available ad libitum in the home cage. Animals were maintained on a 12 h light–dark cycle (lights on from 7 a.m. to 7 p.m.), and all experimental procedures were conducted during the light phase of the cycle. Startle reactivity was studied between PND80 and PND120. All experiments were performed in accordance with the governmental guidelines for care and use of laboratory animals and approved by the ethical committee for animal research of the Faculties of Pharmacy, Biology, and Chemistry at Utrecht University, The Netherlands.
MS procedure
Litters were randomly assigned to either the MS or the control condition. MS groups were separated daily from their mother for 180 min/day (9 a.m. to 12 a.m.), for 13 consecutive days (postnatal days 2 to 14). During separation, the pups were put in clean Macrolon cages filled with bedding, which were placed on a 37°C heating blanket. Pups from each litter were kept together during separation. Control groups underwent normal animal facility rearing (AFR). Each experimental group (wt-control, 1AKO-control, wt-MS, and 1AKO-MS) consisted of pups of at least four separate litters (SW strain four litters per condition; 129S6 strain four to six litters per condition).
Startle apparatus
Four startle devices were used simultaneously (SR-lab, San Diego Instruments, San Diego CA, USA). The startle devices consisted of a plexiglas cylinder (inner diameter 4 cm, length 13 cm) mounted on a plexiglas platform. Each startle device was placed in a ventilated sound attenuated cubicle. Cage movements were measured with a piezoelectric film attached to the plexiglas base of the startle device. A calibration system (San Diego Instruments) was used to ensure comparable startle magnitudes across the four devices. Startle stimuli (50 ms in duration) were presented through a high-frequency speaker located 33 cm above the startle devices. Background noise was 70 dB. Startle magnitudes were sampled each millisecond during a period of 65 ms beginning at the onset of the startle stimulus. A startle response is defined as the peak response during this 65-ms period. Sound intensities were measured using a microphone, which was placed on top of the plexiglas cylinder and fitted to a Bruel and Kjaer sound level meter (Type 2226).
Startle procedures
Acoustic startle response
Mice were placed in the startle chamber. After a 5-min acclimation period, animals were presented with 80 startle stimuli of varying intensities with a variable interstimulus interval of 20–30 s. The stimulus intensities used were 75, 80, 85, 90, 95, 100, 110, and 120 dB noise. All trials were presented in ten blocks of eight stimuli and in a pseudo-random order within each block.
Habituation
Mice were placed in the startle chamber, and after an acclimatization period of 5 min, 100 startle stimuli of 110 dB noise were presented (duration 50 ms) with an interstimulus interval of 3 s.
Prepulse inhibition
Animals were placed in the startle chamber and, after a 5-min acclimation period, were presented with startle stimuli (110 dB) that were presented alone or preceded by noise prepulses (20 ms) of 2, 4, 8, and 16 dB above background (i.e., 72, 74, 78, and 86 dB) with 100 ms between onset of the prepulse and startle stimulus. The different trials were presented in 12 blocks of six trials and in a pseudo-random order within each block. The interstimulus intervals ranged from 25 to 35 s.
Foot shock-induced sensitization
Animals were placed in the startle chamber and allowed to acclimatize for 5 min. Then 40 startle stimuli (100 dB) were presented with an interstimulus interval of 30 s followed by ten foot shocks (0.8 mA, 500 ms, ISI 1 s). Then a second set of 40 startle stimuli (100 dB) were presented. Twenty-four hours later, animals were again presented with a set of 40 startle stimuli (100 dB) to evaluate the level of contextual fear.
Data analyses
Data of the two strains were analyzed separately as the experiments were not completely run in parallel. All mice were tested in all tests (N = 112, n = 14 per condition. For each strain, n = 56, with n = 28 wt, and n = 28 1AKO mice, half of which were assigned to AFR and half of which to MS). Due to technical problems, data of some mice were not collected, resulting in smaller group sizes, as indicated below.
Differences in startle reactivity (n = 10–14 per condition) were analyzed using a repeated measures analysis of variance (ANOVA) with stimulus intensity (eight levels) as within-subject factor and condition (two levels) and genotype (two levels) as between-subjects factors.
For startle habituation (n = 6–13 per condition), startle magnitudes were assessed as ten successive blocks of ten trials each. To test differences in habituation, a repeated measures ANOVA was performed with block (ten levels) as within-subject factor and genotype (two levels) and rearing condition (two levels) as between-subject factors. Additionally, paired t-tests were performed to determine when the startle response no further declined relative to the preceding block.
Percent PPI (n = 6–10 per condition) was calculated for each prepulse intensity as percent change compared to the mean startle reflex in response to the 110 dB startle stimulus. Differences in percent PPI were analyzed using a repeated measures ANOVA with prepulse intensity (four levels) as within-subject factor and rearing condition and genotype as between-subjects factors. Pearson’s correlations were used to determine if there was a relationship between acoustic startle and mean PPI levels.
To determine the degree of foot shock-induced sensitization (n = 8–14 per condition), responses to 40 stimuli were collapsed resulting in a pre-shock, post-shock, and 24-h post-shock mean startle amplitude. Subsequently, a repeated measures ANOVA was performed with time as within-subject factor (three levels: pre-, post- and 24-h post-shock), and rearing condition (two levels) and genotype (two levels) as between-subjects factors.
Post-hoc tests consisted of independent t-tests (acoustic startle, habituation, and PPI) or paired t-tests (foot shock sensitization) with Bonferroni correction for alpha. The level of significance was set at p < 0.05.
Results
Acoustic startle response
129S6 strain (n = 10–14 per condition)
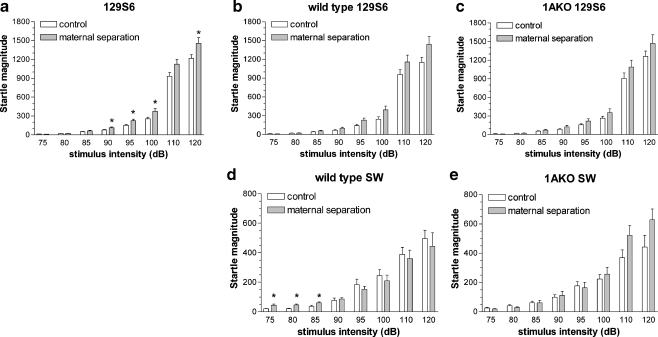
As shown in Fig. 1a, the acoustic startle response to the different stimulus intensities was dependent on rearing condition (F 7,336 = 3.5, p = 0.001). This effect of rearing condition was similar for wild-type and 1AKO mice (Fig. 1b, c). Post-hoc analysis showed that MS mice had a higher startle response than AFR mice (Fig. 1a). This effect was significant at 90, 95, 100, and 120 dB. 1AKO mice responded similarly to the startle stimuli as wild-type mice (Fig. 1b, c).
