Abstract
Infections in skilled nursing facilities (SNFs) are common and result in frequent hospital transfers, functional decline, and death. Colonization with multidrug-resistant organisms (MDROs) – including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and multidrug-resistant gram-negative bacilli (R-GNB) – is also increasingly prevalent in SNFs. Antimicrobial resistance among common bacteria can adversely affect clinical outcomes and increase health care costs. Recognizing a need for action, legislators, policy-makers, and consumer groups are advocating for surveillance cultures to identify asymptomatic patients with MDROs, particularly MRSA in hospitals and SNFs. Implementing this policy for all SNF residents may be costly, impractical, and ineffective. Such a policy may result in a large increase in the number of SNF residents placed in isolation precautions with the potential for reduced attention by health care workers, isolation, and functional decline. Detection of colonization and subsequent attempts to eradicate selected MDROs can also lead to more strains with drug resistance. We propose an alternative strategy that uses a focused multicomponent bundle approach that targets residents at a higher risk of colonization and infection with MDROs, specifically those who have an indwelling device. If this strategy is effective, similar strategies can be studied and implemented for other high-risk groups.
BACKGROUND
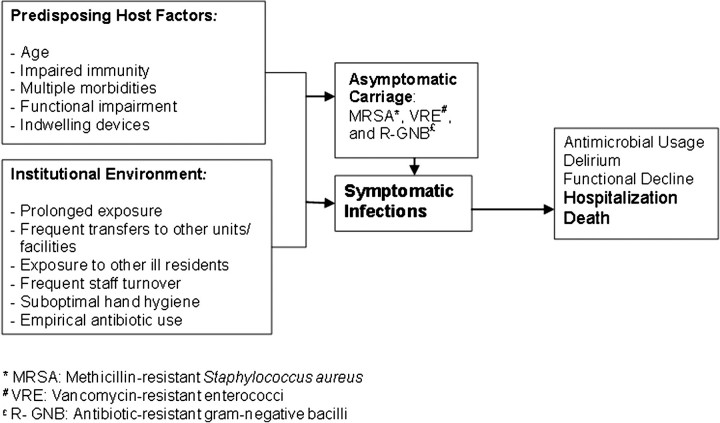
With 1.5 million residents in 16,100 skilled nursing facilities (SNFs) and a burgeoning proportion of short-stay residents, SNFs have become a crucial part of the US health care system [1]. In fact, at any given time, there are more patients in SNFs than in hospitals. Placement of a vulnerable host in an institutional setting with sustained exposure to health care creates numerous opportunities for healthcare-associated infections and acquisition of new multidrug-resistant organisms (MDROs) (Figure 1) [2–4].
Figure 1.
Pathway to antimicrobial resistance and infections in skilled nursing facilities.
Current infection prevention practices in SNFs generally have been adopted from acute care—a much different clinical setting with a much broader-based population. The epidemiology of infections in SNFs differs from that of acute care, and interventions and strategies used in acute care are often impractical and inefficient when applied to the more residential setting of a SNF. SNF staff care for chronic functionally impaired aging residents for a prolonged duration and with fewer resources – including level of access to services such as laboratory and imaging–whereas hospitals serve a broader range of acutely ill patients for a short duration with substantially more infection prevention resources and ready access to support services such as laboratory, pharmacy, and imaging.
MDRO infection prevention and control strategies in acute care hospitals are focused primarily on invasive devices and procedures (including surgery), and they more often include pathogen-specific surveillance initiatives, such as those targeting methicillin-resistant Staphylococcus aureus (MRSA). On the basis of risk assessment, some acute care facilities use proactive screening of all patients to identify MRSA colonization and to institute isolation precautions for those found to be colonized or infected. This is resource intensive and relatively infrequent in SNFs outside of the Department of Veterans Affairs (VA) system. Indeed, several states have passed legislation that mandates screening for MRSA colonization in all hospitalized patients and residents in SNFs, despite lack of scientific evidence that this approach is efficient, cost-effective, and safe for this population [5–7]. A single pathogen focus, although commendable given calls to decrease the prevalence and incidence of MRSA, often diverts limited resources and ignores the wide range of other MDROs, such as vancomycin-resistant enterococci (VRE) and the increasingly prevalent antibiotic-resistant gram-negative bacilli (R-GNB) [2, 4, 8, 9]. In addition, quality of care can suffer as a result of isolation practices. Studies in acute care hospitals have shown that patients in contact precautions have fewer vital sign measurements and fewer physician visits, compared with the number of measurements and visits for patients who are not in contact precautions [10–12]. Although similar studies in SNFs are lacking, older adults are potentially at an even greater risk of adverse psychosocial consequences as a result of isolation practices.
