Abstract
Purpose
The purpose of this study was to investigate whether synovium interposition between repaired tendon ends can integrate into the tendon repair and improve tendon healing strength in a canine tendon explant culture model.
Methods
Eighty flexor digitorum profundus tendons from ten mixed-breed dogs were used for this study. The flexor digitorum profundus tendons were assigned to two groups: repaired tendons with synovium implanted between the cut tendon ends and repaired tendons without any implantation between the tendon ends. The repaired tendons were cultured for either two or four weeks and then assessed mechanically for rupture strength and histologically.
Results
The strength of the repaired tendons with the synovium interposition was significantly higher than the repaired tendons without interposition at both two and four weeks. The strength of the repaired tendons at four weeks was significantly higher than that at two weeks in both groups.
Conclusion
Interpositional synovial grafts have the potential to accelerate tendon healing when it is implanted at the repair site. The exact mechanism of this effect remains to be elucidated.
Keywords: Flexor Tendon, Healing, Synovial Interposition, Mechanical Test, In Vitro
INTRODUCTION
Injuries to the finger flexor tendons are common, especially in the young and working-age population. Urgent primary repair with controlled postoperative mobilization is the current treatment of choice and satisfactory outcomes can be expected in most cases1–6. However, gap formation and rupture after tendon repair remain difficult problems, especially in zone II injuries.
Bulky repairs enhance adhesion formation, while weaker repairs due to tenuous suture strength might increase risk of rupture, both leading to impaired digit and hand function. Even with an ideal repair, tendon healing time, typically 2–3 months, can have a major impact on function and livelihood. Thus, methods that promote rapid tendon healing are constantly being sought by hand surgeons.
Recent studies have shown that synovial cells in the synovial sheath have the potential to migrate to the injury site and accelerate connective tissue healing7–9. Previous in vitro tendon explant studies also investigated tendon intrinsic healing capacity, and its participation in its repair process10, 11. We hypothesized that synovial cells, placed within the tendon repair site, could integrate into the tendon repair and accelerate tendon healing. The purpose of this study was therefore to test this hypothesis and to investigate the effect of direct implantation of synovial tissue between repaired tendon ends, using a canine tendon explant culture model. We chose as our outcome measures the strength and histological appearance of the sutured explant repairs.
METHODS
Eighty flexor digitorum profundus (FDP) tendons were used in this study. The tendons were harvested from the 2nd, 3rd, 4th and 5th digits of both forepaws of ten adult mixed breed dogs who had had procedures performed in the course of other, Institutional Animal care and Use Committee (IACUC) approved, studies. We selected studies which were unlikely to affect tendon viability in the paws. These studies, most of which were short term investigations of cardiac physiology, did not involve the paws and did not involve the administration of medications which might be considered cytotoxic.
The dog tendon assignment was designed prior to the study (Table 1). The synovium of the flexor sheath was also harvested. The tendons from 8 dogs were used for mechanical testing. Each mechanical testing group (C-2, control-2 weeks; C-4, control-4 weeks; S-2, synovium interposition-2 weeks; S-4, synovium interposition-4 weeks) had 16 tendons. The tendons from 2 dogs were analyzed histologically. (H-C-2, histology for control 2 weeks; H-C-4, histology for control 4 weeks; H-S-2, histology for synovium interposition 2 weeks; H-S-4, histology for synovium interposition 4 weeks). Each histology group had 4 tendons.
Table 1.
