Abstract
Many methods of cardiac output measurement have been developed, but the number of methods useful for human pharmacological studies is limited. The ‘holy grail’ for the measurement of cardiac output would be a method that is accurate, precise, operator independent, fast responding, non-invasive, continuous, easy to use, cheap and safe. This method does not exist today. In this review on cardiac output methods used in pharmacology, the Fick principle, indicator dilution techniques, arterial pulse contour analysis, ultrasound and bio-impedance are reviewed.
Keywords: cardiac output, measurement method, methods
Introduction
‘It is a source of regret that measurement of flow is much more difficult than measurement of pressure. This has led to an undue interest in blood pressure measurements. Most organs however, require flow rather than pressure.’ This statement by Jarisch in 1928 [1] is still fully valid. Many methods of cardiac output measurement have been developed, but the number of methods useful for human pharmacological studies is limited. Methods proposed to achieve this goal include the Fick principle, ultrasound, indicator dilution techniques, arterial pulse contour analysis and bio-impedance. To gain widespread acceptance, these methods should ideally be accurate, precise, operator independent, fast responding, non-invasive, continuous, easy of use, cheap and without complications. The methods may allow testing of circulatory changes on pharmacological interventions. In this review on cardiac output, the methods used in pharmacology are described.
Fick's cardiac output measurement
Direct Fick for oxygen
In 1870, Adolf Fick described a method to estimate cardiac output based on a mass balance for oxygen. He postulated that oxygen uptake in the lungs, i.e. the oxygen (O2) consumption in ml of pure gaseous oxygen min−1, is entirely transferred to the blood stream through the lung. With no consumption of oxygen in the lungs the oxygen consumption of the body is equal to the product of blood flow (cardiac output) and arterio-venous oxygen content difference. Therefore cardiac output can be computed as follows:
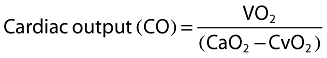 |
where VO2 is the oxygen uptake, CaO2 and CvO2 (ml O2 l−1 blood) are the oxygen content of arterial and venous blood, respectively (also see Figure 1).
Figure 1.
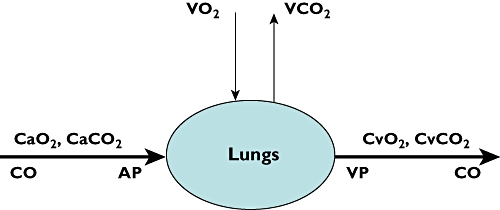
Graphical description of the Fick principle. Oxygen enters the lungs (VO2) and is transported to peripheral tissue of the body (CvO2–CaO2). At the same time carbon dioxide produced by the rest of the body (CaCO2–CvCO2) is cleared by the lungs (VCO2). From these concentrations blood flow can be calculated using the formula described in the text
At first sight the method seems simple to execute. VO2 can be determined by breathing or mechanical ventilation within a spirometer incorporating a carbon dioxide absorber or, more conveniently, via an indirect calorimetry monitor. Also, the calculation of the arterial and venous oxygen content of the blood is a straightforward process and is readily available to physicians. However, the method is laborious and many variables need to be determined. During the acquisition of data the circulation needs to be stable. Some points to consider are (i) the large number of variables involved in the computation result in a large chance on permutation of errors, (ii) ventilation of subjects with inspiratory O2 fractions larger than 60% have been reported to decrease the accuracy of the method [2], (iii) the technique requires an invasive pulmonary artery catheter to sample mixed venous blood. Accurate measurement of VO2 as well as reliable sampling of arterial and venous blood sample is labour intensive. Nevertheless, in a laboratory with skilled researchers, the method is considered the most accurate method to which other methods are compared.
Partial carbon dioxide rebreathing
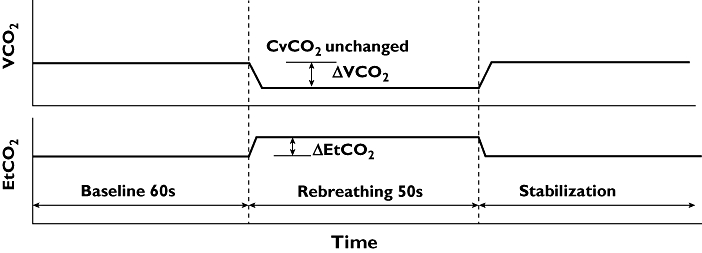
The Fick principle can be applied to all gasses that obey Henry's law and diffuse through the lungs, especially carbon dioxide (CO2). The NICO (Novametrix Medical Systems Inc. Wallingford, CT, USA) is the most studied cardiac output monitor based on the Fick principle for CO2 and uses intermittent partial rebreathing of CO2. This monitor utilizes a specific disposable rebreathing loop in which a CO2 infra-red light absorption sensor, a differential pressure transducer for air flow measurement and a pulse oximeter are placed. VCO2 is calculated from the simultaneously measured minute ventilation by the differential transducer and its CO2 concentration (Figure 2). The arterial content of CO2 (CaCO2) is estimated from end tidal CO2 (EtCO2) after a correction (S), i.e. the slope of the CO2 dissociation curve. Measurement of under normal and under rebreathing conditions allows elimination of measurement of CvCO2.