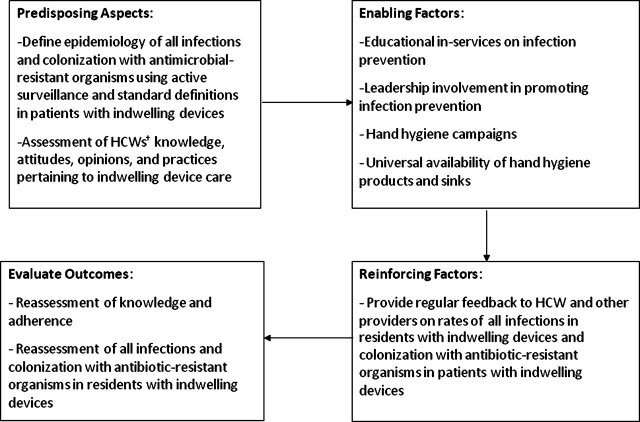
Evidence-based, safe, and practical alternative approaches to infection prevention are needed in SNFs, and at the same time we must make institutional care safer for older adults. In this article, we discuss the merits of one such approach – defining a high-risk group, conducting a needs assessments to evaluate health care workers’ (HCWs) knowledge and practices pertaining to care of this high-risk group, and implementing institutionally acceptable strategies to reduce the rate of infections and MDRO colonization in that group. This approach follows the theoretical framework derived from PRECEDE (predisposing, reinforcing, and enabling factors in educational and health diagnosis and evaluation), a health education model. This model has been used in various studies and research programs, with success in enhancing adherence to complex health behaviors (Figure 2) [13].
Figure 2.
PRECEDE (predisposing, reinforcing, and enabling factors in educational and health diagnosis and evaluation) model to implement interventions in high risk groups. HCW, health care worker.
MOVING FROM A PATHOGEN-BASED TO A RISK FACTOR-BASED MODEL
Defining a High-Risk Group: Residents with Indwelling Urinary Catheters and Feeding Tubes
SNF residents colonized with MDROs share several risk factors: indwelling devices, prior antimicrobial usage, recent hospitalization, and functional impairment [14, 15]. This section reviews the epidemiology of infections, in general, as well as MDRO colonization in one such high-risk group, those with indwelling devices, such as urinary catheters and feeding tubes [16–21].
Urinary catheters are used both short term and long term in SNF residents [13, 15, 17]. Recent studies involving all SNFs from 4 states found indwelling catheters in 12%–13% of all newly admitted patients [17]. Within VA Community Living Centers, 14% of 11,500 residents have an indwelling urinary catheter [17, 21]. The majority of patients with indwelling urinary catheters have persistent bacteriuria [22, 23]. In most SNFs, the leading infection site is the urinary tract, and infections are often associated with having an indwelling urinary catheter [24]. It is estimated that 50% of SNF residents with urinary catheters will have symptomatic catheter-associated urinary tract infections (UTIs) each year. SNF residents with indwelling catheters are more likely to have UTIs or bacteriuria with MDROs than are residents without these devices [25, 26]. These residents are also commonly colonized with MDROs, including MRSA, VRE, and R-GNB, at other body sites [27, 28], and colonizing organisms may be transferred to other residents [29].
Oropharyngeal dysfunction, dementia, anorexia, and stroke are frequently found in SNF residents. Enteral feeding tubes, either nasogastric or percutaneous gastrostomy (PEG) tubes, are often used to provide nutrition to these patients [30, 31]. Approximately 6%–8% of all SNF residents have a feeding tube, with rates of 7%–41% in cognitively impaired SNF residents [18, 20]. Within VA SNFs, approximately 7% of all residents have a feeding tube [21].
PEG tube sites are routinely colonized with organisms; >90% become colonized [22, 32–35]. These organisms are typically S. aureus, including MRSA, and gram-negative organisms, such as Proteus mirabilis and Pseudomonas aeruginosa. Data show that residents with PEGs are often colonized with MDROs at other body sites, such as nares, oropharynx, and groin, which increases the chances of transmission between residents by means of the hands of HCWs [27]. Patients with MRSA at other body sites are also more likely to have MRSA infection at the PEG tube site [34]. Although nutritional requirements are met, rates of reflux and aspiration of gastric contents increase with the use of feeding tubes, which can lead to aspiration pneumonia [36, 37].
Hands of HCWs can act as vectors in the transmission of MDROs from one resident to another. In hospitals, hands of staff have been found to be colonized with MRSA (3%–20%) and VRE (13%–41%) [38, 39]. In one study in an SNF, 65% of HCWs’ hands were colonized with GNB [35]. In another SNF in which R-GNB were common, one-third of nurses carried similar strains on their hands, suggesting horizontal transmission of pathogens [40]. Although evidence is scant, HCWs may be more likely to acquire organisms from SNF residents who are colonized at multiple sites. Thus, an intervention to break the chain of spread of microorganisms, especially MDROs, among HCWs and residents of SNFs (Predisposing Aspects, PRECEDE MODEL, Figure 2) is needed.