Tendon Assignment Table
Each experimental group had 16 tendons. (C-2, control-2 weeks; C-4, control-4 weeks; S-2, synovium interposition-2 weeks; S-4, synovium interposition-4 weeks) The tendons from 2 dogs were analyzed histologically. (H-C-2, histology for control 2 weeks; H-C-4, histology for control 4 weeks; H-S-2, histology for synovium interposition 2 weeks; H-S-4, histology for synovium interposition 4 weeks)
| Right paws | Left Paws | |||||||
|---|---|---|---|---|---|---|---|---|
| dog number | 2nd. digit | 3rd. Digit | 4th. digit | 5th. digit | 2nd. digit | 3rd. Digit | 4th. digit | 5th. digit |
| 1 | C-2 | C-2 | C-2 | C-2 | S-2 | S-2 | S-2 | S-2 |
| 2 | S-2 | S-2 | S-2 | S-2 | C-2 | C-2 | C-2 | C-2 |
| 3 | C-2 | C-2 | C-2 | C-2 | S-2 | S-2 | S-2 | S-2 |
| 4 | S-2 | S-2 | S-2 | S-2 | C-2 | C-2 | C-2 | C-2 |
| 5 | C-4 | C-4 | C-4 | C-4 | S-4 | S-4 | S-4 | S-4 |
| 6 | S-4 | S-4 | S-4 | S-4 | C-4 | C-4 | C-4 | C-4 |
| 7 | C-4 | C-4 | C-4 | C-4 | S-4 | S-4 | S-4 | S-4 |
| 8 | S-4 | S-4 | S-4 | S-4 | C-4 | C-4 | C-4 | C-4 |
| 9 | H-C-2 | H-C-2 | H-C-2 | H-C-2 | H-S-2 | H-S-2 | H-S-2 | H-S-2 |
| 10 | H-S-4 | H-S-4 | H-S-4 | H-S-4 | H-C-4 | H-C-4 | H-C-4 | H-C-4 |
Tissue Harvest
Immediately after euthanasia and after sterile preparation and draping, the 2nd, 3rd, 4th and 5th digits from each fore paw were exposed through a lateral incision. The FDP tendons were transected at the metacarpophalangeal (MCP) joint and distal attachment respectively and removed from the flexor sheath. The flexor synovium around the FDP between the distal and proximal pulleys was harvested. The harvested tissues were immediately stored in minimal essential medium (MEM) with Earle’s salts (GIBCO, Grand Island, NY), 10% fetal calf serum and 5% antibiotics (Antibiotic-Antimycotic, GIBCO, Grand Island, NY).
Tendon Repair Technique
Following tissue harvest, the FDP tendons were lacerated at the proximal interphalangeal joint level; here the tendon is composed of two fibrous bundles. Then, the tendons were repaired using two single loop sutures of 6/0 Ethilon (Ethicon, Somerville NJ), one in each bundle. In the synovial implantation group, before tying the loops to close the tendon ends, a synovial patch was implanted between the lacerated tendon ends. The size of synovium patch was trimmed to match the diameter of the tendon (roughly 2–3 mm diameter, depending on the specific tendon). We made no effort to consistently orient the synovial surfaces so that, for example, the parietal surface might always face the proximal portion of the tendon.
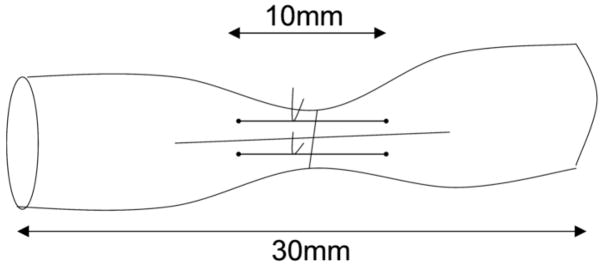
Then the two sutures were tied together to approximate the tendon ends without excessive tension or bunching. In the control group, the tendon was repaired without any interposition. All repaired tendons were trimmed to a 30 mm length, with the repair site at the center (Figure 1).
Figure 1.
Placement of holding suture at the transection site.
Tissue Culture
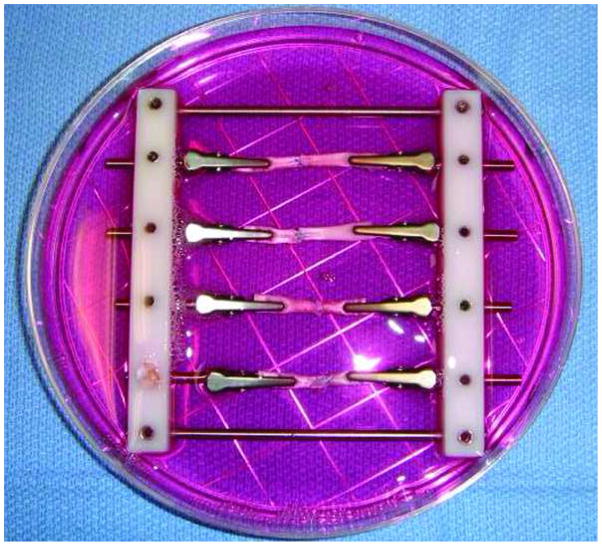
Groups of four repaired tendons, two synovium implanted and two control, were then mounted in custom-made frames to maintain the tendon in a straight alignment (Figure 2). Each frame was placed in a 100 mm Petri dish for incubation in minimal essential medium (MEM) with Earle’s salts (GIBCO, Grand Island, NY), 10% fetal calf serum, 5% antibiotics (Antibiotic-Antimycotic, GIBCO, Grand Island, NY) and incubated at 37 in a 5% CO2 humidified atmosphere. The culture medium was changed every 72 hours. Specimens were cultured for either two or four weeks.