Fig. 1.
Acoustic startle response. Mean startle magnitude (in arbitrary units ± SEM) as a function of startle stimulus intensity in 129S6 (a, b, c) and SW mice (d, e), exposed to AFR (control, white bars) or MS (gray bars). Panel a shows the effects on wild-type and 1AKO 129S6 together, as the effects of MS were independent of genotype. Panels b–e show the effect of MS for the separate genotypes. Differences between wild-type and 1AKO mice can be inferred by comparing the white bars in panels b and c for 129S6, and white bars in panels d and e for SW. * p < 0.05 relative to corresponding control
SW strain (n = 14 per condition)
Repeated measures ANOVA revealed a significant three-way interaction of intensity with rearing condition and genotype (F 7,364 = 2.5, p = 0.016). Furthermore, ANOVAs showed that at the lower intensities the effect of rearing condition was dependent on genotype. The startle response of MS wild-type mice, but not MS-1AKO mice, was significantly higher at 75, 80, and 85 dB (see Fig. 1d, e). In 1AKO mice, the response to the different intensities was dependent on rearing condition (F 7,182 = 2.7, p = 0.01), but post-hoc analysis did not detect significant differences between MS and AFR mice at separate intensities (Fig. 1e).
Habituation
129S6 strain (n = 6–13 per condition)
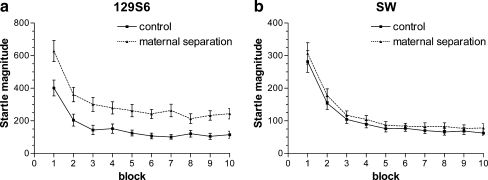
129S6 mice showed clear habituation of the acoustic startle response as evidenced by a significant effect of block (F 9,288 = 48.2, p < 0.001). As shown in Fig. 2a, the acoustic startle response was significantly higher in the MS group during all blocks (F 1,32 = 5.7, p = 0.023), but habituation of the startle response was similar for AFR and MS mice. After presentation of 30 stimuli (three blocks), stable levels of startle responses were achieved. Furthermore, 1AKO mice did not differ from wild-type mice and no significant interaction effects were found (see Table 1).
Fig. 2.
Habituation of the acoustic startle response. Mean startle magnitude (in arbitrary units ± SEM) upon repeated presentation of a 110 dB startle stimulus in 129S6 (a) and SW mice (b) exposed to AFR (control, black squares) or MS (black triangles). In each block 10 startle stimuli were presented. Effects on wild-type and 1AKO mice are taken together, as the effects of MS were independent of genotype. The overall significant effect of rearing on startle reactivity in 129S6 is not indicated in Fig. 2a, as it was independent of block
Table 1.
Habituation
| 129S6 | SW | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | MS | Control | MS | |||||
| WT | 1AKO | WT | 1AKO | WT | 1AKO | WT | 1AKO | |
| B1 | 471 ± 108 | 381 ± 56.2 | 685 ± 104 | 584 ± 84.0 | 273 ± 47.2 | 288 ± 47.5 | 288 ± 34.4 | 291 ± 57.3 |
| B2 | 238 ± 73.0 | 194 ± 43.9 | 405 ± 69.5 | 329 ± 58.9 | 150 ± 33.5 | 157 ± 24.5 | 161 ± 23.5 | 194 ± 37.3 |
| B3 | 138 ± 14.0 | 146 ± 38.0 | 313 ± 58.2 | 291 ± 63.8 | 107 ± 25.4 | 103 ± 13.7 | 108 ± 17.1 | 118 ± 18.2 |
| B4 | 176 ± 23.6 | 145 ± 37.1 | 285 ± 48.7 | 275 ± 56.8 | 86.8 ± 20.1 | 91.2 ± 13.3 | 96.7 ± 14.7 | 106 ± 16.9 |
| B5 | 142 ± 20.7 | 120 ± 23.0 | 265 ± 48.5 | 261 ± 57.2 | 57.5 ± 9.6 | 88.5 ± 15.2 | 81.6 ± 13 | 81.4 ± 17.1 |
| B6 | 121 ± 23.8 | 103 ± 22.1 | 229 ± 32.8 | 252 ± 44.2 | 60.5 ± 9.0 | 88.5 ± 13.8 | 78.8 ± 11.9 | 91.6 ± 17.2 |
| B7 | 93.5 ± 10.6 | 104 ± 17.8 | 261 ± 51.8 | 266 ± 57.4 | 62.9 ± 14.6 | 75.1 ± 12.9 | 72.9 ± 14.1 | 98.2 ± 25.7 |
| B8 | 131 ± 28.1 | 118 ± 26.1 | 213 ± 33.9 | 213 ± 47.6 | 58.2 ± 16.4 | 71.0 ± 12.6 | 70.5 ± 13.3 | 91.6 ± 16.1 |
| B9 | 120 ± 24.6 | 100 ± 23.9 | 243 ± 30.8 | 226 ± 47.0 | 58.3 ± 13.2 | 75.6 ± 14.3 | 71.8 ± 14.3 | 70.0 ± 11.3 |
| B10 | 127 ± 61.5 | 112 ± 23.2 | 252 ± 41.4 | 237 ± 48.5 | 55.8 ± 9.2 | 66.7 ± 9.3 | 69.7 ± 13.7 | 61.3 ± 6.8 |
Mean startle magnitude (±SEM) per block (B) for 129S6 and SW mice. Data are given for rearing condition [control and maternal separation (MS)] and genotype [wild-type (WT) and 1AKO mice] separately
SW strain (n = 6–13 per condition)
As shown in Fig. 2b, SW mice showed clear habituation of the acoustic startle response as evidenced by a significant effect of block (F 9,306 = 87.2, p < 0.001). Habituation of the startle response was similar for AFR and MS mice. Stable levels of startle responses were achieved after presentation of 50 stimuli (five blocks). Furthermore, 1AKO mice did not differ from wild-type mice and no significant two- or three-way interactions were found (see Table 1).