Healthcare Workers’ Knowledge on Research-Proven Infection Prevention Practices Pertaining to Device Care
Recommendations based predominantly on evidence from acute care have been published by leading organizations, such as the Centers for Disease Control and Prevention (CDC) and Healthcare Infection Control Practices Advisory Committee (HICPAC), for the prevention of infections and complications associated with both urinary catheters and feeding tubes [24, 41, 42]. However, evidence suggests there may be gaps in knowledge about some of these recommended practices among HCWs in SNFs. For example, studies show that HCWs were often unaware of research-proven recommendations to not disconnect the catheter from its collection bag, to not routinely irrigate the catheter, and to perform hand hygiene even after casual contact [43, 44]. In a study of HCWs in long-term care facilities in the United Kingdom, 35% reported performing regular changes of catheter bags and 55% reported routinely performing bladder irrigations, contrary to UK National Institute for Clinical Excellence recommendations [44]. This intervention compromises the closed-drainage system, and routine irrigations can harm the patient by causing more UTIs. HCWs in this setting are also often unaware of updated recommendations regarding indications for hand hygiene, in particular the use of alcohol-based hand rub [39].
HCWs in SNFs have been shown to learn infection prevention and control practices both through formal didactic methods and through informal methods, such as learning from their nursing managers and supervisors [43]. This suggests that a multipronged approach that includes structured educational in-services, informal discussions with supervisors, and identification of effective linkages, such as medical directors, infection preventionists, long-term care organizations, and nursing mentors, may be required to promote the use of recommended infection prevention practices (Enabling Factors, PRECEDE MODEL, Figure 2).
STRATEGIES TO PREVENT COLONIZATION AND INFECTION WITH MULTIDRUG-RESISTANT ORGANISMS IN HIGH-RISK RESIDENTS
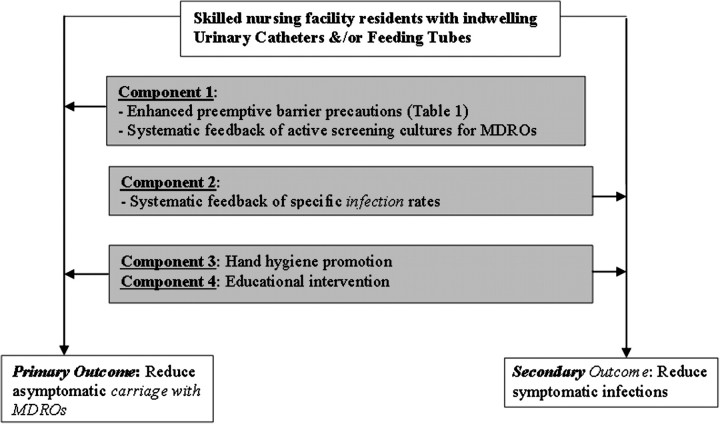
Several strategies, by themselves or in combination as a “bundle” or “multicomponent intervention,” can be individualized and implemented to reduce colonization and infection with MDROs in high-risk residents in SNFs (Figure 3).
Figure 3.
Proposed multicomponent bundle to reduce antimicrobial resistance and infections in high-risk residents of skilled nursing facilities. MDRO, multidrug-resistant organism.
Preemptive Barrier Precautions and Active Screening To Identify Asymptomatic Colonization with Resistant Organisms
The role of single pathogen-based active screening cultures to identify asymptomatic carriers of MRSA with subsequent institution of barrier or isolation precautions has been a subject of much debate [45, 46]. This strategy has been shown to be successful in several European countries. In Denmark, after a 33% increase in bloodstream infections in the 1960s, a comprehensive program including active surveillance cultures was introduced [47]. The rate of MRSA infections decreased to 1% and has remained so for >2 decades. Similar results have been reported from other countries [48].
Nevertheless, several critical gaps and questions remain regarding adoption of this practice in SNFs. Should SNFs conduct pathogen-based screening for all of their residents? Should screening focus only on MRSA or also incorporate other MDROs, such as r-GNB? In SNFs, infections with r-GNB are more common than infections with MRSA [26]. How often should residents be re-screened? Should they be screened from only 1 site? Research shows that single-site screening can miss 30% of patients colonized with MRSA at other sites [27]. Are SNFs equipped to analyze the data in a timely fashion? What are the unintended consequences of placing residents into contact precautions? If there are a limited number of single occupancy rooms in a facility, should the resident colonized with MDRO be cohorted with someone else?