Figure 2.
Measurement of cardiac output with the use of carbon dioxide rebreathing
Fick's equation applied to carbon dioxide is
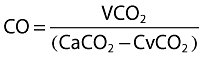 |
where VCO2 is the CO2 production, CaCO2 and CvCO2 the arterial and mixed venous CO2 content in blood.
Assuming cardiac output is not changed by CO2 rebreathing, CvCO2 does not differ between normal and rebreathing conditions (CO2 diffuses very fast in blood, 22× faster than O2) and arterial CaCO2 can be approximated by end-tidal CO2 multiplied by the slope (S) of the CO2 dissociation curve the equation above can be rewritten to
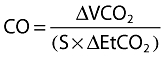 |
where ΔVCO2 is the change in VCO2 and ΔEtCO2 is the change in end tidal CO2 between normal breathing and CO2 rebreathing.
The method actually calculates effective lung perfusion. The effects of unknown ventilation/perfusion inequality and anatomic shunts may explain underestimation of CO and the method shows a lack of agreement with reference techniques [3]. To correct for shunt behaviour the subjects must be fully under mechanical ventilation and arterial blood samples are needed, making this method (less) invasive. However, clinically acceptable cardiac output estimation seems possible in intubated mechanically ventilated patients with minor lung abnormalities [4].
Indicator dilution techniques
Today four different modalities of the indicator dilution technique are commercially available, i.e. the pulmonary artery catheter (PAC) thermodilution method with bolus injection of cold fluid, the PAC continuous thermodilution method, the transpulmonary bolus thermodilution method and the transpulmonary lithium bolus dilution method. All these methods have in common that the computation of cardiac output is based on a mass balance.
where mi is the amount of indicator injected, q(t) is instantaneous blood flow and c(t) is concentration as function of time.
Application of this equation assumes complete mixing of blood and indicator, with no loss of indicator between place of injection and place of detection. If we further assume blood flow to be constant then we find the well-known Stewart-Hamilton equation:
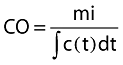 |
where ∫c(t)dt is the area under the indicator dilution curve. Errors made in the application of indicator dilution methods are primarily related to violation of the assumption mentioned above, inaccurate implementation of the method [5] and anatomic abnormalities [6].
Intermittent pulmonary thermodilution
Since the introduction of the pulmonary artery catheter (PAC) equipped with a thermistor by Swan & Ganz in 1970 [7] the thermodilution method has become the standard method to determine cardiac output in patients. The thermodilution method is based on the law of conservation of thermal energy. With the intermittent thermodilution technique a certain amount of cold fluid is injected into the blood stream near the entrance of the right atrium and the resulting dilution curve is detected in the pulmonary artery. With temperature as indicator the Stewart-Hamilton equation can be rewritten as follows:
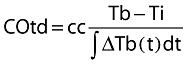 |
where COtd is cardiac output by thermodilution, Tb is the temperature of blood in the pulmonary artery before injection of injectate, Ti the temperature of the injectate, and ∫ΔTb(t)dt the area under the dilution curve (Figure 3) and cc is the computation constant. The computation constant contains corrections for specific mass and heat of injectate and blood, respectively, injected volume and loss of indicator in the PAC and has to be entered in the thermodilution cardiac output computer.
Figure 3.
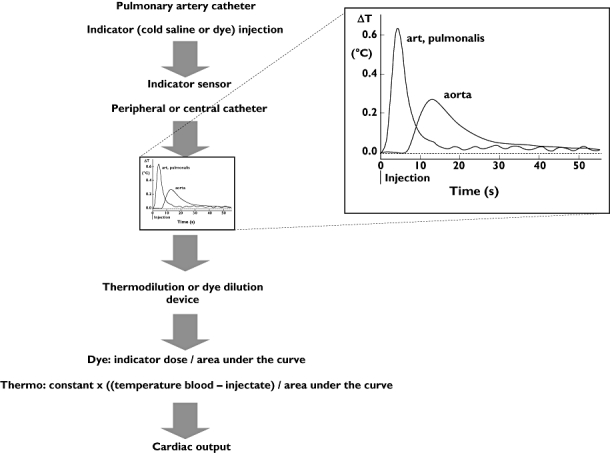
Indicator dilution to measure cardiac output. A dye solution or cold saline is injected and detected by a (dye or thermal) sensor downstream of the injection site. The dilution signal is fed to a cardiac output device. To compute cardiac output the dose injected is divided by the area under the indicator dilution curve. The inset shows the difference in temperature changes for two different locations of detection (see text)
Investigators have previously explored methods of minimizing the errors in the intermittent thermodilution technique [8–12]. The best method is to average the results of three or four thermodilution measurements with the injection of cold fluid equidistantly distributed over the ventilatory cycle. For such an approach injections of fluid must be done with an injector under computer control. Use of such a set-up results in a coefficient of variation or 1 SD-precision of 3.5%, whereas the averaged result of three randomly applied measurements have a 1 SD-precision of about 10% and single measurements a 1 SD-precision of 15%. After 40 years of clinical experience, the conventional thermodilution method has been generally accepted as the clinical standard to which all other methods are compared. However, some serious complications can arise from PAC insertion like arrhythmias, valvular lesions, rupture of the pulmonary artery and lung infarction.