Prepulse inhibition
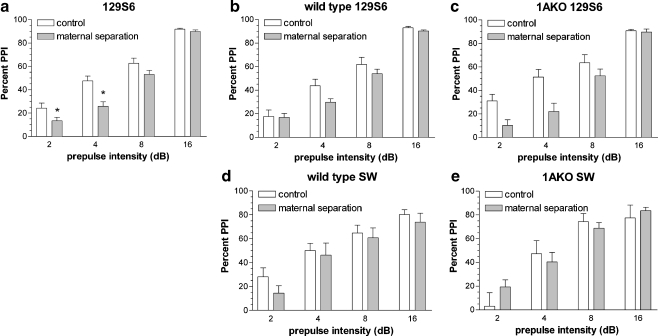
129S6 strain (n = 8–10 per condition)
MS reduced percent PPI similarly in wild-type and 1AKO mice, but the effect of MS was dependent on prepulse intensity (F 3,96 = 5.6, p = 0.001). As shown in Fig. 3a, the differences between AFR and MS mice were significant at 2 and 4 dB prepulse intensity. PPI in 1AKO mice did not differ from that of wild-type mice (see Fig. 3b, c). Startle magnitude was significantly higher in MS mice, irrespective of genotype (F 1,32 = 12.6, p = 0.001; control mice 758 ± 88.7, MS mice 1150.2 ± 119).
Fig. 3.
PPI of the acoustic startle response. Effect of MS (gray bars) on percent PPI measured at different prepulse intensities, in 129S6 (a, b, c) and SW mice (d, e). White bars show the response of AFR mice (control, white bars). In panel a, the effects on wild-type and 1AKO 129S6 mice are taken together, as the effects of MS were independent of genotype. Panels b–e show the effect of MS for the separate genotypes. Differences between wild-type and 1AKO mice can be inferred by comparing the white bars in panels b and c for 129S6, and white bars in panels d and e for SW. Pearson’s correlation showed that startle magnitude and mean PPI levels were not correlated (r = -0.17, NS, n = 63). Data represent means ± SEM. *p < 0.05 relative to corresponding control
SW strain (n = 6–8 per condition)
Rearing condition had no significant effect on percent PPI. Percent PPI increased with increasing prepulse intensities as expected, but this effect was dependent on genotype (F 3,69 = 3, p = 0.035). Post-hoc analyses did not detect significant differences between genotypes at specific prepulse intensities (see Fig. 3d, e). Startle magnitude was similar in control and MS mice and was independent of genotype (control mice 383.1 ± 50.6, MS mice 312.7 ± 56.1).
Foot shock sensitization
129S6 strain (n = 10–12 per condition)
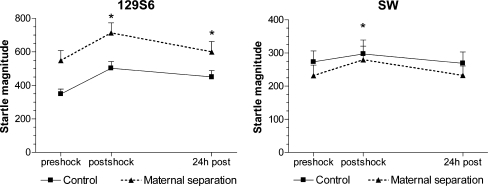
As shown in Fig. 4a, presentation of foot shocks increased the startle response (F 2,80 = 23, p < 0.001), measured post-shock (F 1,40 = 49.1, p < 0.001), and 24 h later (F1,40 = 13.2, p = 0.001). These effects of foot shock were independent of rearing condition and genotype. No significant differences were observed between wild-type and 1AKO mice in this procedure (see Table 2). Foot shock reactivity to the shocks per se was similar for all experimental groups (wt-control 1,536 ± 87.6, 1AKO-control 1,289 ± 72.8, wt-MS 1,346 ± 86.7, 1AKO-MS 1,324 ± 56.7).
Fig. 4.
Foot shock sensitization of the acoustic startle response. Mean startle magnitude (in arbitrary units ± SEM) before, after, and upon re-exposure to the context 24 h later. Left panel shows the effect in the 129S6 strain (wild-type and 1AKO together), while right panel shows the effect in the SW strain (wild-type and 1AKO mice together). Effects on wild-type and 1AKO mice are not shown separately, as the effects of foot shock and MS were independent of genotype. The significant overall effect of MS (black triangles) on startle reactivity in 129S6 mice is not indicated in the left panel, as the effect of foot shock was independent of rearing condition. * p < 0.05 relative to pre-shock for control and MS groups together
Table 2.
Foot shock sensitization
| 129S6 | SW | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | MS | Control | MS | |||||
| WT | 1AKO | WT | 1AKO | WT | 1AKO | WT | 1AKO | |
| Pre-shock | 352 ± 45.3 | 346 ± 39.9 | 608 ± 73.2 | 486 ± 92.9 | 278 ± 45.2 | 260 ± 49.0 | 267 ± 48.8 | 182 ± 28.6 |
| Post-shock | 469 ± 59.0 | 534 ± 55.2 | 795 ± 87.5 | 625 ± 78.0 | 319 ± 52.4 | 285 ± 64.5 | 298 ± 59.0 | 253 ± 55.0 |
| 24-h post | 473 ± 60.2 | 430 ± 50.7 | 646 ± 79.9 | 550 ± 69.4 | 281 ± 63.3 | 258 ± 53.9 | 255 ± 42.8 | 200 ± 30.9 |
Mean startle magnitude (±SEM) pre-shock, post-shock, and 24-h post (24-h post-shock) for 129S6 and SW mice. Data are given for rearing condition [control and maternal separation (MS)] and genotype [wild-type (WT) and 1AKO mice] separately
SW strain (n = 8–14 per condition)
As shown in Fig. 4b, presentation of foot shocks significantly enhanced the startle response (F 2,72 = 3.6, p = 0.03), which could be ascribed to an effect on post-shock startle magnitude (F 1,42 = 5.3, p = 0.03). 24 h later, the startle response did not differ significantly from the startle response measured before the foot shocks on day 1. The effect of foot shocks on the startle response was independent of rearing condition and genotype. No significant differences were observed between wild-type and 1AKO mice in this procedure. Foot shock reactivity to the shocks per se was similar for all experimental groups (wt-control 1,398 ± 139.3, 1AKO-control 1,415 ± 178, wt-MS 1,351 ± 117, 1AKO-MS: 1,370 ± 150).
Discussion
In the present study, we determined the susceptibility of 1AKO mice on different background strains to the effects of repeated MS. MS markedly and consistently increased startle reactivity and reduced PPI in later life, but only in 129S6 mice. These effects were independent of 5-HT1A receptor deletion. In SW mice, MS had no effect on startle plasticity. 1AKO mice did not differ from wild-type mice in any of the tests.
Effect of MS on startle reactivity and foot shock sensitization
In all tests, 129S mice exposed to MS showed a consistent and robust increase in baseline startle response. The affective modulation of the startle response, as measured with shock-induced sensitization, however, was not altered by MS.