A risk factor-based approach, rather than a pathogen-based approach, could be considered and could offer cost-effective strategies to answer some of the preceding questions. In this approach, it is assumed that residents with devices are either already colonized with MDROs or at a high risk of acquiring MDROs. As a result, SNFs would not have to wait for screening culture results in order to institute enhanced barrier precautions. They can institute preemptive enhanced barrier precautions, without isolation, on all residents with indwelling devices (Table 1). This could include diligent hand hygiene, glove use, and gown use. Facilities may individualize enhanced barrier precautions on the basis of the population that they serve. However, in high-risk patients, glove use should be encouraged particularly during assistance with activities of daily living, such as feeding and transfers. Monitoring adherence to these practices by using device-specific care checklists or random observations could also be incorporated. Although the hours devoted to infection prevention vary by site, facilities can be creative in implementing random observations, such as observations during walking rounds or during hands-on teaching demonstrations (Reinforcing Factors and Evaluate Outcomes, PRECEDE MODEL, Figure 2).
Table 1.
Comparison of Preemptive Barrier Precautions for High-Risk vs General Residents of Skilled Nursing Facilities
| Enhanced precautions for residents with indwelling devices | Standard precautions for all residents |
| Place enhanced barrier precautions signs on clinical charts, nursing stations, resident rooms. | None. |
| Hand hygiene before and after providing any patient care. Hand hygiene performed before donning gloves and after they are removed. | Hand hygiene before and after providing any patient care. Hand hygiene performed before donning gloves and after they are removed. |
| Gloves to be worn upon entry into rooms of patients with devices. Glove use encouraged when providing any assistance with activities of daily living, such as transfers, grooming, feeding, during physical and occupational therapy and feeding. Gloves must be changed before caring for different patients. | Gloves to be used when contact with blood or potentially infectious materials could occur. Gloves must be changed before caring for different patients. |
| Protective gown to be worn to protect skin and to prevent soiling or contamination of clothing during procedures and patient care activities when contact with body fluids, blood, secretions, or excretions is expected.Protective gown to be worn when providing any morning and evening care. Morning and evening care activities include dressing (clothing change, including donning or removing shoes, socks, sweaters), bathing (sponge bath daily and showering twice weekly), toileting, oral hygiene (mouth, teeth, and denture care), and grooming (hair care and glasses). | Protective gown to be worn to protect skin and to prevent soiling or contamination of clothing during procedures and patient care activities when contact with body fluids, blood, secretions, or excretions is expected. |
| When residents leave their rooms for any activities, their wounds and other areas of drainage will be covered. | When residents leave their rooms for any activities, their wounds and other areas of drainage will be covered. |
Active Surveillance to Identify Infections and Dissemination of Surveillance Results
Most data on the effectiveness of active surveillance for symptomatic infections have come from acute care, where efforts directed towards the prevention of UTI, pneumonia, bacteremia, and surgical wound infection have led to significant reductions in infection rates [49]. More recently, studies have shown that introduction of alcohol-based hand gel for hand hygiene coupled with a MRSA infection surveillance feedback program has led to reduced rates of MRSA infection in patients in intensive care units [50]. SNFs currently use many different infection definitions with variable feedback to their administration during quality control meetings and no feedback of aggregate data to HCWs who provide direct care. As one strategy, facilities could conduct focused, active surveillance of infections using standardized definitions in SNF residents with indwelling devices and rapidly disseminate these results to clinical staff, including the infection preventionist, the medical director, SNF physicians and nurse practitioners, and other HCWs (Reinforcing Factors and Evaluate Outcomes, PRECEDE MODEL, Figure 2).
Hand Hygiene Promotion and Educational Interventions To Reduce Infections
Alcohol-based hand rubs and antimicrobial soaps for hand hygiene have been shown to prevent the transfer of pathogens [51]. In one study, gram-negative organisms from a colonized patient's skin were transferred to a piece of catheter material by means of the hands of HCWs in only 17% of the instances after the HCW used alcohol-based hand rub, compared with 92% of the instances after the HCW used plain soap and water for hand cleansing [52]. In another study, which looked at the effectiveness of alcohol-based hand rub in removing organisms from the hands of HCWs, the alcohol-based hand rub was more efficacious in removing S. aureus, GNB, and yeast, compared with the efficacy of plain soap and water [39]. In studies examining MDROs, the use of alcohol-based hand rub for hand cleansing reduced the number of MDROs on the hands of HCWs more effectively than did hand-washing with soap and water in acute care facilities [50, 52]. These studies emphasize the need for hand hygiene with either antimicrobial soap or alcohol-based hand rub to prevent transmission of microorganisms after caring for high-risk patients.
Hand hygiene campaigns and educational initiatives have been shown to enhance hand hygiene compliance and to reduce MRSA infections in hospitals [39, 53, 54]. As a part of this proposed bundle, facilities can promote the universal practice of hand hygiene with either antimicrobial soap and water or alcohol-based hand rub by HCWs when caring for residents with indwelling devices. Studies have shown that educational sessions are beneficial in reducing device-related infections in acute care when the sessions are incorporated within a multipronged intervention [55, 56]. These interventions have used either didactic training sessions or a combination of didactic and hands-on training. Staff education as a part of multicomponent intervention has been shown to improve patient care processes with regard to prevention of SNF-acquired pneumonia, reduction of feeding tube use, and appropriate use of nonsteroidal pain medication and antipsychotic medication in SNFs (Enabling Factors, PRECEDE MODEL, Figure 2) [57–60].