Figure 2.
The custom-made frame used to hold specimens straight alignment in tissue culture.
Biomechanical Testing
At the end of the culture period, the specimens were removed from the culture medium for strength testing. A single loop suture was placed at each end of the test specimen to connect the tendon to a custom-designed mechanical micro-tester (Figure 3). The specimens were set in the testing device with one suture loop connecting to a load transducer (Techniques Inc., Temecula, CA) and the other loop connecting to a motor and potentiometer (Parker Hannifin Corp., Rohnert Park, CA). Before testing, the tendon was placed on a flat saline-moistened plastic dish and the two repair sutures were cut carefully without disrupting the repair site, in order to assess the strength of the healing tissue rather than the suture (Figure 3). This was not done in a blinded fashion, as the synovial patch could not be obscured from view while cutting the sutures.
Figure 3. Test Setup.
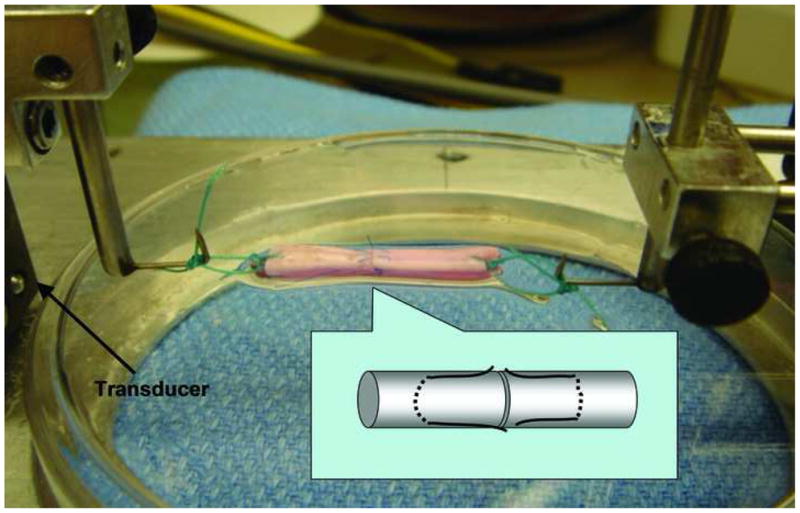
To the left is the transducer, to the right side the motor, which distracted at a rate of 0.1 mm/second. The data from the load transducer was recorded to measure the maximum failure load of the tendon repair site.
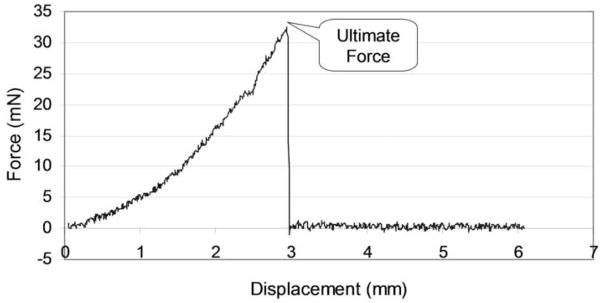
The tendon was then distracted at the speed of 0.1 mm per second until the tendon repair site separated, as noted both visually and on the force/displacement curves generated by the testing device (Figure 4). The ultimate force to failure was then recorded.
Figure 4. Sample Data.
The force increased gradually until failure of the repair site
Histological Analysis
Four tendons in each group were analyzed histologically. These tendons were fixed in 10% formalin, embedded in paraffin and sectioned longitudinally. Sections were stained with hematoxylin and eosin; then examined for cell distribution and cell counts in the tendon ends.
Statistical Analysis
A mixed linear model was used to analyze the strength of load among the 2 treatment groups (without, or with synovium) at 2 time points (2 or 4 weeks) while dog and digit of the tendon were considered as random effects, so that we could control the variance from dog and paw. If a significant treatment effect and time point effect was observed, post ad hoc pairwise comparisons was also studied using the Least Significant Difference (LSD) multiple comparison procedure. A p value ≤ 0.05 was considered as statistical significant.