PAC continuous cardiac output
The Vigilance system (Edwards Lifescience, Irvine, CA, USA) combines heat-dilution principles with stochastic system identification to measure cardiac output [13]. Small amounts of thermal energy (heat-indicator) are transported directly into the blood in a pseudo random on-off pattern to form the input signal (see Figure 4). The resulting blood temperature changes are detected with a thermistor in the pulmonary artery. This signal is small in proportion to the resident pulmonary artery thermal noise. To overcome this problem, a cross correlation is carried out on the input signal and the temperature data measured in the pulmonary artery, resulting in a thermodilution curve, as would have been found after a bolus injection. From this dilution curve, cardiac output is computed using the classical Stewart-Hamilton equation. The entire process is automated, requiring no user intervention. A detailed explanation of the technique is given by Yelderman[13]. The ‘continuous’ cardiac output measurement makes extensive use of averaging techniques. Therefore the displayed cardiac output number represents the averaged value of the previous 1 to 6 min [13]. Under extreme clinical situations this delay can run up to 12 min [14]. This property of the technique makes the method continuous but not instantaneous.
Figure 4.
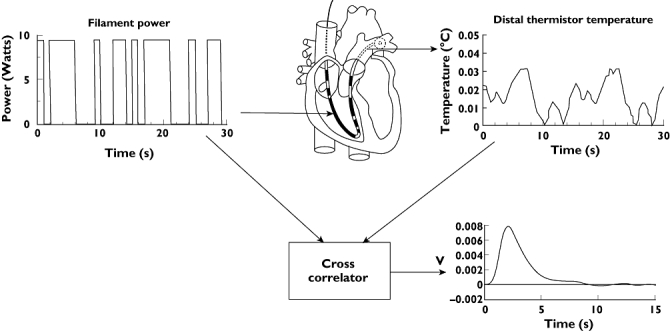
Schematic diagram of the working principle of the continuous thermodilution method
Concerns for the pulmonary thermodilution techniques
Recently, the use of both pulmonary artery thermodilution cardiac output methods has been under discussion. Many physicians believe that the PAC due to its multi-purpose role is useful for the diagnoses, treatment and assessment of volume status in critical ill patients [15]. However, this is not confounded by studies. In contrast, different investigators raised doubts about the safety of the PAC. Indeed, most recent studies do not show a difference in morbidity and mortality between patients with and without a PAC [16–18]. On the other hand, in these trials the introduction of the PAC could not be associated with an increase in morbidity and mortality. The inability to demonstrate the merit of the PAC in predicting outcome does not necessarily mean that the monitors using the PAC are not functioning [17]. It may also indicate a persisting lack of correct and consistent interpretation of PAC-derived data among physicians [19] or ineffectiveness of our current therapeutic options in reversing critical disease states. Thus, further investigation into the role of the PAC is feasible, likely safe, and should proceed forthwith [15, 20].
Intermittent transpulmonary thermodilution
With this intermittent thermodilution technique a certain amount of cold fluid is injected into the blood stream near the entrance of the right atrium and the dilution curve is detected in the femoral artery [21–23]. CO is computed with the Stewart-Hamilton equation equal to the intermittent pulmonary thermodilution technique. In theory, the transpulmonary thermodilution technique should be less accurate due to unpredictable loss of indicator over the lungs, but more precise than pulmonary thermodilution [8, 9] because the dilution curves are less affected by the respiration cycle. However the decreased signal-to-noise ratio of the dilution curve, i.e. a broader but smaller high of the curve (see Figure 3), may undo this advantage.