To the best of our knowledge, there is only one report on the effects of MS on startle reactivity and plasticity in mice. Millstein et al. (2006) studied the effect of MS on PPI in five different inbred mouse strains and measured basal startle response at one stimulus intensity. These authors used two different control groups: a facility-reared mice as we did and a so-called daily handled group. In their study, MS did not increase startle reactivity in any of the strains relative to facility-reared mice. However, MS increased startle reactivity relative to the “handling” group in the 129S1 and FVB/NJ strains (Millstein et al. 2006). Several studies indicate that handling, which is basically separation of pups for 15 min/day for 14 days, may result in resilience to environmental stressors in later life (Holmes et al. 2005; Pryce et al. 2005). As such, handled control groups often show an attenuated behavioral response to stressors relative to facility-reared controls (for review see Holmes et al. 2005). Genetically, 129S1 and 129S6 strains are closely related. The fact that MS increased startle reactivity in 129S6 (this study) and 129S1 mice, albeit relative to a different control group (Millstein et al. 2006), may suggest that 129S strains are more vulnerable to early life stressors. In other species, including rhesus monkeys (Sanchez et al. 2005) and rats (Caldji et al. 2000; Kalinichev et al. 2002), repeated MS also increased startle reactivity, although in rats, this effect is not found consistently (de Jongh et al. 2005).
MS did not alter affective startle modulation as measured with foot shock sensitization. The foot shock-induced increase in startle reactivity, termed “shock sensitization” is a cross-species phenomenon (Davis 1989; Dirks et al. 2001b; Greenwald et al. 1998). Shock sensitization involves rapid contextual fear learning, reflecting anxiety that develops to the environment where aversive stimuli were presented (Richardson 2000; Risbrough et al. 2009). Context conditioning is interesting as it models particular aspects of anxiety such as anxious apprehension. The potential threat associated with context conditioning involves other brain systems than cued fear, which models actual threat (Davis et al. 2010; Veening et al. 2009). As such, mutant mice studies implicated a role for both CRF1 and CRF2 receptors in context conditioning, but not in cued fear (Risbrough et al. 2009). Considering the reported changes in CRF systems following early life adversity (Holmes et al. 2005; Nemeroff 2004), the finding that MS did not alter foot shock sensitization was therefore somewhat surprising. Mice had been exposed to the context during the other tests, a factor that may reduce the level of contextual conditioning (Richardson 2000). But it seems unlikely that this pre-exposure obscured a potential effect of MS on affective startle modulation, as normal shock sensitization was induced in control mice. Therefore, the present data suggest that MS does not alter context conditioning in later life. This observation is in line with a study comparing maternal and genetic effects using different mouse strains. It was shown that foot shock sensitization is mainly influenced by genetic factors, whereas startle reactivity is affected both by genetic and prenatal and postnatal factors (Rose et al. 2008). Accordingly, mouse strains may differ in the magnitude of the response and also in the time frame in which the shock sensitization occurs (Dirks et al. 2001a), as was also found in the present study. To the best of our knowledge, effects of MS on context conditioning in mice have not been reported before. In rats, MS had no effect on context-induced freezing (Kosten et al. 2006; Stevenson et al. 2009a, b) and on reaction to the foot shocks per se (Kosten et al. 2006). The results of the present study confirm and extend those findings: repeated MS in mice has no effect on context conditioning, using startle response as readout.
Studies on the long-term behavioral effects of repeated MS on anxiety-like behavior are scarce. Some studies reported an increase in anxiety- and depression-like behavior following MS (Romeo et al. 2003; MacQueen et al. 2003), but absence of effects has also been reported (Parfitt et al. 2004; Venerosi et al. 2003). In a recent study, the effect of repeated MS on anxiety- and depression-like behavior was assessed comparing five inbred mouse strains (Millstein and Holmes 2007). Overall, MS had no clear effect on anxiety- or depression-like behaviors in any of the strains, as measured in approach avoidance paradigms and the forced swim test. Thus, repeated neonatal MS of mice does not seem to have marked effects on anxiety-like behavior in approach avoidance tests.
The lack of effect of MS on shock sensitization may seem in line with these findings. However, in humans, an increase in baseline startle reactivity has been proposed as a potential vulnerability factor for the development of anxiety disorders (Grillon and Baas 2003; Guthrie and Bryant 2005; Pole et al. 2009). Furthermore, patients with anxiety disorders and individuals at risk for anxiety disorders often show an elevation in baseline startle both in a neutral (Butler et al. 1990; Morgan et al. 1995; Ludewig et al. 2005) and stressful environment. These changes typically occur in the absence of enhanced fear-potentiated startle (for review, see Grillon and Baas 2003). Thus, increased startle reactivity in the absence of altered affective startle modulation, as observed in maternally separated 129S mice, may reflect increased anxiety levels per se or enhanced vulnerability to exaggerated anxiety. In this respect, it is interesting to note that the effects of MS on startle plasticity in 129S6 mice resemble the effects of childhood abuse on startle plasticity in humans (Jovanovic et al. 2009). In PTSD and MDD patients with a history of childhood abuse, startle reactivity was increased, whereas habituation was normal. Childhood abuse also did not alter fear potentiated startle or fear inhibition in this patient group (Jovanovic et al. 2009). In healthy police cadets with a history of childhood trauma, a similar increase in startle reactivity was reported (Pole et al. 2007).
Effect of MS on habituation and PPI
In 129S6 mice, MS impaired PPI but had no effect on habituation of the acoustic startle response. In SW mice, MS did not alter PPI or habituation.
As MS not only reduced PPI but also increased startle reactivity in 129S6 mice, it could be argued that the changes in baseline startle response confounded percent PPI. However, startle magnitude and percent PPI were not significantly correlated in this study. Other studies also demonstrated that the level of PPI is independent of the magnitude of the startle response in mice (Paylor and Crawley 1997; Crawley et al 1997; Pietropaolo and Crusio 2009). Regarding the effects of long-term stressors on baseline startle and PPI in mice, it was shown that isolation rearing reduced PPI, without an effect on baseline startle (Varty et al 2006). And results of a stress manipulation study in rats further demonstrate that an increase in startle reactivity not necessarily results in impaired PPI. It was shown that isolation rearing and isolation housing both increased basal startle magnitude, whereas PPI was only reduced in the isolation reared rats (Wilkinson et al, 1994).
