Abstract
Rationale
By enhancing brain anandamide tone, inhibitors of fatty acid amide hydrolase (FAAH) induce anxiolytic-like effects in rodents and enhance brain serotonergic transmission. Mice lacking the faah gene (FAAH−/−) show higher anandamide levels. However, their emotional phenotype is still debated and their brain serotonergic tone remained unexplored.
Objectives and methods
In this study, we tested FAAH−/− mice in the social interaction and the open field tests performed under different lighting conditions (dim and bright) since variations of the experimental context were proposed to explain opposite findings. Moreover, by microdialysis performed under dim light, we analyzed their serotonergic transmission in frontal cortex (FC) and ventral hippocampus (vHIPP).
Results
In both light conditions, FAAH−/− mice showed reduced emotionality, compared to wt controls, as suggested by the increased rearing and reduced thigmotaxis displayed in the open field and by the longer time spent in social interaction. Basal serotonergic tone was higher in the FC of mutant mice as compared to control mice, while no difference was observed in the vHIPP. K+-induced depolarization produced similar increases of serotonin in both areas of both genotypes. An acute treatment with the CB1 antagonist rimonabant completely abolished the emotional phenotype of FAAH−/− mice and prevented the K+-stimulated release of serotonin in their FC and vHIPP, without producing any effect in wt mice.
Conclusions
Our results support the role of FAAH in the regulation of emotional reactivity and suggest that anandamide-mediated hyperactivation of CB1 is responsible for the emotional phenotype of FAAH−/− mice and for their enhanced serotonergic tone.
Keywords: Serotonin, Microdialysis, Frontal cortex, Ventral hippocampus, Anxiety, Open field, Social interaction, Rimonabant, Fatty acid amide hydrolase (FAAH) knockout
Introduction
Brain anandamide tone plays important roles in the modulation of emotionality and mood through the activation of cannabinoid receptor type 1 (CB1). The fatty acid amide hydrolase (FAAH) is the critical regulator of anandamide levels (Freund et al. 2003). In accordance, mutant mice lacking faah gene (FAAH−/− mice) show various signs of exaggerated anandamide tone, such as increased nociceptive threshold, enhanced memory extinction and increased sensitivity to the effects of exogenously administered anandamide (Cravatt et al. 2001; Varvel et al. 2007).
Many observations indicate that FAAH may serve as a new target for the treatment of anxiety and mood disorders (Gaetani et al. 2003, 2009). In fact, the administration of the FAAH inhibitor URB597 produced anxiolytic- and antidepressant-like effects in several behavioural tests for rodents (Kathuria et al. 2003; Naidu et al. 2007; Patel and Hillard 2006; Moreira et al. 2008). These effects were accompanied by elevations of brain anandamide levels (Kathuria et al. 2003) and stimulation of monoaminergic neuronal activity in brain regions controlling mood and emotionality (Berton and Nestler 2006; Gobbi et al. 2005).
In accordance with these results, FAAH−/− mice exhibited reduced anxiety-like behaviour in the elevated plus maze and the light–dark tests (Moreira et al. 2008). Their phenotype was reversed by the systemic administration of the CB1 antagonist rimonabant. Similarly, rimonabant antagonized the effects of URB597 in rodents, thus suggesting an involvement of CB1-receptor-mediated signalling. Opposite observations on FAAH−/− mice were reported by Naidu et al. (2007). Differences in the experimental contexts have been proposed as key factors determining such discrepancies (Moreira et al. 2008). In keeping with this hypothesis, the anxiolytic effects of URB597 were mostly evident under aversive testing condition, i.e. when rats had no habituation to the experimental room or were not previously handled or when a bright light illuminated the testing environment (Naidu et al. 2007; Naderi et al. 2008; Haller et al. 2009).
In this study, we explored whether the emotional phenotype of FAAH−/− mice can vary with the experimental context. To this aim, we evaluated male wild-type (wt) and FAAH−/− mice in two different ethological tests of anxiety, the open field test and the social interaction test. Since a bright illumination of the behavioural apparatus is well established to increase fear in rodents (Valle 1970), both tests were performed in a nonfamiliar environment illuminated with either dim or bright lights. Moreover, considering the likelihood of finding a reduced emotionality in FAAH−/− mice accordingly with the observation reported in the literature, we performed both tests during the light phase (resting phase) when, in general, rodents show a higher anxiety-related behaviour and are more sensitive to a new environmental challenge (Bertoglio and Carobrez 2002; Roedel et al. 2006).
To further characterize the emotional phenotype of FAAH−/− mice, we searched for a neurochemical correlate in their brain serotonin (5-HT) tone, as previous studies showed an endophenotypic augmentation of spontaneous 5-HT neuronal discharge activity (Bambico et al. 2009) in these mice. Therefore, by in vivo microdialysis, we analyzed basal and K+-stimulated 5-HT extracellular levels in the frontal cortex (FC) and ventral hippocampus (vHIPP), two regions receiving 5-HT inputs from the raphe nuclei and highly involved in the regulation of anxiety-related behaviour. In all experiments, the involvement of CB1 receptor activation was tested by evaluating the effects of rimonabant administration.
Materials and methods
Animals
All experiments were carried out on male adult FAAH knockout (FAAH−/−) mice and their wt counterparts (FAAH+/+). All mice used were from the F9 generation, weighed 30–35 g during the time of the experiments. FAAH−/− mice were generated as previously described (Cravatt et al. 2001) and were backcrossed into C57BL6/6 J background. Mutant and wt mice were bred and maintained in the animal facilities of the University of California (Irvine, CA, USA) under standard conditions (12:12 light–dark cycle, lights on at 07:30; temperature at 20 ± 2°C; 50–60% relative humidity, ad libitum access to food and water) until maturation. Thereafter, a batch of the colony was transported to the animal facilities of the University of Foggia, where animals were maintained under similar conditions and group-housed (four to six per cage). One week prior to the behavioural tests, the mice were single-housed. Experimental procedures commenced about 3 months after arrival in the laboratory facility when the mice were approximately 6–7 months old. All experiments on FAAH−/− mice were performed with age-matched wt controls (Bambico et al. 2010).
All the experimental procedures met the Italian National Laws (DL 116/92) and the European Communities Council Directive (86/609/EEC). All efforts were made to minimize the number of animals used in the study and their suffering.