The transpulmonary thermodilution method is vulnerable to the same sources of error and variability as pulmonary thermodilution because the two techniques rely on the same physical principles. CO by the transpulmonary method slightly overestimates the results of the pulmonary method due to a small extra loss of indicator between injection and detection site in the aorta or femoral artery. To gain sufficient precision the results of three measurements need to be averaged. These three measurements take approximately 3–10 min. Therefore, the transpulmonary thermodilution method lacks the ability to monitor cardiac output continuously. The intermittent transpulmonary thermodilution is incorporated in the PiCCO-system (Pulsion Medical Systems, Munich, Germany).
Transpulmonary lithium dilution
The lithium dilution method is based on the venous bolus injection of a small dose (1–2 ml) of an isotonic lithium chloride (LiCl) solution (150–300 mmol) and the resulting arterial lithium concentration–time curve is measured by a lithium sensor in a pre-existing peripheral arterial line. Cardiac output is calculated by the Stewart-Hamilton equation.
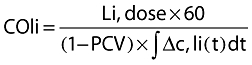 |
where Li, dose is amount of lithium injected, ∫Δc,li(t)dt the area under the lithium dilution curve and PCV the packed cell volume (calculated as the haemoglobin concentration (g dl−1) divided by 34). This correction is needed because lithium is only diluted in the plasma and not in the red and white cells of blood [24]. The pharmacokinetics of intravenous lithium administration are described [25]. No side effects have been reported. To achieve a good precision with this technique, the results of three measurements should be taken [26]. The lithium dilution method is incorporated in the LiDCO system (LiDCO, London, UK).
A concern related to the lithium dilution method is the need for repetitive blood samples. Furthermore, the lithium dilution technique is contraindicated in patients receiving high doses of neuromuscular blocking agents, because of interference with the sensing electrode. The technique cannot be used in patients receiving lithium therapy and is not licensed for subjects weighing less than 40 kg.
Pulse contour cardiac output
The pulse contour devices are perhaps the most promising with respect to their ease of use. The estimation of cardiac output via pulse contour analysis is an indirect method; CO is computed from an arterial pressure pulsation on the basis of a criterion or model. The origin of the pulse contour method for estimation of beat-to-beat stroke volume goes back to the classical Windkessel model described by Otto Frank in 1899 [27]. In principle the aortic pressure waveform is the input of the Windkessel models of the systemic circulation. In medical practice, the pressure waveform is not obtained from the aorta but from a peripheral artery (radial or femoral), which requires a backward filtering from the peripheral to aortic pressure. Not much is known about the algorithms applied. At present there are four commercial pulse contour cardiac output computers available: PiCCO, PRAM, LidCO, Vigileo and Modelflow.
The PiCCO system
The PiCCO system (Pulsion Medical Systems, Munich, Germany) uses a modified version of Wesseling's cZ algorithm [28, 29]. It analyzes the actual shape and area under the pressure waveform and uses individual aortic compliance and systemic vascular resistance. The PiCCO algorithm is summarized in the following equation.
where COpi cardiac output, K calibration factor, HR heart rate, P arterial blood pressure, ∫P(t)dt area under the systolic part of the pressure curve, SVR systemic vascular resistance, C(P) pressure dependent arterial compliance and dP/dt describes the shape of the pressure wave. The calibration factor (K) is determined with transpulmonary thermodilution and recalibration is needed after profound changes in SVR and at regular (≥1 h) intervals [30–32]. Invasive catheterization is thus still required. For the PiCCO device both the radial and the femoral artery approach can be used [33]. A basic overview of the computation of pulse contour cardiac output is shown in Figure 5.
Figure 5.
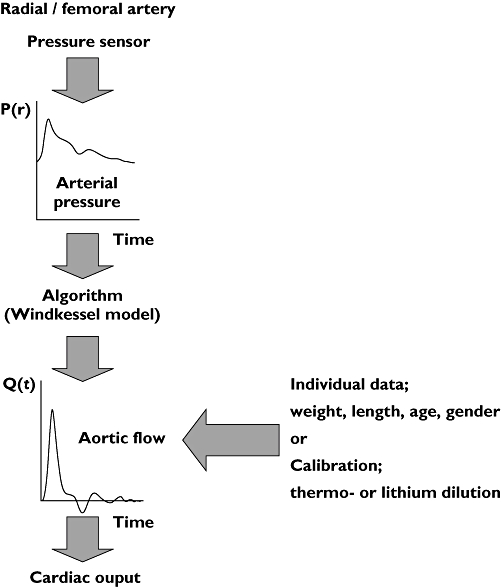
General working principle to estimate cardiac output by pulse contour analysis. A pressure signal is conducted from the pressure sensor to a pulse contour cardiac output device. Together with either calibration values obtained by transpulmonary thermodilution (PiCCO) or lithium dilution (LidCO) and personal patient data, the algorithm estimates aortic flow over a certain interval. This is shown on the device as cardiac output
The pressure recording analytical method (PRAM)
PRAM (Vytech Health, Padova, Italy) is a modified version of Wesselings cZ algorithm [28, 29]. Stroke volume (SV) is proportional to the area under the diastolic part of the arterial pressure wave divided by characteristic impedance (Z). The proportionally factor is usually obtained by calibration with an independent SV measurement (for instance by intermittent thermodilution). However in contrast to other methods PRAM does not rely on calibration or demographic data. With PRAM characteristic impedance is obtained from morphological data of the pressure curve of a whole heart beat [34] and is calculated as Z = (P/t) × K(t). Stroke volume (SV) is therefore computed as:
where A is the area under the systolic part of the pressure curve, P/t is the analytical description of the pressure wave form of pressure (P) with time (t) for each heart beat and K(t) is a factor inversely related to the instantaneous acceleration of the cross sectional area of the aorta.