Impaired PPI and habituation have been associated with schizophrenia and other neuropsychiatric disorders, consistent with a deficit in information processing (Braff et al. 1995, 2001; Geyer and Braff 1987; Geyer et al. 1990). But impaired PPI has also been associated with high trait anxiety, PTSD, obsessive–compulsive disorder and panic disorder (Duley et al. 2007; Franklin et al. 2009; Hoenig et al. 2005; Ludewig et al. 2002, 2005). When PPI is reduced and gating functions are impaired, this could result in increased anxious apprehension, which may relate to high trait anxiety. In this light, the observed reduction in PPI in maternally separated 129S6 mice could predispose or add to increased anxiety-like behavior in later life.
In rats, MS (once for 24 h) disrupted PPI and habituation (Ellenbroek et al. 1998, 2004), although this could not be replicated by others (Lehmann and Feldon 2000). As in the present study, the disruption of PPI appeared to be strain-dependent (Ellenbroek and Cools 2000). The one study that determined the effect of MS on PPI in mice did not find alterations in PPI in any of the five inbred strains studied including the 129S1 strain (Millstein et al. 2006). The procedures used by Millstein and co-workers were not very different from those in the present study. Most marked differences included the time frame of MS (from P0 to P13 versus P2–P14 in the present study), and the intensity of the startle probe relative to background noise (55 versus 40 dB in the present study). However, as SW mice appeared to be resistant to the MS procedure in the present study, it is more likely that subtle differences in genetic background between 129S1 and 129S6 contributed to the effects of MS between the two 129S6 strains than procedural variations.
Effects of deletion of 5-HT1A receptor on MS and startle plasticity
1AKO 129S6 and 1AKO SW mice did not differ from wild-type mice in startle reactivity (Fig. 1), habituation (Table 1), PPI (Fig. 3), or shock sensitization (Table 2). Previous studies already showed that deletion of 5-HT1A receptors does not affect startle plasticity in 1AKO mice on a 129S6 background (Dirks et al. 2001c; Dulawa et al. 2000), despite the anxiety-like behavior of these 1AKO mice in approach avoidance tests (Groenink et al. 2003a), and the effects of 5-HT1A receptor agonists on PPI and affective startle modulation (de Jongh et al. 2002; Dulawa et al. 2000; Gogos et al. 2008). The absence of effect of 5-HT1A receptor deletion on context conditioning, as measured with shock sensitization, is in line with studies reporting a normal freezing response under conditions of cue- and context-dependent fear in 1AKO mice on different backgrounds (Groenink et al. 2003a; Klemenhagen et al. 2006). Interestingly, 1AKO mice show an enhanced freezing response to contexts containing both conditioned and novel cues, suggesting that these animals may have a deficit in the processing of aversive stimuli (Tsetsenis et al. 2007).
The fact that 5-HT1A receptor deletion did not interact with exposure to MS was somewhat surprising. First, a functional promoter polymorphism in the 5-HT1A receptor has been associated with increased anxiety in healthy and patient populations (Lemonde et al. 2003; Rothe et al. 2004; Strobel et al. 2003), confirming the function of this receptor in modulating critical anxiety circuits. Second, the same promoter polymorphism has recently been associated with attenuated PPI in humans (Bräuer et al. 2009). Furthermore, we and others showed that absence or blockade of 5-HT1A in early life has considerable effects on anxiety in later life and that the first 2 to 3 weeks of life are especially important in this process (Gross et al. 2002; Vinkers et al. 2010).
It may be argued that the effects of MS in 129S6 mice are such that a further enhancement of the effects by 5-HT1A receptor deletion could not be detected. However, the absence of an interaction effect in SW mice that were resilient to the effects of MS per se argues against this. Our findings suggest that MS and 5-HT1A receptor deletion exert their effect on different neural pathways, resulting in different behavioral changes that do not seem to interact. However, it cannot be excluded that an interaction between rearing condition and 5-HT1A receptor expression does occur when studying other behavioral measures. Also, a potential limitation of the present study is that the experimental animals were derived from homozygous breeding. A recent study showed that 5-HT1A receptor deficiency in dams can produce maladaptive stress responses via two different mechanisms: inheritance of the receptor deficiency and nongenetic transmission during prenatal and postnatal development (Gleason et al 2010). However, in the present study, 5-HT1AKO did not differ from wild-type mice and 1AKO mice were not more sensitive to the effects of early life stress. Although this does not exclude the possibility that maternal factors influenced the behavior, this seems unlikely as maternal environment seems to enhance the risk for anxiety disorders (either genetically or nongenetically) rather than to protect against maladaptation (Gleason et al 2010).
Strain differences in MS and startle plasticity
It is well known that mouse strains vary widely in their emotional reactivity (Bothe et al. 2005; Crawley et al. 1997; van Bogaert et al. 2006), as well as in startle reactivity (Pietropaolo and Crusio 2009; Rose et al. 2008) and PPI (Millstein et al. 2006; Paylor and Crawley 1997). In the present study, comparable strain differences were observed. Basal startle response of 129S6 was markedly higher than that of SW mice, and shock sensitization procedure had longer-lasting effects in 129S6 than in SW mice. These findings are in line with the fact that 129S6 are considered more anxious than SW mice (Groenink et al. 2003a; van Bogaert et al. 2006). Besides strain differences in the expression of anxiety, the genetic differences between strains may also interfere with receptor deletion. Although 5-HT1A receptor deletion had no effect on startle plasticity in either strain, 1AKO SW and 1AKO 129S6 markedly differ in their benzodiazepine sensitivity (Sibille et al. 2000; Pattij et al. 2002b). The present study further stresses the importance of genetic background as a determining factor in the impact of early life adversity on later life. Besides a small interaction between rearing condition, genotype, and startle intensity on startle reactivity, MS had no effect in SW mice, whereas it markedly and consistently increased startle reactivity in 129S6 mice. It may well be that the genetic makeup of the 129S6 strain determines not only its anxious phenotype but also its vulnerability to early life stressors.
In conclusion, we measured startle reactivity, habituation, PPI, and shock-induced sensitization to determine whether MS results in abnormalities in the processing of sensory information that could contribute to enhanced anxiety-like behavior. Results showed that MS increased startle reactivity and impaired PPI, an effect that was dependent on genetic background. Although the present findings await replication, the fact that comparable alterations in startle modulation have been observed in humans exposed to childhood trauma illustrates the added value of using startle reactivity and plasticity in translational research.