RESULTS
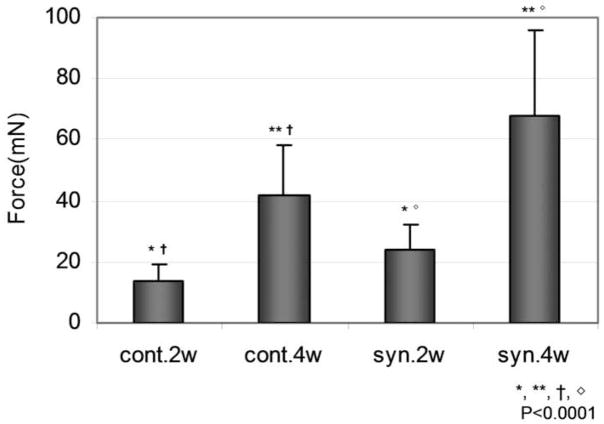
The breaking strength of the repaired control tendons was 13.58 ± 5.89 mN at 2 weeks and 41.88 ± 16.49 mN at 4 weeks. The breaking strength of the repaired synovial interposition tendons was 24.25 ± 7.89 mN at 2 weeks and 67.81 ± 28.11 mN at 4 weeks. The strength of the repaired tendons with the synovium patch was significantly higher than the repaired tendons without a patch at both two and four weeks. The strength of the repaired tendons at four weeks was significantly higher than that at two weeks in both the with and without synovium patch groups (Figure 5).
Figure 5. Load to Failure.
cont. = Without Synovium syn. = Synovium Interposition
2w = 2 Weeks 4w = 4 Weeks
Histologically, cellularity appeared to be less at the repair site in the tendons repaired without implantation (Figure 6) than in the tendons repaired with synovium implantation (Figure 7). The synovial tissue appeared to be incorporating into the repair site, but due to the small number of specimens we did not quantify this measurement. These cells had a fibroblastic appearance (Figure 7). No inflammatory cells were seen in either control or implant tendons; such cells would not be expected in an in vitro model.
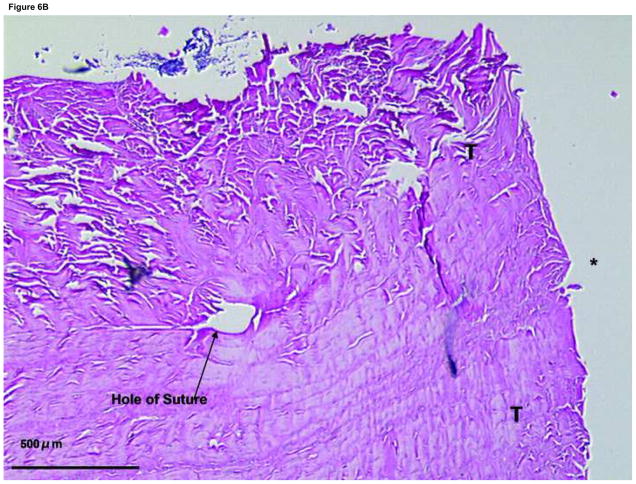
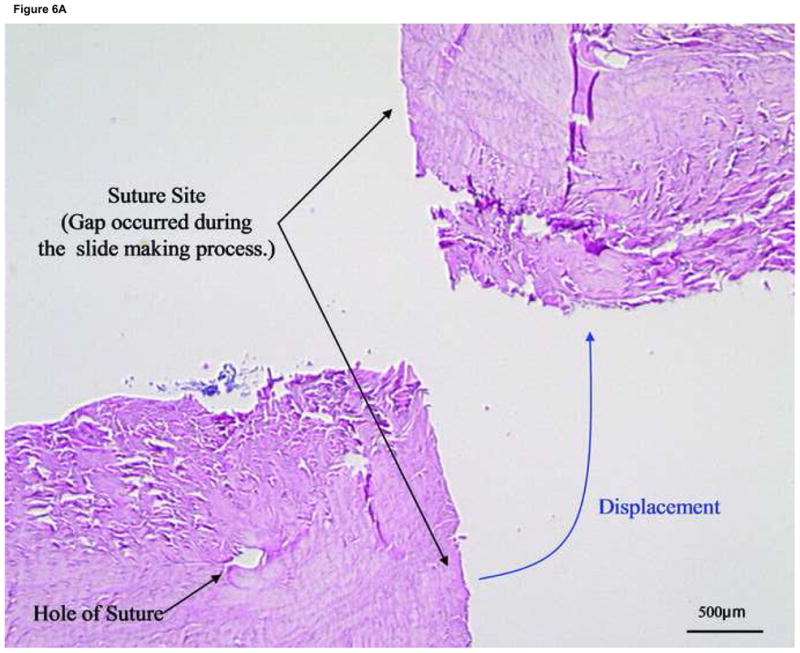
Figure 6. Histology of the suture site after 4 weeks tissue culture in control group.