IMPLEMENTING EVIDENCE-BASED PRACTICES IN SKILLED NURSING FACILITIES
Designing and implementing evidence-based practices in SNFs can be challenging on several fronts [61]. First, SNFs take care of a population that carries a high risk of infection because of higher rates of chronic diseases, increasing severity of illness, impaired mental and functional status, and presence of indwelling devices, such as feeding tubes and urinary catheters. Second, SNFs often face numerous organizational challenges, including suboptimal full-time equivalents for registered nurses, nursing aides, and therapists; high staff turnover rates; changing case mix; limited availability of information systems; and variable availability of laboratory and radiologic services [62]. Third, there is a paucity of infection prevention research in this setting. Acquisition of informed consent from family, care-givers, or other designated durable powers of attorney is very common. Facility staff, residents, and their families often voice mistrust and skepticism about research, in general. However, these barriers can be overcome by involving SNF administrators, as well as resident advocate groups and families, during the planning phase. Additionally, staff turnover may affect the efficacy of interventions. Using information technology, such as DVDs and pod casts, as well as developing infection prevention tool-kits to be provided during new employee orientation can help train new staff.
Challenges notwithstanding, developing practical, transportable, and easily implementable models is crucial. Individualized approaches to infection prevention that focus a multicomponent intervention in high-risk populations may be a way to incorporate evidence-based practices in this setting [63]. Such interventions, if shown to be effective, can then be tested for other risk groups, such as those who have wounds, severe functional impairment, or recent hospitalization, and have the potential to lead to substantial cost savings. Further research is required to define and design interventions for groups at high risk of acquiring other pathogens, such as Clostridium difficile. Research in this setting will guide policy and legislative actions to enhance the quality of life of SNF residents.
Acknowledgments
Financial support.National Institute of Aging (NIA) K23 Career Development Award (NIA RO1 AG032298 to L.M.); Association of Specialty Professors (ASP)/American Geriatrics Society (AGS) T. Franklin Williams Research Scholarship (to L.M.); Department of Veterans Affairs, Health Services Research and Development Service (SAF 04-031); Ann Arbor Veterans Affairs Medical Center (VAMC)/University of Michigan Patient Safety Enhancement Program; National Institute of Diabetes and Digestive and Kidney Diseases (R21-DK078717 to S.S.); and the National Institute of Nursing Research (Drs. Saint and Krein by award R01-NR010700 to S.S. and S.L.K.).
Potential Conflicts of Interest. S.F.B. reports that she is a member of the board of the National Institutes of Aging/Yale University; a consultant for GlaxoSmithKline and Merck, and a source for expert testimony for Centers for Medicare/Medicaid Services, and has received honoraria for lectures to France Foundation/University of Nebraska and Centers for Health Protection Hong Kong. L.M. reports that she has received institutional grant support from Cepheid, and that Gojo is donating personal-use alcohol-based hand gel bottles to nursing homes that are implementing a multicomponent bundle. R.N.O. reports that he serves as consultant to Applied Epidemiology Solutions, Arizant Healthcare, Ecolab, Mintie, Epidemiology Consulting Services in Beverely Hills, Premier, and Trademark Medical LLC; has received honoraria for lectures for Baxter Healthcare, CareFusion, University of Pittsburgh Medical Center, MI Society for Infection Prevention & Control, Multidisciplinary Alliance Against Device-Related Infections (MAADRI), Becton Dickinson, Association for Professionals in Infection Control & Epidemiology (APIC)-IA, APIC-IN, and APIC-OK; and has received accommodations/travel expenses from APIC, Health Guidelines Revision Committee (Facility Guidelines Inst), HICPAC, and CDC. All other authors: no conflicts.
