Abstract
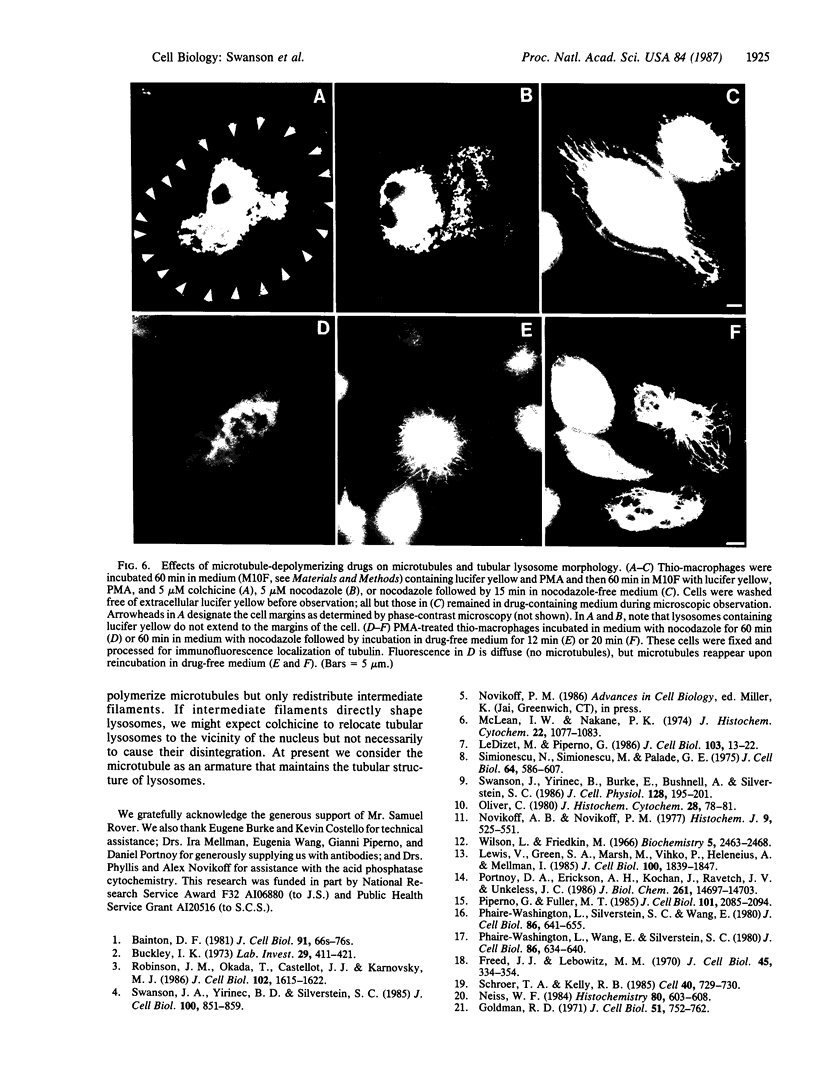
Pinocytosis of the fluorescent dye lucifer yellow labels elongated, membrane-bound tubular organelles in several cell types, including cultured human monocytes, thioglycolate-elicited mouse peritoneal macrophages, and the macrophage-like cell line J774.2. These tubular structures can be identified as lysosomes by acid phosphatase histochemistry and immunofluorescence localization of cathepsin L. The abundance of tubular lysosomes is markedly increased by treatment with phorbol 12-myristate 13-acetate (10 ng/ml). When labeled by pinocytosis of microperoxidase and examined by electron microscopic histochemistry, the tubular lysosomes have an outside diameter of approximately 75 nm and a length of several micrometers; they radiate from the cell's centrosphere in alignment with cytoplasmic microtubules and intermediate filaments. Incubation of phorbol myristate acetate-treated macrophages at 4 degrees C or in medium containing 5 microM colchicine or nocodazole at 37 degrees C leads to disassembly of microtubules and fragmentation of the tubular lysosomes. Return of the cultures to 37 degrees C or removal of nocodazole from the medium leads to reassembly of microtubules and the reappearance of tubular lysosomes within 10-20 min. We conclude that microtubules are essential for the maintenance of tubular lysosome morphology and that, in macrophages, a significant proportion of the lysosomal compartment is contained within these tubular structures.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton D. F. The discovery of lysosomes. J Cell Biol. 1981 Dec;91(3 Pt 2):66s–76s. doi: 10.1083/jcb.91.3.66s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley I. K. The lysosomes of cultured chick embryo cells. A correlated light and electron microscopic study. Lab Invest. 1973 Oct;29(4):411–421. [PubMed] [Google Scholar]
- Freed J. J., Lebowitz M. M. The association of a class of saltatory movements with microtubules in cultured cells. J Cell Biol. 1970 May;45(2):334–354. doi: 10.1083/jcb.45.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. D. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol. 1971 Dec;51(3):752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDizet M., Piperno G. Cytoplasmic microtubules containing acetylated alpha-tubulin in Chlamydomonas reinhardtii: spatial arrangement and properties. J Cell Biol. 1986 Jul;103(1):13–22. doi: 10.1083/jcb.103.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis V., Green S. A., Marsh M., Vihko P., Helenius A., Mellman I. Glycoproteins of the lysosomal membrane. J Cell Biol. 1985 Jun;100(6):1839–1847. doi: 10.1083/jcb.100.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Neiss W. F. A coat of glycoconjugates on the inner surface of the lysosomal membrane in the rat kidney. Histochemistry. 1984;80(6):603–608. [PubMed] [Google Scholar]