The value of K(t) is found from the ratio between expected and measured mean arterial blood pressure. This relationship approaches an arctangent function (similar to that of Langewouters et al. [35]. The expected mean blood pressure which is constant depends on the site of measurement, i.e. for adults 100 mmHg for the aortic pressure and 90 mmHg for a peripheral pressure. With PRAM stroke volume is calculated for each beat and CO per beat is then derived by multiplying SV with heart rate of the same beat. CO is presented as the mean value of 12 beats.
As the internal calibration of PRAM is derived from the morphology of the pressure curve, this makes the method vulnerable to sources of errors related to signal quality and in patients with heart diseases that are suspected to affect the arterial pressure waveform (for instance in patients with aortic valve stenosis or valve insufficiencies).
The LiDCO's pulsco system
The LiDCO-system (LiDCO, London, UK) calculates continuous cardiac output by analysis of the arterial blood pressure trace. Using a non-linear relationship between arterial pressure and volume, given by Remington & Noback[36], nominal changes in arterial volume within every cardiac cycle are calculated from the pressure waveform. These nominal changes are converted to actual stroke volume by multiplying the nominal stroke volume or nominal cardiac output by a calibration factor. This patient-specific calibration is derived from an independently measured cardiac output, for instance by the conventional thermodilution or by the transpulmonary lithium indicator dilution method. In this case invasive catheterization with a PAC or an additional peripheral venous catheter is still necessary. Recent data suggest recalibration every 8 h or whenever major haemodynamic changes occur [37].
Vigileo/FloTrac system
The FloTrac/Vigileo (Edwards Lifesciences, Irvine, CA, USA) is a pulse contour technique utilizing a dedicated pressure sensor (FloTrac) and a monitor to compute stroke volume and cardiac output (Vigileo). It does not require an independent calibration. The cardiac output algorithm is based on the principle that aortic pulse pressure is proportional to stroke volume and inversely related to aortic compliance. The system obtains the pressure signal from any standard peripheral arterial line. From the arterial pressure the standard deviation (σAP) around mean arterial pressure (MAP) is computed over a 20 s interval. This σAP is multiplied by a conversion factor Khi to calculate stroke volume. Khi incorporates a multivariate polynomial equation which assesses the impact of the patient's ever-changing vascular tone on pulse pressure. It is calculated by analyzing the patient's heart rate, standard deviation σAP, mean arterial pressure, pressure dependent arterial compliance estimated by the demographics of the patients with the Langewouters equation [35], BSA body surface area calculated from weight and height, skewness (symmetry) and kurtosis (distinctness of a peak) of the beat-to-beat arterial waveform. Khi is updated and applied to the stroke volume algorithm on a rolling 60 s average.
Cardiac output is calculated by multiplying stroke volume with heart rate. The extensive use of arterial pressure signal processing makes the FloTrac algorithm highly dependent upon a high-fidelity pressure signal. Therefore, attention to the quality of the pressure monitoring signal by testing for optimal dampening and flushing of the arterial line is important.
Modelflow method
Fifteen years ago Wesseling and co-workers [29] discovered that a straightforward extension of the classical Windkessel model could be adequate for pulse contour analysis. Modelflow (FMS, Amsterdam, the Netherlands) is a three-element Windkessel model of the arterial circulation. The model includes three principal components of opposition: characteristic impedance which represents the opposition of the aorta to pulsatile inflow, Windkessel compliance which represents the opposition of the aorta to volume increases, and peripheral resistance which represents the opposition of the vascular beds to the drainage of blood. Aortic compliance is not constant but depends not only on demographic data of the patient (gender, age, weight and height) but also on arterial pressure itself [35]. Aortic characteristic impedance, in contrast to compliance increases moderately with pressure. Systemic peripheral resistance depends on many factors including circulatory filling, metabolism, sympatic tone and the presence of vasoactive drugs. The Modelflow method simulates this behaviour. The Modelflow method uses a peripheral arterial pressure and can be applied uncalibrated by using demographic data of the subject as well as calibrated. For calibration an independent measure of cardiac output [38] or a measure of the cross sectional area of the aorta can be used [39]. A more detailed description of the method can be found elsewhere [29, 38].