Acknowledgments
Conflicts of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. Behavioral differences among fourteen inbred mouse strains commonly used as disease models. Comp Med. 2005;55:326–334. [PubMed] [Google Scholar]
- Braff DL, Swerdlow NR, Geyer MA. Gating and habituation deficits in the schizophrenia disorders. Clin Neurosci. 1995;3:131–139. [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Bräuer D, Strobel A, Hensch T, Diers K, Lesch KP, Brocke B. Genetic variation of serotonin receptor function affects prepulse inhibition of the startle. J Neural Transm. 2009;116:607–613. doi: 10.1007/s00702-009-0222-0. [DOI] [PubMed] [Google Scholar]
- Butler RW, Braff DL, Rausch JL, Jenkins MA, Sprock J, Geyer MA. Physiological evidence of exaggerated startle response in a subgroup of Vietnam veterans with combat-related PTSD. Am J Psychiatry. 1990;147:1308–1312. doi: 10.1176/ajp.147.10.1308. [DOI] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chipman P, Jorm AF, Tan XY, Easteal S. No association between the serotonin-1A receptor gene single nucleotide polymorphism rs6295C/G and symptoms of anxiety or depression, and no interaction between the polymorphism and environmental stressors of childhood anxiety or recent stressful life events on anxiety or depression. Psychiatr Genet. 2010;20:8–13. doi: 10.1097/YPG.0b013e3283351140. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Davis M. Sensitization of the acoustic startle reflex by footshock. Behav Neurosci. 1989;103:495–503. doi: 10.1037/0735-7044.103.3.495. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jongh R, Groenink L, van Der Gugten J, Olivier B. The light-enhanced startle paradigm as a putative animal model for anxiety: effects of chlordiazepoxide, flesinoxan and fluvoxamine. Psychopharmacology (Berl) 2002;159:176–180. doi: 10.1007/s002130100914. [DOI] [PubMed] [Google Scholar]
- de Jongh R, Geyer MA, Olivier B, Groenink L. The effects of sex and neonatal maternal separation on fear-potentiated and light-enhanced startle. Behav Brain Res. 2005;161:190–196. doi: 10.1016/j.bbr.2005.02.004. [DOI] [PubMed] [Google Scholar]
- De Vry J. 5-HT1A receptor agonists: recent developments and controversial issues. Psychopharmacology (Berl) 1995;121:1–26. doi: 10.1007/BF02245588. [DOI] [PubMed] [Google Scholar]
- Dirks A, de Jongh R, Groenink L, van der GJ H, TH OB. Footshock-induced sensitization of the acoustic startle response in two strains of mice. Behav Brain Res. 2001;123:17–21. doi: 10.1016/S0166-4328(01)00193-0. [DOI] [PubMed] [Google Scholar]
- Dirks A, de Jongh R, Groenink L, van der Gugten J, Hijzen TH, Olivier B. Footshock-induced sensitization of the acoustic startle response in two strains of mice. Behav Brain Res. 2001;123:17–21. doi: 10.1016/S0166-4328(01)00193-0. [DOI] [PubMed] [Google Scholar]
- Dirks A, Pattij T, Bouwknecht JA, Westphal TT, Hijzen TH, Groenink L, van der Gugten J, Oosting RS, Hen R, Geyer MA, Olivier B. 5-HT1B receptor knockout, but not 5-HT1A receptor knockout mice, show reduced startle reactivity and footshock-induced sensitization, as measured with the acoustic startle response. Behav Brain Res. 2001;118:169–178. doi: 10.1016/S0166-4328(00)00326-0. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Gross C, Stark KL, Hen R, Geyer MA. Knockout mice reveal opposite roles for serotonin 1A and 1B receptors in prepulse inhibition. Neuropsychopharmacology. 2000;22:650–659. doi: 10.1016/S0893-133X(99)00164-5. [DOI] [PubMed] [Google Scholar]
- Duley AR, Hillman CH, Coombes S, Janelle CM. Sensorimotor gating and anxiety: prepulse inhibition following acute exercise. Int J Psychophysiol. 2007;64:157–164. doi: 10.1016/j.ijpsycho.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Cools AR. The long-term effects of maternal deprivation depend on the genetic background. Neuropsychopharmacology. 2000;23:99–106. doi: 10.1016/S0893-133X(00)00088-9. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, van den Kroonenberg PT, Cools AR. The effects of an early stressful life event on sensorimotor gating in adult rats. Schizophr Res. 1998;30:251–260. doi: 10.1016/S0920-9964(97)00149-7. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, de Bruin NM, van Den Kroonenburg PT, van Luijtelaar EL, Cools AR. The effects of early maternal deprivation on auditory information processing in adult Wistar rats. Biol Psychiatry. 2004;55:701–707. doi: 10.1016/j.biopsych.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Franklin JC, Bowker KB, Blumenthal TD. Anxiety and prepulse inhibition of acousitc startle in normative sample: the importance of signal-to-noise ratio. Pers Indiv Differences. 2009;46:369–373. doi: 10.1016/j.paid.2008.11.004. [DOI] [Google Scholar]
- Geyer MA, Braff DL. Startle habituation and sensorimotor gating in schizophrenia and related animal models. Schizophr Bull. 1987;13:643–668. doi: 10.1093/schbul/13.4.643. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR, Mansbach RS, Braff DL. Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res Bull. 1990;25:485–498. doi: 10.1016/0361-9230(90)90241-Q. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Phifer J, Bradley B, Ressler KJ. Risk and resilience: genetic and environmental influences on development of the stress response. Depress Anxiety. 2009;26:984–992. doi: 10.1002/da.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason G, Liu B, Bruening S, Zupan B, Auerbach A, Mark W, OhJE Gal-Toth J, Toth M. The serotonin1A receptor gene as a genetic and prenatal maternal environmental factor in anxiety. Proc Natl Acad Sci. 2010;107:7592–7597. doi: 10.1073/pnas.0914805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos A, Bogeski M, van den Buuse M. Role of serotonin-1A receptors in the action of antipsychotic drugs: comparison of prepulse inhibition studies in mice and rats and relevance for human pharmacology. Behav Pharmacol. 2008;19:548–561. doi: 10.1097/FBP.0b013e32830cd822. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Bradley MM, Cuthbert BN, Lang PJ. Startle potentiation: shock sensitization, aversive learning, and affective picture modulation. Behav Neurosci. 1998;112:1069–1079. doi: 10.1037/0735-7044.112.5.1069. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–1579. doi: 10.1016/S1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Groenink L, Pattij T, De Jongh R, Van der Gugten J, Oosting RS, Dirks A, Olivier B. 5-HT1A receptor knockout mice and mice overexpressing corticotropin-releasing hormone in models of anxiety. Eur J Pharmacol. 2003;463:185–197. doi: 10.1016/S0014-2999(03)01281-0. [DOI] [PubMed] [Google Scholar]