T: tendon end, S: synovium, *: tendon to tendon apposition site
(A) With the suture cut, the end to end healing strength of the tendon is very low. We have no examples, therefore, of specimens in which the cut ends did not separate during sectioning. (×20)
(B) Suture site of a control group specimen. (×40) The synovium cannot be seen on this section. Note limited cellularity as compared to Figure 7.
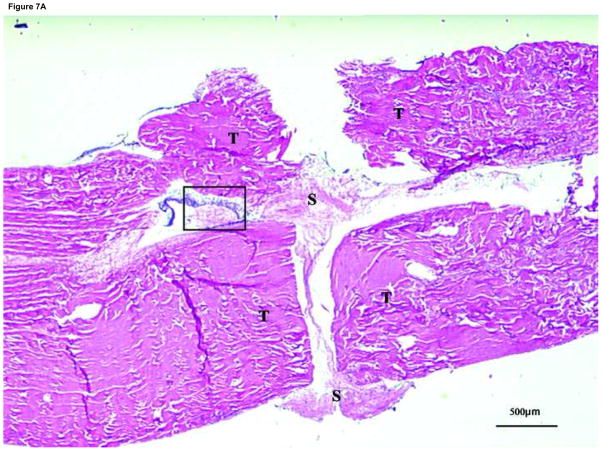
Figure 7. Histology of the suture site after 4 weeks in tissue culture with synovium implantation.
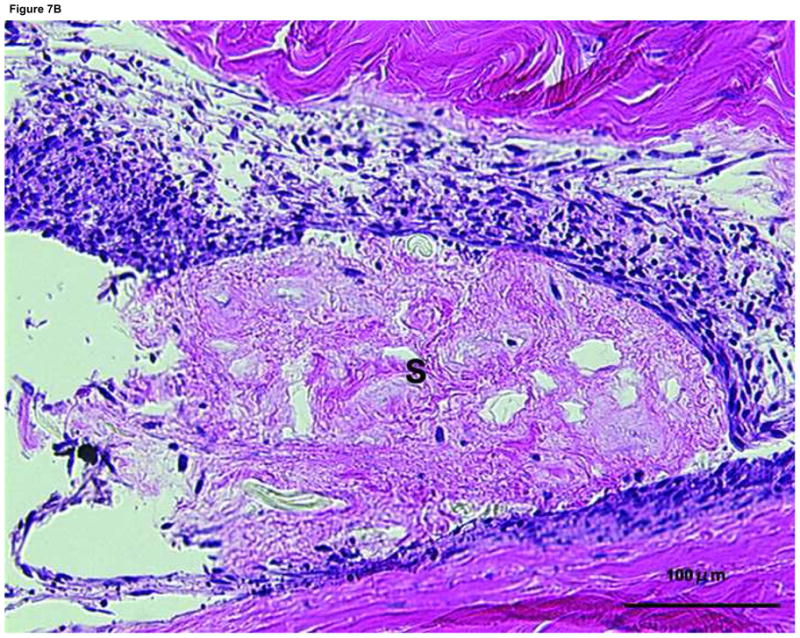
T: tendon end, S: synovium, *: tendon to tendon apposition site
(A) Suture site specimen in the synovium interposition group. Note fibroblast-like cells were around the interposed synovium and migrating into the tendon ends. (×20)
(B) Higher magnification view (X200) of area with the box in Figure 7(A).
DISCUSSION
Although the mechanism of flexor tendon healing still remains controversial10, 12–17 it is well known that intrinsic flexor tendon healing is affected by the hypocellular nature of this tendon tissue18–20. The consequent delay in healing complicates early postoperative rehabilitation, as the tendon tensile strength remains weak for several weeks after repair. During this healing phase the loading needed to effect mobilization may result in gap formation or rupture of the repair site. Strong suture techniques have been developed to overcome these problems21–23, but the increased suture bulk increases gliding resistance24, 25, which can limit tendon gliding and aggravate adhesion formation26. Accelerating the healing process could reduce the need for bulky sutures, reduce the risk of gap or rupture and improve hand function following flexor tendon injury and repair.