References
- 1.Jones AL, Dwyer LL, Bercovitz AR, et al. The National Nursing Home Survey: 2004 overview. Vital Health Stat. 2009;13:1–155. [PubMed] [Google Scholar]
- 2.Bonomo RA. Multiple antibiotic-resistant bacteria in long-term care facilities: an emerging problem in the practice of infectious diseases. Clin Infect Dis. 2000;31:1414–22. doi: 10.1086/317489. [DOI] [PubMed] [Google Scholar]
- 3.Rogers MA, Mody L, Chenoweth C, et al. Incidence of antibiotic-resistant infection in long-term residents of skilled nursing facilities. Am J Infect Control. 2008;36:472–5. doi: 10.1016/j.ajic.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith PW, Bennett G, Bradley S, et al. SHEA/APIC guideline: infection prevention and control in the long-term care facility, July 2008. Infect Control Hosp Epidemiol. 2008;29:785–814. doi: 10.1086/592416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Department of Veterans Affairs. VHA directive 2007–002: Methicillin-resistant Staphylococcus aureus (MRSA) initiative. https://www1.va.gov/vhapublications/ViewPublication.asp?pub_ID=1525. Accessed 20 January 2010. [Google Scholar]
- 6.Diekema DJ, Climo M. Preventing MRSA infections: finding it is not enough. JAMA. 2008;299:1190–2. doi: 10.1001/jama.299.10.1190. [DOI] [PubMed] [Google Scholar]
- 7.Weber SG, Huang SS, Oriola S, et al. Legislative mandates for use of active surveillance cultures to screen for methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: Position statement from the Joint SHEA and APIC Task Force. Am J Infect Control. 2007;35:73–85. doi: 10.1016/j.ajic.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Sengstock DM, Thyagarajan R, Apalara J, et al. Multidrug-resistant Acinetobacter baumannii: an emerging pathogen among older adults in community hospitals and nursing homes. Clin Infect Dis. 2010;50:1611–6. doi: 10.1086/652759. [DOI] [PubMed] [Google Scholar]
- 9.Lautenbach E, Marsciano R, Tolomeo P, et al. Epidemiology of antimicrobial resistance among gram-negative organisms recovered from patients in a multistate network of long-term care facilities. Infect Control Hosp Epidemiol. 2009;30:790–3. doi: 10.1086/599070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290:1899–905. doi: 10.1001/jama.290.14.1899. [DOI] [PubMed] [Google Scholar]
- 11.Saint S, Higgins LA, Nallamothu BK, et al. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31:354–6. doi: 10.1016/s0196-6553(02)48250-8. [DOI] [PubMed] [Google Scholar]
- 12.Kirkland KB, Weinstein JM. Adverse effects of contact isolation. Lancet. 1999;354:1177–8. doi: 10.1016/S0140-6736(99)04196-3. [DOI] [PubMed] [Google Scholar]
- 13.Green LW, Kreuter MW. Health promotion planning: an educational and ecological approach. 3rd ed. Mountain View, CA: Mayfield; 1999. [Google Scholar]
- 14.Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, Enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med. 2002;136:834–44. doi: 10.7326/0003-4819-136-11-200206040-00013. [DOI] [PubMed] [Google Scholar]
- 15.Lasseter G, Charlett A, Lewis D, et al. Staphylococcus aureus carriage in care homes: identification of risk factors, including the role of dementia. Epidemiol Infect. 2010;138:686–96. doi: 10.1017/S0950268810000233. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Medicare and Medicaid Services. Online Survey, Certification and Reporting (OSCAR) data, December 2009: medical condition---bladder and bowel status. http://www.ahcancal.org/research_data/oscar_data/NursingFacilityPatientCharacteristics/MC_bladder_bowel_Dec2009.pdf. Accessed 20 January 2010. [Google Scholar]
- 17.Maki DG, Tambyah PA. Engineering out the risk for infection with urinary catheters. Emerg Infect Dis. 2001;7:342–7. doi: 10.3201/eid0702.010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell SL, Teno JM, Roy J, et al. Clinical and organizational factors associated with feeding tube use among nursing home residents with advanced cognitive impairment. JAMA. 2003;290:73–80. doi: 10.1001/jama.290.1.73. [DOI] [PubMed] [Google Scholar]
- 19.Rogers MA, Mody L, Kaufman SR, et al. Use of urinary collection devices in skilled nursing facilities in five states. J Am Geriatr Soc. 2008;56:854–61. doi: 10.1111/j.1532-5415.2008.01675.x. [DOI] [PubMed] [Google Scholar]
- 20.Teno JM, Feng Z, Mitchell SL, et al. Do financial incentives of introducing case mix reimbursement increase feeding tube use in nursing home residents? J Am Geriatr Soc. 2008;56:887–90. doi: 10.1111/j.1532-5415.2008.01647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsan L, Davis C, Langberg R, et al. Prevalence of nursing home-associated infections in the Department of Veterans Affairs nursing home care units. Am J Infect Control. 2008;36:173–9. doi: 10.1016/j.ajic.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Smith PW, Seip CW, Schaefer SC, et al. Microbiologic survey of long-term care facilities. Am J Infect Control. 2000;28:8–13. doi: 10.1016/s0196-6553(00)90005-1. [DOI] [PubMed] [Google Scholar]