General concerns for pulse contour methods
All pulse contour systems are based on a mathematical model and not on a mass balance as the indicator dilution and Fick method are. This implies that deviations of the model to the physiological reality have consequences for the estimated cardiac output. Growing knowledge of the arterial circulation and increasing computation possibilities have led to different software versions of the different methods. This complicates reviewing these methods. We selected only those papers that make use of recent software versions. Furthermore, with a peripheral arterial pressure as input of the model instead of aortic pressure, loss of signal quality may be crucial. An example of the effect of loss of signal quality on blood pressure and cardiac output is shown in Figure 6.
Figure 6.
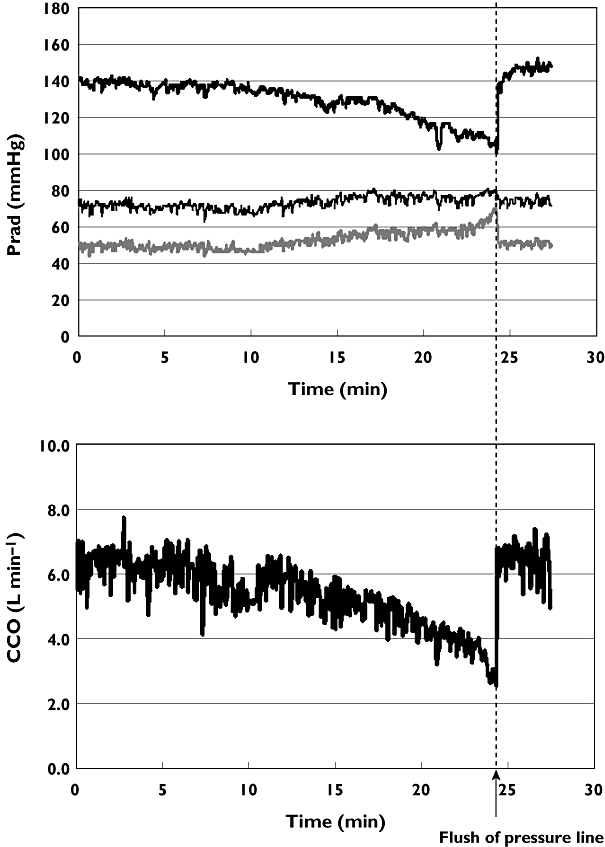
Effects of dampened radial artery pressure on LidCO pulse contour output of an individual patient. Upper panel systolic (Sys), diastolic (Dia) and mean (MAP) radial artery pressure (Prad). Bottom panel cardiac output by PulseCO (CCO). Sys ( ); MAP (
); MAP ( ); Dia (
); Dia ( )
)
Echo-Doppler ultrasound methods
Transoesophageal Doppler
In the last decade the Transoesophageal Doppler (TOD) is the most frequently used ultrasound method (Figure 7); a small ultra-sound transducer, mounted at the tip of a flexible probe, is orally or nasally positioned in the oesophagus along the descending aorta. Insertion depth is typically 35 to 45 cm for adults, depending on the route of insertion (oral vs. nasal). The transducer is pointed towards the aorta by rotation to obtain the optimal aortic velocity signal. The blood flow velocity is calculated with the Doppler equation.
Figure 7.
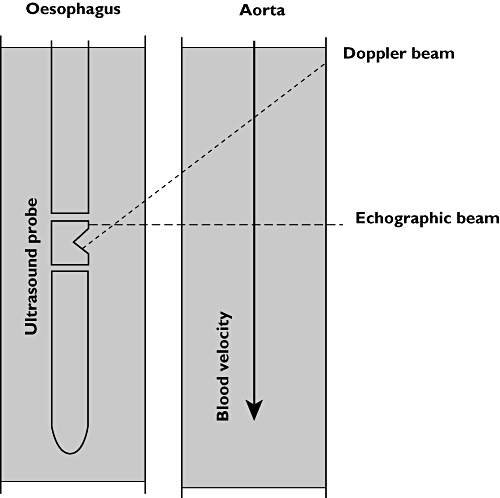
Transoesophageal probe geometry. Blood flow velocity is measured by the Doppler beam using the well known Doppler principle. Aortic diameter is determined by the echographic beam by measuring the distance between the backward scatter of the proximal and distal aortic wall. From this distance the cross sectional area of the aorta is calculated
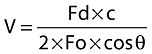 |
where V is the velocity of blood, Fo is the transmitted frequency, Fd is the change in frequency (Doppler shift), cosθ is the angle between the direction of the ultra-sound beam and blood flow and c is the velocity of ultra-sound in blood.