This study was designed to better understand the mechanical properties of the healing tendon very early in the healing process. What we have shown here is that, while repaired tendons with sutures in place may have breaking strengths on the order of tens of Newtons in the first few weeks after repair, when there is no functioning suture present the healing tendon itself at this time has a strength two orders of magnitude less. It may take many weeks for the intrinsic healing to match the suture construct strength. Until that time, the tendon is at risk of failure due to suture breakage or pull out. Indeed, these are the common modes of failure of a tendon repair. If it were possible to accelerate the intrinsic healing process, this window of vulnerability might be reduced from the current 6–8 weeks in duration to something less. This would in turn allow faster rehabilitation and return to function.
In this in vitro tendon explant study, we wished to pilot a preparation that might accelerate this intrinsic healing, prior to initiating an in vivo study in a relevant large animal model, such as the dog. Because we were specifically interested in studying this intrinsic healing and not the healing of the tendon-suture construct, we removed the suture. This explains the low breaking strengths we recorded. Nonetheless, we have shown that the synovial patch does appear to incorporate, and to improve the intrinsic healing strength in vitro.
As noted above, the goal of this study was not to identify a patch that could substitute for a suture. Rather, it was to identify a patch that might accelerate the healing process, so that tendon rehabilitation could proceed more rapidly. To test the effect of the patch on the rate of healing, it is necessary to remove the sutures and test the strength of the patch alone, compared to the strength of a cut tendon with no patch. For this reason, the breaking strengths demonstrated here are far lower than those reported in traditional studies of tendon healing, in which the suture is left intact and the breaking strength reflects the material properties of the suture construct.
We have been unable to identify any other studies in which the strength of healing tendon tissue was studied alone, without any contribution from a suture repair. Thus we are unable to put our results in the context of other studies. However, in this study we have shown a significant improvement in breaking strength of the healing tendon itself from the synovial patch. If this patch could accelerate the union of a cut tendon by even a few weeks, it could have a significant impact on tendon rehabilitation and return to activity after tendon injury.
The role of synovial cells in tendon healing has been studied extensively27, 28. Ghadially et al reported that fibroblastic repair tissue derived from the synovium aid the repair of injured tissue7. Richard et al investigated the role of the synovium in flexor tendon healing in vivo and found that synovial cells migrated into the lacerated tendon ends and enhanced tendon healing9. Banes et al demonstrated that synovial cells are able to synthesize fibronectin, which can induce cell spreading and adhesion29. More recent studies have shown that stem cells obtained from synovium have a high proliferation and differentiation potential30–34. This synovial cell migration normally takes several weeks to occur11.
Ultimately, the clinical question addressed here is, does the delivery of a cellularized ‘patch’ between the tendon ends help tendon healing, by delivering more cells to the repair site, or hurt, by further separating the cut tendon ends? In this in vitro explant study, it appeared that the patch actually did help, at least a little. We believe that the synovial implantation may have improved strength both through the delivery of cells and through the presence of cytokines in the synovium. Grossly, it appeared to us that there was greater cellularity (fibroblast-like cells) at the repair site with synovium implantation compared to the tendons repaired without implantation. However, due to the small number of specimens we did not quantify the number of cells at the repair sites. In addition, since we did not label the cells, we cannot be sure of their origin. In a future study, we will study decellularized synovium to distinguish the effect of cell and extracellular matrix on tendon healing in this model.
The principal advantage of this in vitro system is that we can easily isolate and study parameters that might affect healing, such as different cell and tissue types in the patch, the effect of growth factors and, to a limited extent, the effect of applied static or intermittent loading, while reducing research cost and avoiding the use and sacrifice of valuable research animals. The principal disadvantage is, clearly, that it is not an in vivo system. Some in vivo factors cannot be studied in vitro, such as the inflammatory response to injury, the effect of blood supply and the systemic response to tendon injury. We plan to use this in vitro model to narrow down the range of possibilities of tendon “patches” to test subsequently in vivo. Furthermore, since this study focused on the healing strength; we only assessed four samples in each group for histological evaluation. Any quantitative assessment, such as cell counting, would not be reliable with such a small sample size, as only extremely large effect sizes could be identified and even this would be tempered by any tendency for interspecimen variation. Qualitatively, though, it appeared to us that the cellularity was higher in the synovium patch group compared to the control. Finally, we did not study the secretion of cytokines by the tendon explants or our synovial patches. Thus the effect of the synovium patch on tendon healing in vivo is unknown. However, we believe that our results justify further investigation and we are planning such studies. If the findings in the current in vitro model are confirmed in vivo, synovial interposition could provide a potentially useful and clinically simple technique to accelerate and improve tendon healing. However, the actual mechanism by which synovial implantation affects healing in vivo needs to be studied in further detail.