Chemicals
N-Piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-pyrazolecarboxamide (rimonabant) was dissolved in 5% Tween–80/5% polyethylene glycol/saline (by volume) and injected intraperitoneally (i.p.) in a volume of 10 ml/kg of body weight. All HPLC chemicals were from Sigma-Aldrich (Milan, Italy) and Levanchimica (Bari, Italy).
Behavioural testing
Open field test
The open field test is one of the most popular tests for the study of emotionality in rodents. The number of risk assessment postures directed to the open field provides a good measure of the approach response toward novelty that is exploration. Anxiety behaviour in the open field is triggered by two factors: individual testing (the animal is separated from its social group) and agoraphobia (as the arena is very large relative to the animal’s breeding or natural environment) (Prut and Belzung 2003).
All behavioural tests were performed between 08:00 and 14:00. The apparatus was illuminated either by four 40-W neon lamps (bright light condition, 200 lx at the arena level) or by a red lamp of 40 W (dim light condition, 1 lx at the arena level). The open field apparatus consisted of a square arena (28 × 28-cm white Plexiglas box with 15-cm-high walls) with the floor divided into 16 squares (7 × 7 cm) by black lines drawing over it. On the day of experiments, wt mice (n = 9–12 animals per treatment group) and FAAH−/− mice (n = 14–16 animals per treatment group) were transferred to the experimental room immediately before testing. Each mouse received a single i.p. injection of rimonabant (1 mg/kg) or vehicle and 25 min later placed at the centre of the arena, where it was left undisturbed for 5 min. The mice were monitored and videotaped with a camera (Canon MV750i) mounted on a tripod about 1 m above the centre of the arena floor. The following ethological parameters were measured by an observer unaware of genotypes and treatments: (1) the frequency and the duration of rearing (including wall rearing), (2) the thigmotaxis (time spent in the corners and along the walls of the arena), (3) the time spent in self-grooming (wiping, licking, combing or scratching any part of its own body) and (4) locomotor activity (total number of squares crossed with both forepaws).
Social interaction test
Immediately following the open field test, each mouse was left in the arena to undergo the social interaction test. An unfamiliar conspecific juvenile partner (15–17 g) was placed at the centre of the arena and the mice were video-recorded undisturbed for a 5-min period (File and Seth 2003).
The social interaction test is the first animal test of anxiety that endeavoured to use ethologically relevant sources of anxiety and to use a natural form of behaviour as the dependent measure. The endpoints analyzed were the latency to the first social contact and the time spent by the tested mice in social interaction measured as the total time spent sniffing, following, grooming, mounting and crawling under or over the partner. Passive interaction (when the tested mouse sat or lied with body contact, but without interaction, with the juvenile partner) was not included. An increase in social interaction is indicative of an anxiolytic effect, whereas a specific decrease in social interaction indicates an anxiogenic effect (File and Seth 2003).
Motor activity for four consecutive days
To investigate the habituation profile of spontaneous motor activity in the open field test, wt mice (n = 12) and FAAH−/− mice (n = 15) were repeatedly subjected to the open field test for four consecutive days, being transferred to the experimental room just prior to the daily session and returned to their housing room at the end of the session. Each day, the mice were left undisturbed in the arena for 30 min under a dim light condition and their motor activity was measured across 5-min time bins. The overall motor activity was assessed as the total number of squares crossed with both forepaws (horizontal movement) and the frequency and the duration of rearing events (vertical movement).
Microdialysis in freely moving mice
Mice (n = 5–7 animals per group) were anaesthetized by equithensin (3 ml/kg, i.p.) and double-implanted with a CMA/7 guide cannula (CMA Microdialysis, Stockholm, Sweden), vertically placed into the right vHIPP (anterior–posterior, −3.0 mm; lateral, +3.0 mm; ventral, −1.8 mm from bregma), and a hand-made microdialysis linear probe (AN69 Hospal S.p.A; 20 kDa cutoff; 3-mm membrane length), horizontally placed into the FC (anterior–posterior, +5.9 mm; ventral, −4.0 mm from the interaural line) according to the stereotaxic atlas of Franklin and Paxinos (1997). Two days after surgery, the CMA/7 probe (6 kDa cutoff; 2-mm membrane length) was inserted and dialyses were carried out in awake freely moving mice, perfusing the probes with Krebs–Ringer phosphate buffer at a flow rate of 1 μl/min and collecting dialysates every 30 min. The constituents of the buffer were (in mM) 145 NaCl, 2.7 KCl, 1 MgCl2, 1.2 CaCl2 and 2 Na2HPO4, at pH 7.4.
After a 120-min stabilization period, four samples were collected from each probe to establish stable baseline levels of 5-HT. Thereafter, mice were treated with rimonabant (1 mg/kg, i.p.) or vehicle (time, zero) and six samples were further collected. At 180 min after the acute treatment, the mice were subjected to a second i.p. administration of the same drug received before and the probes were transiently perfused for 30 min with KCl (100 mM)-enriched Krebs–Ringer buffer. Four samples were further collected when the perfusion was returned to the previous Krebs–Ringer buffer. After the completion of the microdialysis, each probe placement was verified histologically.
HPLC analysis
The levels of 5-HT were determined by microbore HPLC using a SphereClone 150 × 2-mm column (3 μm packing) with a Unijet cell (BAS, Bioanalytical Systems, Kenilworth Warwickshire, UK) equipped with a 6-mm-diameter glassy carbon electrode (set at + 650 mV) and connected to an electrochemical amperometric detector (INTRO, Antec Leyden, The Netherlands). The flow rate of the mobile phase [85 mM sodium acetate, 0.34 mM EDTA, 15 mM sodium chloride, 0.81 mM octanesulphonic acid sodium salt, 2% acetonitrile (v/v), 9% methanol (v/v), pH 4.85] was 220 μl/min and the total runtime was 40 min.
Statistical analysis
All data were expressed as mean ± SEM. Within-group variability was analysed through Levene test for homogeneity of variances. Most of the data from open field and social interaction tests failed to meet the homoscedasticity assumption; therefore, they were analyzed by Kruskal–Wallis ANOVA followed by orthogonal chi-square (χ 2) partitioning to test the main effects of light (L), genotype (G), treatment (T) and the interactions L × G, L × T, G × T and L × T × G. Mann–Whitney U test with Bonferroni’s correction was used as post hoc test for multiple comparisons.