Three different models of oesophageal CO monitoring have been offered. Two of these systems i.e. the Deltex monitor (Cardio Q, Deltex Medical, Chichester, UK) and the monitor of Medicina (TECO, Berkshire, UK), use a nomogram to obtain the cross sectional area (CSA) of the ascending aorta based on the patient's age weight and height, whereas the Hemosonic (Arrow International, Reading, PA, currently not available) uses the M-mode echo for the measurement of the diameter of the aorta at the point of the velocity measurement. From the aortic diameter cross section area is calculated assuming a circular aorta. Aortic blood flow (l min−1) is found by multiplying velocity with heart rate and cross sectional area of the aorta at the insonation point. Cardiac output is calculated from aortic blood flow by assuming a constant distribution of blood between cephalic and caudal circulation.
It is however questionable whether this partitioning of blood streams is constant under a variety of patho-physiological circumstances [40, 41]. Most obvious concerns with the technique are angle of insonation and the fixation of the transducer with respect to the blood flow, especially during movements of the subject. This has led to the conclusion that the method is operator dependent [42] and that additional training is required. Another point of concern is the use of a nomogram to estimate CSA. It is clear that a nomogram for CSA is based on group averages which may include large individual differences. Also CSA has been found pressure dependent [35]. Lastly, the technique is poorly tolerated in awake non-intubated subjects and cannot be used in subjects with an oesophageal disorder.
In a meta-analysis by Dark & Singer in 2004 [43], the authors concluded that the TOD estimates absolute cardiac output with minimal bias but limited agreement. However, the semi-invasive TOD technique enables trend monitoring of CO as long as the probe position is not changed.
Transthoracic Doppler
Transthoracic Doppler (TTD) is an entirely non-invasive method using an ultrasound probe positioned in the jugular notch to obtain blood velocity in the outflow of the left ventricle. The method is in essence equal to oesophageal Doppler technique. Cardiac output is calculated by measuring the cross sectional area of the aortic valve together with the velocity profile in the outflow track. However, it may be very difficult to identify the aortic root in some subjects. In these cases the outflow over the pulmonary valve may be used. Although it is possible to orientate the ultrasound beam in the assumed 0 degree direction of blood flow and perpendicular on the valve, in practice this is difficult to realize. The alignment is affected by operator skill, anatomy and subject movements (for instance during breathing). Consequently the technique has a larger inter- and intra-observer variability and larger limits of agreement compared with reference methods than the transoesophageal method. The portable and non-invasive character of the method allows use in many settings with patients in the supine position.
Thoracic electrical bioimpedance
Electrical bioimpedance was introduced five decades ago as an inexpensive and non-invasiveness cardiac output method. A high-frequency alternating electrical current with low amplitude is applied to the thorax via two electrodes. The resulting voltage is measured with two other electrodes, positioned in between the current electrodes. The measured changes in bio-impedance are thought to be related to changes in cardiac related blood volume. A mathematical conversion is used to translate the change in bioimpedance into cardiac output. Several formulae exist for this conversion. These formulae and their nuances go well beyond the scope of this review. A more detailed description can be found in a review of de Waal and co-workers [44]. The over-simplification of physiological reality by mathematical equations, motion artefacts, abnormal thoracic anatomy, cardiac valve disease, thoracic shunts and arrhythmias contribute to the inaccuracy of this method. In a large meta-analysis of three decades of validation studies on thoracic impedance cardiography, Raaijmakers et al. [45] concluded that a better physical-physiological model in combination with improvements on the impedance CO-equation are still needed.
We expect that this aspect accounts also for the recently developed bio-reactance technology (Biorectance, Cheetah Medical Inc., Indianapolis USA). This method is based on the observation that blood volume changes induce small changes in frequency and phase of the electrical signal propagating across the thorax. These small changes have been shown to correlate with stroke volume [46].
How to evaluate the different cardiac output measurement methods?
Bland & Altman [47, 48] proposed that bias (the mean difference between the techniques) ± 2 SD-precision is an appropriate indication of agreement between techniques. Here bias is the systematic error and the standard deviation (SD) of the differences is the random error between methods. Thus the limits of agreement (bias ± 2 SD) involve the combination of errors of each measurement technique.
In the present review on cardiac output methods a lack of consistency was found in the presentation of results. Regularly the method under study is compared to thermodilution by linear regression analysis also known as calibration statistic, presenting the regression coefficients of the line together with the correlation coefficient. Bland & Altman [47, 48] in their statistical notes pointed out that it could be highly misleading to analyze data pairs by combining repeated observations from several patients and then calculating standard regressions and correlation coefficients.