- 23.Trick WE, Weinstein RA, DeMarais PL, et al. Colonization of skilled-care facility residents with antimicrobial-resistant pathogens. J Am Geriatr Soc. 2001;49:270–6. doi: 10.1046/j.1532-5415.2001.4930270.x. [DOI] [PubMed] [Google Scholar]
- 24.Gould CV, Umscheid CA, Agarwal RA, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. http://www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf. Accessed 28 July 2010. [DOI] [PubMed] [Google Scholar]
- 25.Saint S, Kaufman SR, Rogers MA, et al. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54:1055–61. doi: 10.1111/j.1532-5415.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- 26.Terpenning MS, Bradley SF, Wan JY, et al. Colonization and infection with antibiotic-resistant bacteria in a long-term care facility. J Am Geriatr Soc. 1994;42:1062–9. doi: 10.1111/j.1532-5415.1994.tb06210.x. [DOI] [PubMed] [Google Scholar]
- 27.Mody L, Kauffman CA, Donabedian S, et al. Epidemiology of Staphylococcus aureus colonization in nursing home residents. Clin Infect Dis. 2008;46:1368–73. doi: 10.1086/586751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mody L, Maheshwari S, Galecki A, et al. Indwelling device use and antibiotic resistance in nursing homes: identifying a high-risk group. J Am Geriatr Soc. 2007;55:1921–6. doi: 10.1111/j.1532-5415.2007.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fryklund B, Haeggman S, Burman LG. Transmission of urinary bacterial strains between patients with indwelling catheters–nursing in the same room and in separate rooms compared. J Hosp Infect. 1997;36:147–53. doi: 10.1016/s0195-6701(97)90121-3. [DOI] [PubMed] [Google Scholar]
- 30.Kaw M, Sekas G. Long-term follow-up of consequences of percutaneous endoscopic gastrostomy (PEG) tubes in nursing home patients. Dig Dis Sci. 1994;39:738–43. doi: 10.1007/BF02087416. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell SL, Kiely DK, Lipsitz LA. The risk factors and impact on survival of feeding tube placement in nursing home residents with severe cognitive impairment. Arch Intern Med. 1997;157:327–32. [PubMed] [Google Scholar]
- 32.Chaudhary KA, Smith OJ, Cuddy PG, et al. PEG site infections: the emergence of methicillin-resistant Staphylococcus aureus as a major pathogen. Am J Gastroenterol. 2002;97:1713–6. doi: 10.1111/j.1572-0241.2002.05830.x. [DOI] [PubMed] [Google Scholar]
- 33.Hull M, Beane A, Bowen J, et al. Methicillin-resistant Staphylococcus aureus infection of percutaneous endoscopic gastrostomy sites. Aliment Pharmacol Ther. 2001;15:1883–8. doi: 10.1046/j.1365-2036.2001.01124.x. [DOI] [PubMed] [Google Scholar]
- 34.Mainie I, Loughrey A, Watson J, et al. Percutaneous endoscopic gastrostomy sites infected by methicillin-resistant Staphylococcus aureus: impact on outcome. J Clin Gastroenterol. 2006;40:297–300. doi: 10.1097/01.mcg.0000210096.44123.b6. [DOI] [PubMed] [Google Scholar]
- 35.Saadeddin A, Freshwater DA, Fisher NC, et al. Antibiotic prophylaxis for percutaneous endoscopic gastrostomy for nonmalignant conditions: a double-blind prospective randomized controlled trial. Aliment Pharmacol Ther. 2005;22:565–70. doi: 10.1111/j.1365-2036.2005.02578.x. [DOI] [PubMed] [Google Scholar]
- 36.Fox KA, Mularski RA, Sarfati MR, et al. Aspiration pneumonia following surgically placed feeding tubes. Am J Surg. 1995;170:564–6. doi: 10.1016/s0002-9610(99)80016-6. [DOI] [PubMed] [Google Scholar]
- 37.Kitamura T, Nakase H, Iizuka H. Risk factors for aspiration pneumonia after percutaneous endoscopic gastrostomy. Gerontology. 2007;53:224–7. doi: 10.1159/000100898. [DOI] [PubMed] [Google Scholar]
- 38.Bonilla HF, Zervos MA, Lyons MJ, et al. Colonization with vancomycin-resistant Enterococcus faecium: comparison of a long-term care unit with an acute-care hospital. Infect Control Hosp Epidemiol. 1997;18:333–9. doi: 10.1086/647621. [DOI] [PubMed] [Google Scholar]
- 39.Mody L, McNeil SA, Sun R, et al. Introduction of a waterless alcohol-based hand rub in a long-term care facility. Infect Control Hosp Epidemiol. 2003;24:165–71. doi: 10.1086/502185. [DOI] [PubMed] [Google Scholar]
- 40.Wingard E, Shlaes JH, Mortimer EA, et al. Colonization and cross-colonization of nursing home patients with trimethoprim-resistant gram-negative bacilli. Clin Infect Dis. 1993;16:75–81. doi: 10.1093/clinids/16.1.75. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Guidelines for preventing health-care-associated Pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53 (RR-3):1–40. [PubMed] [Google Scholar]