The results obtained analyzing the locomotor activity, rearing frequency and rearing duration for 4 consecutive days and the results from microdialysis experiments resulted homoscedastic and were analyzed by two-way ANOVA for repeated measures, with “time” as the within variable and “genotype” or “treatment” as the between variable, depending from the experimental design. Violations of the sphericity assumption were corrected using the Greenhouse–Geisser epsilon correction to adjust the degrees of freedom. Dunnett’s post hoc comparisons were used where appropriate. Overall, basal 5-HT levels were calculated as marginal means of the first four dialysate samples and were analysed by two-tailed Student’s t test. The threshold for statistical significance was set at P < 0.05.
Results
FAAH−/− mice show reduced emotional reactivity in the open field test
The emotional phenotype of FAAH−/− mice was first tested in a nonsocial context using the open field test, a well-established task to evaluate fear reactivity (Choleris et al. 2001). The results obtained by the nonparametric statistical analysis aimed to test the main effects of light (L), genotype (G), treatment (T), and the interactions L × G, L × T, G × T and L × T × G are reported in Table 1.
Table 1.
Results from the nonparametric statistical analysis of data obtained from the open field and social interaction tests
| L | G | T | L×G | L×T | G×T | L×G×T | ||
|---|---|---|---|---|---|---|---|---|
| Rearing frequency | χ 2 | 12.93*** | 16.12*** | 20.66*** | n.s. | n.s. | n.s. | n.s. |
| Rearing duration | χ 2 | 6.88*** | 12.31*** | 25.48*** | n.s. | n.s. | n.s. | n.s. |
| Thigmotaxis | χ 2 | 45.12*** | 7.83** | 7.51** | n.s. | n.s. | n.s. | n.s. |
| Grooming | χ 2 | 15.32*** | 5.80* | 7.55** | n.s. | 4.04* | n.s. | n.s. |
| Locomotor activity | χ 2 | 34.26*** | 25.89*** | n.s. | n.s. | n.s. | n.s. | n.s. |
| Social interaction | χ 2 | 8.15** | 4.46* | 26.20*** | n.s. | n.s. | 11.77** | n.s. |
L light, G genotype, T treatment, χ 2 deriving from the orthogonal partitioning of Kruskal–Wallis H, n.s. P > 0.05 (n = 9–16 per group)
*P < 0.05; **P < 0.01; ***P < 0.001
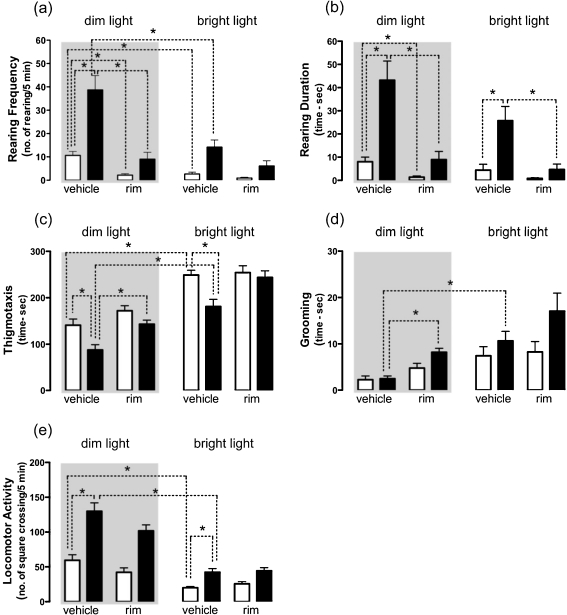
When the mice were tested under a dim light condition, both the rearing frequency and the rearing duration were higher in FAAH−/− than in wt mice (+264% and +440%, respectively; Fig. 1a, b). Such difference disappeared in mice treated with rimonabant and was attenuated in mice exposed to the bright light. In fact, in this condition, only rearing duration remained significantly higher in FAAH−/− mice with respect to control mice (+483%).
Fig. 1.
Effects of the CB1 antagonist rimonabant (rim) on rearing frequency (a), rearing duration (b), thigmotaxis (c), grooming (d), locomotor activity and (e) of FAAH−/− (black bar) and wt (white bar) mice tested in the open field under dim (grey panel) or bright (open panel) light conditions. Data are expressed as mean ± SEM (n = 9–16 per group). *P < 0.05 (Mann–Whitney U test with Bonferroni’s correction)
As for thigmotaxis, FAAH−/− mice spent significantly less time in the corners and along the walls compared to wt mice (Fig. 1c). This difference was evident under both dim and bright light conditions (−38% and −27% in FAAH−/− mice, respectively) but was abolished by rimonabant treatment. As expected, the bright light caused a significant increase of thigmotaxis in both genotypes (+77% in wt and +107% in FAAH−/− mice).
The occurrence of grooming behaviour was similar in both genotypes, when mice were injected with vehicle and tested under a dim light (Fig. 1d). However, either rimonabant treatment in dim light condition or the exposure to the bright light condition produced an increase of grooming behaviour in FAAH−/− mice (+238% and +339%, respectively), but not in wt mice.
A further difference between the two genotypes was found when we measured locomotor activity during the 5-min period of the open field test (Fig. 1e). In particular, although the bright light condition reduced the locomotor activity in mice of both genotypes, the mutant mice showed a significantly higher number of crossings with respect to wt mice when tested either in dim or bright light (+118% and +112%). Rimonabant treatment did not affect the locomotor activity in both genotypes.
FAAH−/− mice show reduced emotional reactivity in the social interaction test
We further evaluated the behavioural phenotype of FAAH−/− mice in a social context, using the social interaction test, which is sensitive to a number of environmental and physiological factors that can affect anxiety (File and Seth 2003).
The results regarding nonparametric analysis aiming to test the main effects of light (L), genotype (G), treatment (T) and the interactions L × G, L × T, G × T and L × T × G are reported in Table 1.
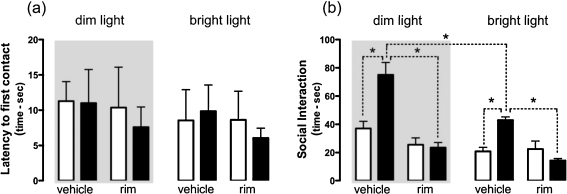
An evident phenotypical difference between the two genotypes was observed in this test. In fact, although the animals from all groups showed similar latencies to the first contact with the conspecific (Fig. 2a), compared to wt, FAAH−/− mice spent significantly more time engaged in active interaction, both when the test was performed in dim light (+83%) and when mice were tested in bright light (+106%) (Fig. 2b). Rimonabant treatment did not affect the social interaction in wt mice, regardless of the lighting condition, whereas it significantly reduced the social activity of FAAH−/− mice, tested either in dim (−65%) or in bright light (−66%), thus abolishing the behavioural differences between the two genotypes. Bright lighting condition significantly reduced the social interaction activity of FAAH−/− mice, without affecting the behaviour in wt mice.