Acknowledgments
This study was supported by a grant from Mayo Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kleinert HE, Kutz JE, Atasoy E, Stormo A. Primary repair of flexor tendons. Orthop Clin North Am. 1973;4:865–876. [PubMed] [Google Scholar]
- 2.Lister GD, Kleinert HE, Kutz JE, Atasoy E. Primary flexor tendon repair followed by immediate controlled mobilization. J Hand Surg [Am] 1977;2:441–451. doi: 10.1016/s0363-5023(77)80025-7. [DOI] [PubMed] [Google Scholar]
- 3.Strickland JW. Flexor tendon repair. Hand Clin. 1985;1:55–68. [PubMed] [Google Scholar]
- 4.Chow JA, Thomes LJ, Dovelle S, et al. A combined regimen of controlled motion following flexor tendon repair in “no man’s land”. Plast Reconstr Surg. 1987;79:447–455. doi: 10.1097/00006534-198703000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Strickland JW. Development of flexor tendon surgery: twenty-five years of progress. J Hand Surg [Am] 2000;25:214–235. doi: 10.1053/jhsu.2000.jhsu25a0214. [DOI] [PubMed] [Google Scholar]
- 6.Baskies MA, Tuckman DV, Paksima N. Management of flexor tendon injuries following surgical repair. Bull NYU Hosp Jt Dis. 2008;66:35–40. [PubMed] [Google Scholar]
- 7.Ghadially FN, Wedge JH, Lalonde JM. Experimental methods of repairing injured menisci. J Bone Joint Surg Br. 1986;68:106–110. doi: 10.1302/0301-620X.68B1.3753606. [DOI] [PubMed] [Google Scholar]
- 8.Churei Y, Yoshizu T, Maki Y, Tsubokawa N. Flexor tendon repair in a rabbit model using a “core” of extensor retinaculum with synovial membrane. An experimental study. J Hand Surg [Br] 1999;24:267–271. doi: 10.1054/jhsb.1998.0006. [DOI] [PubMed] [Google Scholar]
- 9.Harrison RK, Mudera V, Grobbelaar AO, et al. Synovial sheath cell migratory response to flexor tendon injury: an experimental study in rats. J Hand Surg [Am] 2003;28:987–993. doi: 10.1016/s0363-5023(03)00380-0. [DOI] [PubMed] [Google Scholar]
- 10.Manske PR, Lesker PA. Histologic evidence of intrinsic flexor tendon repair in various experimental animals. An in vitro study. Clin Orthop Relat Res. 1984:297–304. [PubMed] [Google Scholar]
- 11.Manske PR, Gelberman RH, Vande Berg JS, Lesker PA. Intrinsic flexor-tendon repair. A morphological study in vitro. J Bone Joint Surg Am. 1984;66:385–396. [PubMed] [Google Scholar]
- 12.Lundborg G. Experimental flexor tendon healing without adhesion formation--a new concept of tendon nutrition and intrinsic healing mechanisms. A preliminary report. Hand. 1976;8:235–238. doi: 10.1016/0072-968x(76)90007-3. [DOI] [PubMed] [Google Scholar]
- 13.Lundborg G, Rank F. Experimental intrinsic healing of flexor tendons based upon synovial fluid nutrition. J Hand Surg [Am] 1978;3:21–31. doi: 10.1016/s0363-5023(78)80114-2. [DOI] [PubMed] [Google Scholar]
- 14.Lundborg G, Hansson HA, Rank F, Rydevik B. Superficial repair of severed flexor tendons in synovial environment. An experimental, ultrastructural study on cellular mechanisms. J Hand Surg [Am] 1980;5:451–461. doi: 10.1016/s0363-5023(80)80075-x. [DOI] [PubMed] [Google Scholar]
- 15.Skoog T, Persson BH. An experimental study of the early healing of tendons. Plast reconstr surg (1946) 1954;13:384–399. doi: 10.1097/00006534-195405000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Potenza AD, Herte MC. The synovial cavity as a “tissue culture in situ”--science or nonsense? J Hand Surg [Am] 1982;7:196–199. doi: 10.1016/s0363-5023(82)80088-9. [DOI] [PubMed] [Google Scholar]
- 17.Chow SP, Hooper G, Chan CW. The healing of freeze-dried rabbit flexor tendon in a synovial fluid environment. Hand. 1983;15:136–142. doi: 10.1016/s0072-968x(83)80002-3. [DOI] [PubMed] [Google Scholar]