- 42.Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of American. Clin Infect Dis. 2010;50:625–63. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 43.Mody L, Saint S, Galecki A, Chen S, Krein SL. Knowledge of evidence-based urinary catheter care practice recommendations among healthcare workers in nursing homes. J Am Geriatr Soc. 2010;58:1532–7. doi: 10.1111/j.1532-5415.2010.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McNulty CA, Bowen J, Foy C, et al. Urinary catheterization in care homes for older people: self-reported questionnaire audit of catheter management by care home staff. J Hosp Infect. 2006;62:29–36. doi: 10.1016/j.jhin.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Muto CA, Jernigan JA, Ostrowsky BE, et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol. 2003;24:362–86. doi: 10.1086/502213. [DOI] [PubMed] [Google Scholar]
- 46.Jackson M, Jarvis WR, Scheckler WE. HICPAC/SHEA–conflicting guidelines: what is the standard of care? Am J Infect Control. 2004;32:504–11. doi: 10.1016/j.ajic.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Harbarth S. Control of endemic methicillin-resistant Staphylococcus aureus–recent advances and future challenges. Clin Microbiol Infect. 2006;12:1154–62. doi: 10.1111/j.1469-0691.2006.01572.x. [DOI] [PubMed] [Google Scholar]
- 48.Verhoef J, Beaujean D, Blok H, et al. A Dutch approach to methicillin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1999;18:461–6. doi: 10.1007/s100960050324. [DOI] [PubMed] [Google Scholar]
- 49.Haley RW, Culver DH, White JW, et al. The efficacy of infection surveillance and control programs in preventing nosocomial infections in US hospitals. Am J Epidemiol. 1985;121:182–205. doi: 10.1093/oxfordjournals.aje.a113990. [DOI] [PubMed] [Google Scholar]
- 50.Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356:1307–12. doi: 10.1016/s0140-6736(00)02814-2. [DOI] [PubMed] [Google Scholar]
- 51.Boyce JM, Pittet D. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA hand hygiene task force. Infect Control Hosp Epidemiol. 2002;23:S3–40. doi: 10.1086/503164. [DOI] [PubMed] [Google Scholar]
- 52.Harrington G, Watson K, Bailey M, et al. Reduction in hospital-wide incidence of infection or colonization with methicillin-resistant Staphylococcus aureus with use of antimicrobial hand-hygiene gel and statistical process control charts. Infect Control Hosp Epidemiol. 2007;28:837–44. doi: 10.1086/518844. [DOI] [PubMed] [Google Scholar]
- 53.Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30:59–64. doi: 10.1097/00003246-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Parras F, Ena J, Bouza E, et al. Impact of an educational program for the prevention of colonization of intravascular catheters. Infect Control Hosp Epidemiol. 1994;15:239–42. [PubMed] [Google Scholar]
- 55.Pronovost P. Interventions to decrease catheter-related bloodstream infections in the ICU: the Keystone Intensive Care Unit Project. Am J Infect Control. 2008;36:S171:e171–5. doi: 10.1016/j.ajic.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 56.Resar R, Pronovost P, Haraden C, et al. Using a bundle approach to improve ventilator care processes and reduce ventilator-associated pneumonia. Jt Comm J Qual Patient Saf. 2005;31:243–8. doi: 10.1016/s1553-7250(05)31031-2. [DOI] [PubMed] [Google Scholar]
- 57.Hutt E, Ruscin JM, Corbett K, et al. A multifaceted intervention to implement guidelines improved treatment of nursing home-acquired pneumonia in a state veterans home. J Am Geriatr Soc. 2006;54:1694–700. doi: 10.1111/j.1532-5415.2006.00937.x. [DOI] [PubMed] [Google Scholar]
- 58.Monteleoni C, Clark E. Using rapid-cycle quality improvement methodology to reduce feeding tubes in patients with advanced dementia: before and after study. BMJ. 2004;329:491–4. doi: 10.1136/bmj.329.7464.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ray WA, Taylor JA, Meador KG, et al. Reducing antipsychotic drug use in nursing homes: a controlled trial of provider education. Arch Intern Med. 1993;153:713–21. [PubMed] [Google Scholar]
- 60.Stein CM, Griffin MR, Taylor JA, et al. Educational program for nursing home physicians and staff to reduce use of nonsteroidal anti-inflammatory drugs among nursing home residents: a randomized controlled trial. Med Care. 2001;39:436–45. doi: 10.1097/00005650-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Mody L, Miller DK, McGloin JM, et al. Recruitment and retention of older adults in aging research. J Am Geriatr Soc. 2008;56:2340–8. doi: 10.1111/j.1532-5415.2008.02015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zoutman DE, Ford BD, Gauthier J. A cross-Canada survey of infection prevention and control in long-term care facilities. Am J Infect Control. 2009;37:358–63. doi: 10.1016/j.ajic.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 63.Bowler WA, Bresnahan J, Bradfish A, et al. An integrated approach to methicillin-resistant Staphylococcus aureus control in a rural, regional-referral healthcare setting. Infect Control Hosp Epidemiol. 2010;31:269–75. doi: 10.1086/650445. [DOI] [PubMed] [Google Scholar]