Fig. 2.
Effects of the CB1 antagonist rimonabant (rim) on the latency to the first social contact (a) and on the time spent in social interaction (b) displayed by FAAH−/− (black bar) and wt (white bar) mice in the social interaction test under dim (grey panel) or bright (open panel) light conditions. Data are expressed as mean ± SEM (n = 9–16 per group). *P < 0.05 (Mann–Whitney U test with Bonferroni’s correction)
FAAH−/− mice show a normal habituation profile of spontaneous motor activity
The higher locomotor activity displayed by FAAH−/− mice during the 5-min open field test raised the question of whether it might be an indicator of a better response to novelty and stressing situation or the consequence of a mere general hyperactive behaviour. Addressing this issue was fundamental to interpret the results obtained from the social interaction test. In fact, if FAAH−/− mice were generally hyperactive, the longer time spent in social interaction could not necessarily reflect a lower emotional reactivity with respect to wt mice. To explore these possibilities, we repeatedly subjected the mice to a 30-min open field test in dim light for four consecutive days to analyse their habituation profile in a novel environment.
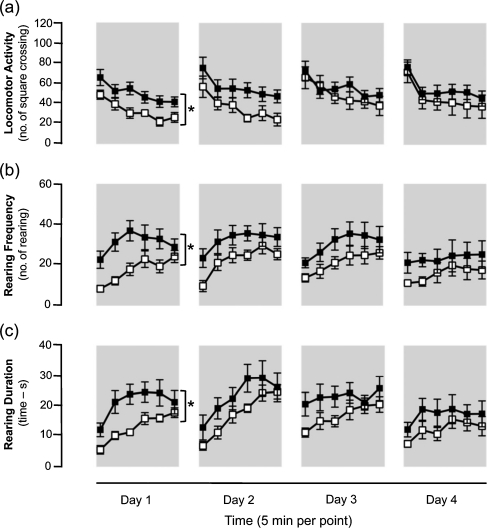
In each day, a regular habituation profile of spontaneous locomotor activity was observed in both genotypes (Fig. 3a). The two-way ANOVA for repeated measures revealed that the locomotor activity of FAAH−/− mice was higher than of wt mice only during the first day, whilst no difference was observed during the following days (1st day: main effect of time F 5,125 = 16.06, P < 0.0001; main effect of genotype F 1,25 = 6.37, P < 0.05; interaction between effects of time and genotype F 5,125 = 0.777, n.s.; 2nd day: main effect of time F 5,125 = 10.342, P < 0.0001; 3rd day: main effect of time F 5,125 = 8.584, P < 0.0001; 4th day: main effect of time F 5,125 = 9.427, P < 0.0001).
Fig. 3.
Four-day locomotor activity (a), rearing frequency (b) and rearing duration (c) of FAAH−/− (black squares) and wt (white squares) mice measured during the 30-min open field test (5 min per point) performed under dim light condition. Data are expressed as mean ± SEM (n = 12–15 per group). *P < 0.05 (two-way ANOVA for repeated measures: main effect of genotype)
Moreover, the analysis of overall rearing activity (often referred as vertical movement) confirmed the genotype difference only during the first day of the open field, whilst no difference was observed during the following days (Fig. 3b, c). In fact, FAAH−/− mice had higher rearing frequency (genotype difference, F 1,125 = 7.076, P < 0.05) and rearing duration (genotype difference, F 1,125 = 7.051, P < 0.05) compared to wt mice.
FAAH−/− mice show enhanced 5-HT-ergic tone in FC and higher sensitivity to rimonabant effects
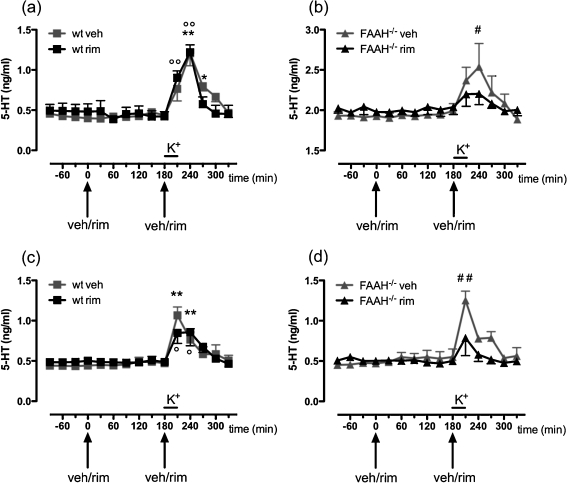
After a 2-h stabilization period, the basal extracellular levels of 5-HT reached a steady state in both FC and vHIPP of all mice. The overall basal 5-HT levels (calculated as the marginal means of the first four dialysates) in the FC of FAAH−/− mice (1.93 ± 0.04 ng/ml) resulted significantly (t 9 = 33.15, P < 0.0001) higher compared to wt mice (0.42 ± 0.01 ng/ml; Fig. 4a, b). Conversely, basal 5-HT level in vHIPP did not differ (t 9 = 1.207, n.s.) between the two genotypes (wt: 0.44 ± 0.02 ng/ml; FAAH−/−: 0.46 ± 0.01 ng/ml; Fig. 4c, d).
Fig. 4.
Effects of vehicle (veh) and rimonabant (rim) administrations on the basal and K+-stimulated (horizontal black line) 5-HT extracellular levels in the FC (a, b) and vHIPP (c, d) of wt (panel a, c) and FAAH−/− (b, d) freely moving mice. Arrows indicate the time of vehicle or rimonabant administrations. Data are expressed as mean ± SEM (n = 5–7 per group). *P < 0.05, **P < 0.01 vs last basal wt veh; °P < 0.05 and °°P < 0.01 vs last basal wt rim; # P < 0.05 and ## P < 0.01 vs last basal FAAH−/− veh (Dunnett’s multiple comparison test). Please note that different scale units were used in b due to the much higher basal 5-HT levels in FAAH−/− mice
Moreover, in both genotypes, the first systemic administration of rimonabant (1 mg/kg, i.p.) did not alter the basal extracellular 5-HT levels either in FC (Fig. 4a, b) or vHIPP (Fig. 4c, d) with respect to animals of the same genotype treated with vehicle.