- Novikoff A. B., Novikoff P. M. Cytochemical contributions to differentiating GERL from the Golgi apparatus. Histochem J. 1977 Sep;9(5):525–551. doi: 10.1007/BF01002901. [DOI] [PubMed] [Google Scholar]
- Oliver C. Cytochemical localization of acid phosphatase and trimetaphosphatase activities in exocrine acinar cells. J Histochem Cytochem. 1980 Jan;28(1):78–81. doi: 10.1177/28.1.6243325. [DOI] [PubMed] [Google Scholar]
- Phaire-Washington L., Silverstein S. C., Wang E. Phorbol myristate acetate stimulates microtubule and 10-nm filament extension and lysosome redistribution in mouse macrophages. J Cell Biol. 1980 Aug;86(2):641–655. doi: 10.1083/jcb.86.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaire-Washington L., Wang E., Silverstein S. C. Phorbol myristate acetate stimulates pinocytosis and membrane spreading in mouse peritoneal macrophages. J Cell Biol. 1980 Aug;86(2):634–640. doi: 10.1083/jcb.86.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G., Fuller M. T. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985 Dec;101(6):2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Erickson A. H., Kochan J., Ravetch J. V., Unkeless J. C. Cloning and characterization of a mouse cysteine proteinase. J Biol Chem. 1986 Nov 5;261(31):14697–14703. [PubMed] [Google Scholar]
- Robinson J. M., Okada T., Castellot J. J., Jr, Karnovsky M. J. Unusual lysosomes in aortic smooth muscle cells: presence in living and rapidly frozen cells. J Cell Biol. 1986 May;102(5):1615–1622. doi: 10.1083/jcb.102.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer T. A., Kelly R. B. In vitro translocation of organelles along microtubules. Cell. 1985 Apr;40(4):729–730. doi: 10.1016/0092-8674(85)90329-0. [DOI] [PubMed] [Google Scholar]
- Simionescu N., Siminoescu M., Palade G. E. Permeability of muscle capillaries to small heme-peptides. Evidence for the existence of patent transendothelial channels. J Cell Biol. 1975 Mar;64(3):586–607. doi: 10.1083/jcb.64.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. A., Yirinec B. D., Silverstein S. C. Phorbol esters and horseradish peroxidase stimulate pinocytosis and redirect the flow of pinocytosed fluid in macrophages. J Cell Biol. 1985 Mar;100(3):851–859. doi: 10.1083/jcb.100.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Yirinec B., Burke E., Bushnell A., Silverstein S. C. Effect of alterations in the size of the vacuolar compartment on pinocytosis in J774.2 macrophages. J Cell Physiol. 1986 Aug;128(2):195–201. doi: 10.1002/jcp.1041280209. [DOI] [PubMed] [Google Scholar]
- Wilson L., Friedkin M. The biochemical events of mitosis. I. Synthesis and properties of colchicine labeled with tritium in its acetyl moiety. Biochemistry. 1966 Jul;5(7):2463–2468. doi: 10.1021/bi00871a042. [DOI] [PubMed] [Google Scholar]