- Groenink L, van Bogaert MJ, Van Der Gugten J, Oosting RS, Olivier B. 5-HT1A receptor and 5-HT1B receptor knockout mice in stress and anxiety paradigms. Behav Pharmacol. 2003;14:369–383. doi: 10.1097/01.fbp.0000087737.21047.75. [DOI] [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Altered fear circuits in 5-HT(1A) receptor KO mice. Biol Psychiatry. 2000;48:1157–1163. doi: 10.1016/S0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Guthrie RM, Bryant RA. Auditory startle response in firefighters before and after trauma exposure. Am J Psychiatry. 2005;162:283–290. doi: 10.1176/appi.ajp.162.2.283. [DOI] [PubMed] [Google Scholar]
- He M, Sibille E, Benjamin D, Toth M, Shippenberg T. Differential effects of 5-HT1A receptor deletion upon basal and fluoxetine-evoked 5-HT concentrations as revealed by in vivo microdialysis. Brain Res. 2001;902:11–17. doi: 10.1016/S0006-8993(01)02271-5. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, An SS, van den Oord EJ, Neale MC, Kendler KS, Chen X. Association study between the serotonin 1A receptor (HTR1A) gene and neuroticism, major depression, and anxiety disorders. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:661–666. doi: 10.1002/ajmg.b.30656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenig K, Hochrein A, Quednow BB, Maier W, Wagner M. Impaired prepulse inhibition of acoustic startle in obsessive–compulsive disorder. Biol Psychiatry. 2005;57:1153–1158. doi: 10.1016/j.biopsych.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci Biobehav Rev. 2005;29:1335–1346. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Joober R, Zarate JM, Rouleau GA, Skamene E, Boksa P. Provisional mapping of quantitative trait loci modulating the acoustic startle response and prepulse inhibition of acoustic startle. Neuropsychopharmacology. 2002;27:765–781. doi: 10.1016/S0893-133X(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Blanding NQ, Norrholm SD, Duncan E, Bradley B, Ressler KJ. Childhood abuse is associated with increased startle reactivity in adulthood. Depress Anxiety. 2009;26:1018–1026. doi: 10.1002/da.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long–Evans rats. Pharmacol Biochem Behav. 2002;73:131–140. doi: 10.1016/S0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Klemenhagen KC, Gordon JA, David DJ, Hen R, Gross CT. Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. Neuropsychopharmacology. 2006;31:101–111. doi: 10.1038/sj.npp.1300774. [DOI] [PubMed] [Google Scholar]
- Knobelman DA, Hen R, Blendy JA, Lucki I. Regional patterns of compensation following genetic deletion of either 5-hydroxytryptamine(1A) or 5-hydroxytryptamine(1B) receptor in the mouse. J Pharmacol Exp Ther. 2001;298:1092–1100. [PubMed] [Google Scholar]
- Knobelman DA, Hen R, Lucki I. Genetic regulation of extracellular serotonin by 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) autoreceptors in different brain regions of the mouse. J Pharmacol Exp Ther. 2001;298:1083–1091. [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/S0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Lee HJ, Kim JJ. Early life stress impairs fear conditioning in adult male and female rats. Brain Res. 2006;1087:142–150. doi: 10.1016/j.brainres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien LK, Holik A, Attarbaschi T, Mossaheb N, Sacher J, Geiss-Granadia T, Kletter K, Kasper S, Tauscher J. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61:1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Feldon J. Long-term biobehavioral effects of maternal separation in the rat: consistent or confusing? Rev Neurosci. 2000;11:383–408. doi: 10.1515/revneuro.2000.11.4.383. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, Ou XM, Albert PR. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig S, Ludewig K, Geyer MA, Hell D, Vollenweider FX. Prepulse inhibition deficits in patients with panic disorder. Depress Anxiety. 2002;15:55–60. doi: 10.1002/da.10026. [DOI] [PubMed] [Google Scholar]
- Ludewig S, Geyer MA, Ramseier M, Vollenweider FX, Rechsteiner E, Cattapan-Ludewig K. Information-processing deficits and cognitive dysfunction in panic disorder. J Psychiatry Neurosci. 2005;30:37–43. [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Ramakrishnan K, Ratnasingan R, Chen B, Young LT. Desipramine treatment reduces the long-term behavioural and neurochemical sequelae of early-life maternal separation. Int J Neuropsychopharmacol. 2003;6:391–396. doi: 10.1017/S1461145703003729. [DOI] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Millstein RA, Ralph RJ, Yang RJ, Holmes A. Effects of repeated maternal separation on prepulse inhibition of startle across inbred mouse strains. Genes Brain Behav. 2006;5:346–354. doi: 10.1111/j.1601-183X.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Grillon C, Southwick SM, Davis M, Charney DS. Fear-potentiated startle in posttraumatic stress disorder. Biol Psychiatry. 1995;38:378–385. doi: 10.1016/0006-3223(94)00321-S. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Freimer NB, Ng W, Ophoff R, Veijola J, Miettunen J, Jarvelin MR, Taanila A, Flint J. 5-HTTLPR genotype and anxiety-related personality traits: a meta-analysis and new data. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:271–281. doi: 10.1002/ajmg.b.30808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, Grasby PM, Nutt DJ. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Br J Psychiatry. 2008;193:229–234. doi: 10.1192/bjp.bp.107.041186. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65(Suppl 1):18–28. [PubMed] [Google Scholar]
- Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, Eckelman W, Herscovitch P, Charney DS, Drevets WC. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24:589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt DB, Levin JK, Saltstein KP, Klayman AS, Greer LM, Helmreich DL. Differential early rearing environments can accentuate or attenuate the responses to stress in male C57BL/6 mice. Brain Res. 2004;1016:111–118. doi: 10.1016/j.brainres.2004.04.077. [DOI] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Kerr TM, Tecott LH. 5-HT(1A) receptor mutant mice exhibit enhanced tonic, stress-induced and fluoxetine-induced serotonergic neurotransmission. J Neurochem. 2001;77:607–617. doi: 10.1046/j.1471-4159.2001.00254.x. [DOI] [PubMed] [Google Scholar]
- Pattij T, Groenink L, Hijzen TH, Oosting RS, Maes RA, van der Gugten J, Olivier B. Autonomic changes associated with enhanced anxiety in 5-HT(1A) receptor knockout mice. Neuropsychopharmacology. 2002;27:380–390. doi: 10.1016/S0893-133X(02)00317-2. [DOI] [PubMed] [Google Scholar]