K+-stimulation significantly increased the extracellular 5-HT release in the FC (main effect of time: F 5,45 = 40.166, P < 0.001; Fig. 4a, b) and vHIPP (main effect of time: F 5,45 = 20.109, P < 0.001; Fig. 4c, d) of all mice treated with vehicle. However, the magnitude of such increase in the FC varied between the two genotypes (interaction between effects of time and genotype: F 5,45 = 3.171, P < 0.05; main effect of genotype: F 1,9 = 190.114, P < 0.001). In fact, in the FC of wt mice (Fig. 4a) treated with vehicle, the 5-HT levels reached 1.21 ng/ml 60 min after the beginning of K+-stimulation (287% of basal values) while the peak of extracellular 5-HT levels in FAAH−/− mice (Fig. 4b) treated with vehicle was 2.54 ng/ml (127% of basal values) at the same time point.
In contrast, hippocampal 5-HT release (Fig. 4c, d) increased to the same extent in vehicle-treated mice of both genotypes (+228% of basal values in wt mice and +261% in FAAH−/− mice).
Surprisingly, the second systemic administration of rimonabant (1 mg/kg, i.p.) given before the K+-stimulation completely prevented the effects of depolarization on 5-HT outflow both in the FC (main effect of time: F 5,55 = 29.774, P < 0.001; interaction between effects of time and treatment: F 5,55 = 13.943, P < 0.001; main effect of treatment: F 1,11 = 5.050, P < 0.05; Fig. 4b) and in the vHIPP (main effect of time: F 5,55 = 8.540, P < 0.01; interaction between effects of time and treatment: F 5,55 = 1.533, n.s.; main effect of treatment: F 1,11 = 9.528, P < 0.05; Fig. 4d) of FAAH−/− mice. Conversely, in wt mice, the second administration of rimonabant did not modify K+-induced 5-HT release in the FC (main effect of time: F 5,50 = 31.111, P < 0.001; Fig. 4a) and vHIPP (main effect of time: F 5,45 = 16.114, P < 0.01; Fig. 4c).
Discussion
In this study, we provide evidence (across social and nonsocial tests) that, compared to wt counterparts, FAAH−/− mice exhibit lower anxiety levels, regardless of the experimental conditions, and show constitutively higher 5-HT-ergic tone in the FC, an important area for the control of mood and emotion.
Interactions between experimental conditions and endocannabinoid modulation have been often reported in a variety of behavioural paradigms aiming to test the effects of CB1 activation or blockade on rodent anxiety (Haller et al. 2004a, b, 2009; Hill and Gorzalka 2004; Martin et al. 2002; Naidu et al. 2007; Patel et al. 2004, 2005; Naderi et al. 2008).
In our experiments, the emotional phenotype of FAAH−/− mice was observed both in the open field and the social interaction tests. The experimental context was varied by changing the illumination intensity, and the lower emotional reactivity of mutant mice prevailed under either dim or bright light condition. In particular, FAAH−/− mice showed higher locomotor and rearing activity and reduced thigmotaxis in the open field test; they as well spent a greater time engaged in active interaction in the social interaction test. An increase in social interaction is generally considered indicative of reduced anxiety when not concomitant with an increase in motor activity (File and Seth 2003). Therefore, the higher locomotor activity observed in FAAH−/− mice tested in the open field raised the hypothesis that their increased social interaction might be a mere consequence of general hyperactivity rather than the expression of reduced emotionality. To exclude this possibility, we repeatedly monitored mouse motor activity in the open field for four consecutive days over a 30-min session each day. The results demonstrated that FAAH−/− mice were slightly hyperkinetic only on the first day, when motor activity is thought to be the net result of exploration drive and anxiety-like inhibition of the animal in a novel environment (Denenberg 1969). In fact, on subsequent days, when exploratory behaviour is less influenced by fear, the difference between genotypes completely disappeared. Moreover, in the social interaction test, we observed that the latency to the first social contact was similar in all groups of animals, thus further suggesting no difference in motor capacities between the two genotypes.
Several studies have repeatedly demonstrated that the pharmacological facilitation of endocannabinoid signalling produces anxiolytic effects in rodents (for review, see Gaetani et al. 2003, 2009; Hill et al. 2009). In most cases, these effects are paralleled by an increased 5-HT neuron firing activity in the raphe nuclei, the major source of 5-HT neurons. In line with these evidences, previous studies showed that FAAH-null mice exhibit an endophenotypic augmentation of spontaneous 5-HT neuronal discharge activity (Bambico et al. 2009) and recent observations on the same mice reported desensitized 5-HT2A/2C receptors in the prefrontal cortex (Bambico et al. 2010).
In the present study, we found that FAAH−/− mice have a fourfold higher 5-HT extracellular level in the FC, with respect to wt mice, a trait evident under both basal and K+-stimulated conditions.
A down-regulation of 5-HT2A/2C receptors in the prefrontal cortex appears consistent with the behavioural profile of FAAH−/− mice and with the enhanced 5-HTergic tone observed in their FC. In support of this hypothesis, it is known that the anti-anxiety effects produced by chronic administration of selective serotonin reuptake inhibitors are related to the increase of 5-HT transmission and the down-regulation of 5-HT2A/2C receptors in the prefrontal cortex (Yamauchi et al. 2004, 2006).
Differently from the FC observation, we did not detect any difference between genotypes when we measured 5-HT outflow in the vHIPP either in basal or K+-stimulated conditions.
Based on these observations and considering the assumption that 5-HT release and activation of 5-HT2C receptors in the vHIPP are associated with increased anxiety-like behaviour (Alves et al. 2004; Rex et al. 2005) while lesions of the vHIPP have anxiolytic effects (Bannerman et al. 2003; Degroot and Treit 2004), we hypothesize that the reduced emotionality observed in FAAH null mice might mostly depend on their higher 5-HT-ergic tone in FC. A support to this hypothesis can be found in previous microinjection studies revealing that low doses of the FAAH inhibitor URB597 produce anxiolytic-like effects in rats when injected in the prefrontal cortex (Rubino et al. 2008), but not in the vHIPP (Roohbakhsh et al. 2009). In keeping with our hypothesis, it has been proven that stimulation of CB1 receptors within the FC increases the activity of 5-HT neurons in the dorsal raphe through a multi-synaptic circuit (for review, see Hill et al. 2009) and, in turn, the 5-HT interaction with 5-HT(2)-type receptors is able to evoke endocannabinoid release (Best and Regehr 2008). Therefore, we might speculate that the hyperactivation of CB1 receptors within the FC of FAAH−/− mice might account for the enhanced 5-HT activity observed in their dorsal raphe (Bambico et al. 2009) as well as the higher 5-HT levels observed in their FC. In fact, based on the similarity between the effects of inhibitors of anandamide hydrolysis and the observations made on FAAH−/− mice, the behavioural and neurochemical phenotype of these mice appears compatible with their constitutively elevated levels of anandamide in the brain. Therefore, it is reasonable to suppose that an increased activation of CB1 receptors can induce in these mice an enhancement of stress-coping behaviours and a tonic increase of 5-HT outflow in the FC.