- 18.Marsolais D, Frenette J. Inflammation and tendon healing. Med Sci (Paris) 2005;21:181–186. doi: 10.1051/medsci/2005212181. [DOI] [PubMed] [Google Scholar]
- 19.Schulze-Tanzil G, Mobasheri A, Clegg PD, et al. Cultivation of human tenocytes in high-density culture. Histochem Cell Biol. 2004;122:219–228. doi: 10.1007/s00418-004-0694-9. [DOI] [PubMed] [Google Scholar]
- 20.Gelberman RH. Flexor tendon physiology: tendon nutrition and cellular activity in injury and repair. Instr Course Lect. 1985;34:351–360. [PubMed] [Google Scholar]
- 21.Winters SC, Seiler JG, 3rd, Woo SL, Gelberman RH. Suture methods for flexor tendon repair. A biomechanical analysis during the first six weeks following repair. Ann Chir Main Memb Super. 1997;16:229–234. doi: 10.1016/s0753-9053(97)80006-3. [DOI] [PubMed] [Google Scholar]
- 22.Winters SC, Gelberman RH, Woo SL, et al. The effects of multiple-strand suture methods on the strength and excursion of repaired intrasynovial flexor tendons: a biomechanical study in dogs. J Hand Surg [Am] 1998;23:97–104. doi: 10.1016/s0363-5023(98)80096-8. [DOI] [PubMed] [Google Scholar]
- 23.Thurman RT, Trumble TE, Hanel DP, et al. Two-, four-, and six-strand zone II flexor tendon repairs: an in situ biomechanical comparison using a cadaver model. J Hand Surg [Am] 1998;23:261–265. doi: 10.1016/s0363-5023(98)80124-x. [DOI] [PubMed] [Google Scholar]
- 24.Aoki M, Manske PR, Pruitt DL, Larson BJ. Work of flexion after tendon repair with various suture methods. A human cadaveric study. J Hand Surg [Br] 1995;20:310–313. doi: 10.1016/s0266-7681(05)80084-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C, Amadio PC, Zobitz ME, An KN. Gliding characteristics of tendon repair in canine flexor digitorum profundus tendons. J Orthop Res. 2001;19:580–586. doi: 10.1016/S0736-0266(00)00055-3. [DOI] [PubMed] [Google Scholar]
- 26.Zhao C, Amadio PC, Momose T, et al. The effect of suture technique on adhesion formation after flexor tendon repair for partial lacerations in a canine model. J Trauma. 2001;51:917–921. doi: 10.1097/00005373-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Khan U, Edwards JC, McGrouther DA. Patterns of cellular activation after tendon injury. J Hand Surg [Br] 1996;21:813–820. doi: 10.1016/s0266-7681(96)80199-9. [DOI] [PubMed] [Google Scholar]
- 28.Khan U, Occleston NL, Khaw PT, McGrouther DA. Differences in proliferative rate and collagen lattice contraction between endotenon and synovial fibroblasts. J Hand Surg [Am] 1998;23:266–273. doi: 10.1016/S0363-5023(98)80125-1. [DOI] [PubMed] [Google Scholar]
- 29.Banes AJ, Donlon K, Link GW, et al. Cell populations of tendon: a simplified method for isolation of synovial cells and internal fibroblasts: confirmation of origin and biologic properties. J Orthop Res. 1988;6:83–94. doi: 10.1002/jor.1100060111. [DOI] [PubMed] [Google Scholar]
- 30.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 31.Mochizuki T, Muneta T, Sakaguchi Y, et al. Higher chondrogenic potential of fibrous synovium- and adipose synovium-derived cells compared with subcutaneous fat-derived cells: distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006;54:843–853. doi: 10.1002/art.21651. [DOI] [PubMed] [Google Scholar]
- 32.Shirasawa S, Sekiya I, Sakaguchi Y, et al. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. J Cell Biochem. 2006;97:84–97. doi: 10.1002/jcb.20546. [DOI] [PubMed] [Google Scholar]
- 33.Yoshimura H, Muneta T, Nimura A, et al. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 34.Ju YJ, Muneta T, Yoshimura H, et al. Synovial mesenchymal stem cells accelerate early remodeling of tendon-bone healing. Cell Tissue Res. 2008;332:469–478. doi: 10.1007/s00441-008-0610-z. [DOI] [PubMed] [Google Scholar]