- Pattij T, Groenink L, Oosting RS, van der GJ M, RA OB. GABA(A)-benzodiazepine receptor complex sensitivity in 5-HT(1A) receptor knockout mice on a 129/Sv background. Eur J Pharmacol. 2002;447:67–74. doi: 10.1016/S0014-2999(02)01893-9. [DOI] [PubMed] [Google Scholar]
- Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl) 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain: I. Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-X. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, Crusio WE. Strain-dependent changes in acoustic startle response and its plasticity across adolescence in mice. Behav Genet. 2009;39:623–631. doi: 10.1007/s10519-009-9291-y. [DOI] [PubMed] [Google Scholar]
- Pineyro G, Blier P. Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev. 1999;51:533–591. [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Metzler TJ, Best SR, Henn-Haase C, Marmar CR. Associations between childhood trauma and emotion-modulated psychophysiological responses to startling sounds: a study of police cadets. J Abnorm Psychol. 2007;116:352–361. doi: 10.1037/0021-843X.116.2.352. [DOI] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, Marmar CR. Prospective prediction of posttraumatic stress disorder symptoms using fear potentiated auditory startle responses. Biol Psychiatry. 2009;65:235–240. doi: 10.1016/j.biopsych.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: potential animal models in depression research. Neurosci Biobehav Rev. 2005;29:649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson R. Shock sensitization of startle: learned or unlearned fear? Behav Brain Res. 2000;110:109–117. doi: 10.1016/S0166-4328(99)00189-8. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Geyer MA, Hauger RL, Coste S, Stenzel-Poore M, Wurst W, Holsboer F. CRF1 and CRF2 receptors are required for potentiated startle to contextual but not discrete cues. Neuropsychopharmacology. 2009;34:1494–1503. doi: 10.1038/npp.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm Behav. 2003;43:561–567. doi: 10.1016/S0018-506X(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Rose C, Rohl FW, Hanke J, Schwegler H, Yilmazer-Hanke DM. Maternal and genetic effects on the acoustic startle reflex and its sensitization in C3H/HeN, DBA/2JHd and NMRI mice following blastocyst transfer. Behav Genet. 2008;38:596–611. doi: 10.1007/s10519-008-9222-3. [DOI] [PubMed] [Google Scholar]
- Rothe C, Gutknecht L, Freitag C, Tauber R, Mossner R, Franke P, Fritze J, Wagner G, Peikert G, Wenda B, Sand P, Jacob C, Rietschel M, Nothen MM, Garritsen H, Fimmers R, Deckert J, Lesch KP. Association of a functional 1019C > G 5-HT1A receptor gene polymorphism with panic disorder with agoraphobia. Int J Neuropsychopharmacol. 2004;7:189–192. doi: 10.1017/S1461145703004061. [DOI] [PubMed] [Google Scholar]
- Rutter M. Gene–environment interplay. Depress Anxiety. 2010;27:1–4. doi: 10.1002/da.20641. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, Winslow JT. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biol Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Sibille E, Pavlides C, Benke D, Toth M. Genetic inactivation of the Serotonin(1A) receptor in mice results in downregulation of major GABA(A) receptor alpha subunits, reduction of GABA(A) receptor binding, and benzodiazepine-resistant anxiety. J Neurosci. 2000;20:2758–2765. doi: 10.1523/JNEUROSCI.20-08-02758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson CW, Meredith JP, Spicer CH, Mason R, Marsden CA. Early life programming of innate fear and fear learning in adult female rats. Behav Brain Res. 2009;198:51–57. doi: 10.1016/j.bbr.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Stevenson CW, Spicer CH, Mason R, Marsden CA. Early life programming of fear conditioning and extinction in adult male rats. Behav Brain Res. 2009;205:505–510. doi: 10.1016/j.bbr.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Strobel A, Gutknecht L, Rothe C, Reif A, Mossner R, Zeng Y, Brocke B, Lesch KP. Allelic variation in 5-HT1A receptor expression is associated with anxiety- and depression-related personality traits. J Neural Transm. 2003;110:1445–1453. doi: 10.1007/s00702-003-0072-0. [DOI] [PubMed] [Google Scholar]
- Tsetsenis T, Ma XH, Lo Iacono L, Beck SG, Gross C. Suppression of conditioning to ambiguous cues by pharmacogenetic inhibition of the dentate gyrus. Nat Neurosci. 2007;10:896–902. doi: 10.1038/nn1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bogaert MJ, Groenink L, Oosting RS, Westphal KG, van der Gugten J, Olivier B. Mouse strain differences in autonomic responses to stress. Genes Brain Behav. 2006;5:139–149. doi: 10.1111/j.1601-183X.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- Varty GB, Powell SB, Lehmann-Masten V, Buell MR, Geyer MA. Isolation rearing of mice induces deficits in prepulse inhibition of the startle response. Behav Brain Res. 2006;25(169):162–167. doi: 10.1016/j.bbr.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Veening JG, Bocker KB, Verdouw PM, Olivier B, De Jongh R, Groenink L. Activation of the septohippocampal system differentiates anxiety from fear in startle paradigms. Neuroscience. 2009;163:1046–1060. doi: 10.1016/j.neuroscience.2009.06.064. [DOI] [PubMed] [Google Scholar]
- Venerosi A, Cirulli F, Capone F, Alleva E. Prolonged perinatal AZT administration and early maternal separation: effects on social and emotional behaviour of periadolescent mice. Pharmacol Biochem Behav. 2003;74:671–681. doi: 10.1016/S0091-3057(02)01068-7. [DOI] [PubMed] [Google Scholar]
- Vinkers CH, Oosting RS, van Bogaert MJ, Olivier B, Groenink L. Early-life blockade of 5-HT(1A) receptors alters adult anxiety behavior and benzodiazepine sensitivity. Biol Psychiatry. 2010;67:309–316. doi: 10.1016/j.biopsych.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Wilkinson LS, Killcross SS, Humby T, Hall FS, Geyer MA, Robbins TW. Social isolation in the rat produces developmentally specific deficits in prepulse inhibition of the acoustic startle response without disrupting latent inhibition. Neuropsychopharmacology. 1994;10:61–72. doi: 10.1038/npp.1994.8. [DOI] [PubMed] [Google Scholar]
- Zhang K, Xu Q, Xu Y, Yang H, Luo J, Sun Y, Sun N, Wang S, Shen Y. The combined effects of the 5-HTTLPR and 5-HTR1A genes modulates the relationship between negative life events and major depressive disorder in a Chinese population. J Affect Disord. 2009;114:224–231. doi: 10.1016/j.jad.2008.07.012. [DOI] [PubMed] [Google Scholar]