In accordance with the hypothesis of CB1 hyperactivation, the distinct behavioural phenotype of FAAH−/− mice in our tests was completely abolished by the systemic administration of the CB1 antagonist rimonabant. Surprisingly, rimonabant treatment caused an increase of thigmotaxis and grooming behaviour in FAAH−/− mice subjected to the open field test under dim light, without affecting both behaviours in wt mice, thus suggesting a hypersensitivity of mutant mice toward the anxiogenic effects produced by the blockade of CB1 receptors. The hypersensitivity of FAAH−/− mice to rimonabant effects was even more evident in our microdialysis study at the beginning of K+-stimulation when we detected a dramatic drop of 5-HT outflow both in the FC and vHIPP following the administration of rimonabant at a dose (1 mg/kg) completely ineffective in wt mice.
This observation is in accordance with the results obtained in rats repeatedly treated with the FAAH inhibitor URB597, in which an increased 5-HT neuronal firing activity was observed and an acute rimonabant administration dramatically decreased the serotonin tone below control levels (Di Marzo et al. 2008). Paradoxically, a few previous studies have also reported a moderately increased 5-HT efflux in the rat FC after the administration of rimonabant (Tzavara et al. 2003) and increased 5-HT extracellular level in the prefrontal cortex of mice lacking CB1 receptor (Aso et al. 2009). In keeping with such observations, behavioural studies have demonstrated antidepressant- and anxiolytic-like effects of CB1 receptor antagonists in rodents (Tzavara et al. 2003; Griebel et al. 2005; Takahashi et al. 2008). However, these few studies are inconsistent with a large body of evidence demonstrating that the genetic or pharmacological interruption of CB1 receptor activity results in enhanced anxiety- and depressive-like signs in rats and mice (Haller et al. 2002, 2004a, b; Marsicano et al. 2002; Martin et al. 2002; Navarro et al. 1997).
Clinical observations corroborate the preclinical findings. In fact, rimonabant treatment as anti-obesity therapy was found to be associated with the development of severe adverse psychiatric events, mainly depression and anxiety (Christensen et al. 2007), which led to the suspension of rimonabant trials. Differences in the baseline endocannabinoid tone might be involved in determining the opposite response of 5-HT transmission and emotionality to the administration of rimonabant in humans and rodents. In particular, one might hypothesize that the blockade of CB1 receptor can produce a dramatic drop of 5-HT activity and stress-coping abilities when the endocannabinoid tone is particularly elevated, while producing no effect or sometimes opposite effects when the endocannabinoid tone is low. It is noteworthy that there is increasing body of data for a hyperactivation of the endocannabinoid system in overweight and obesity disorders, with significant increases of anandamide plasma levels in obese patients (Engeli et al. 2005) and the prevalence of FAAH 385 A mutant allele (Sipe, et al. 2010). The increased sensitivity of FAAH-null mice toward the anxiogenic effects of rimonabant is further supportive of our hypothesis.
It is reasonable to speculate that the blockade of CB1 receptor in a condition of elevated anandamide tone might unmask the action of this molecule on a different cellular target. For example, besides CB1 receptors, a large body of evidence now exist to substantiate the concept that anandamide plays an important role in the modulation of the transient receptor potential vanilloid type 1 (TRPV1) in both the central and peripheral nervous systems (for review, see De Petrocellis and Di Marzo 2009). TRPV1 is expressed in many areas of the brain, including the FC and the HIPP (Mezey et al. 2000; Sanchez et al. 2001; Tóth et al. 2005; Cristino et al. 2006), and its activation produces opposite effects with respect to CB1 activation, including anxiogenic effects (Aguiar et al. 2009). The binding site for anandamide at TRPV1 is located on an intracellular domain (De Petrocellis et al. 2001); thus, if originated from outside the cell, anandamide should activate CB1 first and TRPV1 later, after cellular reuptake; conversely, if biosynthesized from inside the cell, anandamide should activate TRPV1 first (De Petrocellis and Di Marzo 2009). However, at least two main aspects of the system normally limit anandamide action at TRPV1 receptors. Anandamide has very low affinity and potency at TRPV1 compared to CB1 receptors (Ross et al. 2001) and has a short intracellular half-life due to the rapid enzymatic hydrolysis, mostly catalysed by FAAH (Piomelli 2003). These two aspects suggest that, in case of faah gene deletion, the intracellular accumulation of anandamide might indeed correspond to an activation of TRPV1 receptors. However, FAAH−/− mice show several signs of CB1, rather than TRPV1, hyperactivation by anandamide (Cravatt et al. 2001; Varvel et al. 2007). The prevalence of CB1-mediated actions in these mice might be ascribed to the negative modulation of TRPV1 responsiveness indirectly mediated by CB1 activation. The α subunit of the Gi protein coupled to CB1 receptor inhibits adenyl cyclase activity, resulting in the inhibition of protein kinase A (PKA) activation, which, in turn, is required for TRPV1 phosphorylation (for review, see Ross 2003). Reduced PKA-mediated TRPV1 phosphorylation results in the rapid loss of TRPV1 responsiveness (Bhave et al. 2002; Mohapatra and Nau 2003, 2005; Mahmud et al. 2009). The administration of rimonabant in a condition of elevated anandamide tone, such as in the case of FAAH−/− mice, might attenuate the negative control of TRPV1 responsiveness, thus sensitizing these receptors to anandamide. The anxiogenic effects of rimonabant administration observed in mutant mice, but not in wt mice, are consistent with this hypothesis, as an increasing body of evidence suggest a pro-anxiety role of TRPV1 activation mediated by high anandamide levels in the brain (Rubino et al. 2008). Moreover, voltage-dependent priming of TRPV1 receptors is another potent mechanism by which the activation by anandamide may be enhanced (Ahern and Premkumar 2002). This might explain why in FAAH−/− mice rimonabant administration produced a dramatic drop of 5-HT outflow in FC and vHIPP perfused in depolarizing conditions, while it previously did not produce any effect when administered under basal conditions. The mechanism responsible for a TRPV1-mediated decrease of 5-HT release in FC and vHIPP might involve GABA-ergic neurons, as suggested by the finding that TRPV1 agonists can potentiate GABA-dependent paired pulse depression (Al-Hayani et al. 2001) in the CA1 region of the HIPP.
Taken together, our results expand our previous findings on the pharmacological blockade of FAAH by URB597 (Kathuria et al. 2003; Gobbi et al. 2005), indicating that anandamide may participate in the regulation of emotional reactivity and 5-HT transmission and that enhanced 5-HT activity may contribute to the possible therapeutic (antidepressant/anxiolytic) properties of FAAH inactivation. The involvement of TRPV1 activation in the effects produced by rimonabant on FAAH−/− mice deserves further investigations to possibly contribute to unveil the mechanisms responsible for the adverse psychiatric effects observed in humans.
Acknowledgements
This study was supported by Grant FIRB 2006 (to V.C.). The authors thank Dr. Antonio Petrella at the Istituto Zooprofilattico Sperimentale della Puglia e della Basilicata for his invaluable veterinary assistance. Moreover, the authors thank Prof. Lawrence Wilkinson at the School of Psychology, University of Cardiff, UK, for his precious comments and suggestions.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- CB1
Cannabinoid receptor type 1
- FAAH
Fatty acid amide hydrolase
- FAAH−/− mice
Mice lacking the faah gene
- FC
Frontal cortex
- G
Genotype
- L
Light
- PKA
Protein kinase A
- Rim
Rimonabant
- SEM
Standard error of the mean
- T
Treatment
- TRPV1
Transient receptor potential vanilloid type 1
- vHIPP
Ventral hippocampus
- Veh
Vehicle
- Wt
Wild type
- χ2
Chi-square
- 5-HT
Serotonin
Footnotes
Tommaso Cassano and Silvana Gaetani contributed equally to the present study.
References
- Aguiar DC, Terzian AL, Guimarães FS, Moreira FA. Anxiolytic-like effects induced by blockade of transient receptor potential vanilloid type 1 (TRPV1) channels in the medial prefrontal cortex of rats. Psychopharmacology. 2009;205:217–225. doi: 10.1007/s00213-009-1532-5. [DOI] [PubMed] [Google Scholar]
- Ahern GP, Premkumar LS. Voltage-dependent priming of rat vanilloid receptor: effects of agonist and protein kinase C activation. J Physiol. 2002;545:441–451. doi: 10.1113/jphysiol.2002.029561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hayani A, Wease KN, Ross RA, Pertwee RG, Davies SN. The endogenous cannabinoid anandamide activates vanilloid receptors in the rat hippocampal slice. Neuropharmacology. 2001;41:1000–1005. doi: 10.1016/S0028-3908(01)00145-9. [DOI] [PubMed] [Google Scholar]
- Alves SH, Pinheiro G, Motta V, Landeira-Fernandez J, Cruz AP. Anxiogenic effects in the rat elevated plus-maze of 5-HT(2C) agonists into ventral but not dorsal hippocampus. Behav Pharmacol. 2004;15:37–43. doi: 10.1097/00008877-200402000-00005. [DOI] [PubMed] [Google Scholar]
- Aso E, Renoir T, Mengod G, Ledent C, Hamon M, Maldonado R, Lanfumey L, Valverde O. Lack of CB1 receptor activity impairs serotonergic negative feedback. J Neurochem. 2009;109:935–944. doi: 10.1111/j.1471-4159.2009.06025.x. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Duranti A, Tontini A, Tarzia G, Gobbi G. Endocannabinoids in the treatment of mood disorders: evidence from animal models. Curr Pharm Des. 2009;15:1623–1646. doi: 10.2174/138161209788168029. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Cassano T, Dominguez-Lopez S, Katz N, Walker CD, Piomelli D, Gobbi G. Genetic deletion of fatty acid amide hydrolase alters emotional behaviour and serotonergic transmission in the dorsal raphe, prefrontal cortex and hippocampus. Neuropsychopharmacology. 2010;35:2083–2100. doi: 10.1038/npp.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/S0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Carobrez AP. Behavioral profile of rats submitted to session 1–session 2 in the elevated plus-maze during diurnal/nocturnal phases and under different illumination conditions. Behav Brain Res. 2002;132:135–143. doi: 10.1016/S0166-4328(01)00396-5. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Best A, Regehr WG. Serotonin evokes endocannabinoid release and retrogradely suppresses excitatory synapses. J Neurosci. 2008;28:6508–6515. doi: 10.1523/JNEUROSCI.0678-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/S0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/S0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Di Marzo V. Role of endocannabinoids and endovanilloids in Ca2+ signalling. Cell Calcium. 2009;45:611–624. doi: 10.1016/j.ceca.2009.03.003. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Maccarrone M, Davis JB, Finazzi-Agro A, Di Marzo V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J Biol Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- Degroot A, Treit D. Anxiety is functionally segregated within the septo-hippocampal system. Brain Res. 2004;1001:60–71. doi: 10.1016/j.brainres.2003.10.065. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Open-field behaviour in the rat: what does it mean? Ann NY Acad Sci. 1969;159:852–859. doi: 10.1111/j.1749-6632.1969.tb12983.x. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Gobbi G, Szallasi A. Brain TRPV1: a depressing TR(i)P down memory lane? Trends Pharmacol Sci. 2008;29:594–600. doi: 10.1016/j.tips.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Janke J, Batkai S, Pacher P, Harvey-White J, Luft FC, Sharma AM, Jordan J. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–2843. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/S0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. Academic Press, San Diego, CA
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Gaetani S, Cuomo V, Piomelli D. Anandamide hydrolysis: a new target for anti-anxiety drugs? Trends Mol Med. 2003;9:474–478. doi: 10.1016/j.molmed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Gaetani S, Dipasquale P, Romano A, Righett L, Cassano T, Piomelli D, Cuomo V. The endocannabinoid system as a target for novel anxiolytic and antidepressant drugs. Int Rev Neurobiol. 2009;85:57–72. doi: 10.1016/S0074-7742(09)85005-8. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Stemmelin J, Scatton B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol Psychiatry. 2005;57:261–267. doi: 10.1016/j.biopsych.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci. 2002;16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Barna I, Freund TF. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J Neurosci. 2004;19:1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Freund TF. CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav Pharmacol. 2004;15:299–304. doi: 10.1097/01.fbp.0000135704.56422.40. [DOI] [PubMed] [Google Scholar]
- Haller J, Barna I, Barsvari B, Gyimesi Pelczer K, Yasar S, Panlilio LV, Goldberg S. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology (Berl) 2009;204:607–616. doi: 10.1007/s00213-009-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Enhancement of anxiety-like responsiveness to the cannabinoid CB(1) receptor agonist HU-210 following chronic stress. Eur J Pharmacol. 2004;499:291–295. doi: 10.1016/j.ejphar.2004.06.069. [DOI] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, Gorzalka BB. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic–pituitary–adrenal axis. Neuropsychopharmacology. 2009;34:2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Mahmud A, Santha P, Paule CC, Nagy I. Cannabinoid 1 receptor activation inhibits transient receptor potential vanilloid type 1 receptor-mediated cationic influx into rat cultured primary sensory neurons. Neuroscience. 2009;162:1202–1211. doi: 10.1016/j.neuroscience.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin JR, Ballard TM, Higgins GA. Influence of the 5-HT2C receptor antagonist, SB-242084, in tests of anxiety. Pharmacol Biochem Behav. 2002;71:615–625. doi: 10.1016/S0091-3057(01)00713-4. [DOI] [PubMed] [Google Scholar]
- Mezey E, Tóth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci USA. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem. 2003;278:50080–50090. doi: 10.1074/jbc.M306619200. [DOI] [PubMed] [Google Scholar]
- Mohapatra DP, Nau C. Regulation of Ca2+ -dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280:13424–13432. doi: 10.1074/jbc.M410917200. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Naderi N, Haghparast A, Saber-Tehrani A, Rezaii N, Alizadeh AM, Khani A, Motamedi F. Interaction between cannabinoid compounds and diazepam on anxiety-like behaviour of mice. Pharmacol Biochem Behav. 2008;89:64–75. doi: 10.1016/j.pbb.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology (Berl) 2007;192:61–70. doi: 10.1007/s00213-006-0689-4. [DOI] [PubMed] [Google Scholar]
- Navarro M, Hernandez E, Munoz RM, del Arco I, Villanua MA, Carrera MR, Rodriguez de Fonseca F. Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. Neuroreport. 1997;8:491–496. doi: 10.1097/00001756-199701200-00023. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic–pituitary–adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Patel S, Cravatt BF, Hillard CJ. Synergistic interactions between cannabinoids and environmental stress in the activation of the central amygdala. Neuropsychopharmacology. 2005;30:497–507. doi: 10.1038/sj.npp.1300535. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/S0014-2999(03)01272-X. [DOI] [PubMed] [Google Scholar]
- Rex A, Voigt JP, Fink H. Anxiety but not arousal increases 5-hydroxytryptamine release in the rat ventral hippocampus in vivo. Eur J Neurosci. 2005;22:1185–1189. doi: 10.1111/j.1460-9568.2005.04251.x. [DOI] [PubMed] [Google Scholar]
- Roedel A, Storch C, Holsboer F, Ohl F. Effects of light or dark phase testing on behavioural and cognitive performance in DBA mice. Lab Anim. 2006;40:371–381. doi: 10.1258/002367706778476343. [DOI] [PubMed] [Google Scholar]
- Roohbakhsh A, Keshavarz S, Hasanein P, Rezvani ME, Moghaddam AH. Role of endocannabinoid system in the ventral hippocampus of rats in the modulation of anxiety-like behaviours. Basic Clin Pharmacol Toxicol. 2009;105:333–338. doi: 10.1111/j.1742-7843.2009.00449.x. [DOI] [PubMed] [Google Scholar]
- Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Gibson TM, Brockie HC, Leslie M, Pashmi G, Craib SJ, Di Marzo V, Pertwee RG. Structure–activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br J Pharmacol. 2001;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Realini N, Castiglioni C, Guidali C, Vigano D, Marras E, Petrosino S, Perletti G, Maccarrone M, Di Marzo V, Parolaro D. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex. 2008;18:1292–1301. doi: 10.1093/cercor/bhm161. [DOI] [PubMed] [Google Scholar]
- Sanchez JF, Krause JE, Cortright DN. The distribution and regulation of vanilloid receptor VR1 and VR1 5′ splice variant RNA expression in rat. Neuroscience. 2001;107:373–381. doi: 10.1016/S0306-4522(01)00373-6. [DOI] [PubMed] [Google Scholar]
- Sipe JC, Scott TM, Murray S, Harismendy O, Simon GM, Cravatt BF, Waalen J. Biomarkers of endocannabinoid system activation in severe obesity. PLoS One. 2010;5:e8792. doi: 10.1371/journal.pone.0008792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi E, Katayama M, Niimi K, Itakura C. Additive subthreshold dose effects of cannabinoid CB(1) receptor antagonist and selective serotonin reuptake inhibitor in antidepressant behavioral tests. Eur J Pharmacol. 2008;589:149–156. doi: 10.1016/j.ejphar.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Tóth A, Boczán J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Edes I, Csiba L, Blumberg PM. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Brain Res Mol Brain Res. 2005;135:162–168. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Tzavara ET, Wade M, Nomikos GG. Biphasic effects of cannabinoids on acetylcholine release in the hippocampus: site and mechanism of action. J Neurosci. 2003;23:9374–9384. doi: 10.1523/JNEUROSCI.23-28-09374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle FP. Effects of strain, sex, and illumination on open-field behavior of rats. Am J Psychol. 1970;83:103–111. doi: 10.2307/1420860. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Wise LE, Niyuhire F, Cravatt BF, Lichtman AH. Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology. 2007;32:1032–1041. doi: 10.1038/sj.npp.1301224. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Tatebayashi T, Nagase K, Kojima M, Imanishi T. Chronic treatment with fluvoxamine desensitizes 5-HT2C receptor-mediated hypolocomotion in rats. Pharmacol Biochem Behav. 2004;78:683–689. doi: 10.1016/j.pbb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Miyara T, Matsushima T, Imanishi T. Desensitization of 5-HT2A receptor function by chronic administration of selective serotonin reuptake inhibitors. Brain Res. 2006;1067:164–169. doi: 10.1016/j.brainres.2005.10.075. [DOI] [PubMed] [Google Scholar]