Critchley & Critchley [49], in an effort to establish objective criteria for judging the accuracy and reproducibility of cardiac output measurement state that if a ‘new’ method is to replace an older, established method, the new method should itself have errors not greater than the older method. Therefore, knowledge and a careful application of the older method as a reliable reference method are essential for a good evaluation of a new technique. Otherwise, the difference between the evaluated method and the reference method could be determined mainly by the reference method. In an example Critchley & Critchley [49] showed that if the reference technique has a 2 SD-precision of ±20%, then a new method must also have a 2 SD-precision of 20% to be acceptable. According to Pythagoras' law, the limits of agreement in the Bland–Altman plot should be less than ±28%, i.e. √(202+ 202), to conclude for agreement between methods. This example has led to an oversimplification in comparison of methods and many authors concluded that the Bland–Altman limits of agreement should be less than ±30% to accept the new measurement technique. Based on the fact that the 2 SD-precision of the reference method may be less than 20%, the criteria of 30% derived from Bland–Altman analysis is highly misleading. Therefore, evaluation studies should provide the precision of the reference method. In addition to the above discussion about the evaluation of new methods, we should realize that a proper evaluation method of continuous cardiac output methods is still awaited [50].
In Table 1, we summarize results of different methods to estimate cardiac output against the results of the intermittent pulmonary thermodilution method as reference method. From each peer reviewed study we noted or recalculated the bias and limits of agreement for cardiac output, hereto cardiac index was converted to cardiac output. For each method we took the median results of the included studies. Furthermore, we calculated the 2 SD-precision for the different methods assuming the reference method had a 2 SD-precision of 10%, 20% and 30%, respectively. A 2 SD-precision of 10% corresponds to the averaged results of three thermodilution measurements equally spread over the ventilatory cycle whereas 20% corresponds to the average result of three measurements randomly applied and 30% to single estimates [5]. The number of studies included in Table 1 are: CCO-vigilance thermodilution method 13 [13, 51–62], transpulmonary thermodilution method 5 [62–66], transpulmonary lithium dilution method 4 [67–70], the Fick CO2-rebreathing method 5 [3, 71–75], calibrated Modelflow method 5 [29, 38, 76–78], uncalibrated Modelflow 4 [38, 78–80], PiCCOplus 7 [62, 76, 81–84] only results with software version 4.x and later were used, LiDCOplus 5 [69, 70, 85–87], PRAM 3 [34, 88, 89] and FloTrack-Vigileo 9 [79, 84, 90–96], only results of software version 1.07 and later were selected. No data of ultrasound methods were included because not enough of these methods were compared with thermodilution cardiac output except for the HemoSonic [79, 97–99] which is however out of production at the moment. Also, the results of the impedance method were excluded because Raaijmakers et al.[45] in a meta-analysis had already concluded that there was insufficient agreement with reference methods. From the data given in Table 1, it can be seen that none of the methods can replace the averaged results of three measurements with pulmonary artery intermittent thermodilution equally distributed over the ventilatory cycle (2 SD < 10%). Transpulmonary thermodilution, transpulmonary lithium dilution both with the averaged results of three measurements, calibrated Modelflow and LiDCOplus pulse contour may replace the pulmonary artery thermodilution with the results of three randomly applied measurements. All methods can replace single thermodilution estimates with a 2 SD-precision of 30%.
Table 1.
Median results for different methods in comparison with intermittent pulmonary thermodilution cardiac output
| Differences with COpa | Calculated 2SD-precision with | ||||||
|---|---|---|---|---|---|---|---|
| Bias | 2SD-precision | 2 SDpa = 10% | 2 SDpa = 20% | 2 SDpa = 30% | |||
| Method | Number of observations | l min−1 | % | % | % | % | % |
| Indicator dilution | |||||||
| CCO-Vigilance | 3439 | 0.03 | 0.55 | 27 | 25 | 18 | 6 |
| Transpulmonary TD | 818 | 0.43 | 7.74 | 21 | 18 | 7 | 0 |
| Transpulmonary LiD | 245 | −0.03 | −0.55 | 26 | 23 | 16 | 0 |
| Fick | |||||||
| CO2-rebreathing | 601 | −0.25 | −4.35 | 35 | 34 | 29 | 19 |
| Pulse contour | |||||||
| Modelflow-calibrated | 995 | 0.00 | 0.00 | 17 | 16 | 0 | 0 |
| Modelflow-noncalibrated | 924 | 0.31 | 5.63 | 31 | 29 | 23 | 7 |
| PiCCOplus | 1802 | 0.04 | 0.73 | 32 | 30 | 25 | 10 |
| LiDCOplus | 452 | 0.05 | 0.91 | 24 | 22 | 13 | 0 |
| FloTrac-Vigileo | 1777 | 0.25 | 4.55 | 41 | 40 | 36 | 29 |
COpa, cardiac output by intermittent pulmonary thermodilution.
Conclusions
Many methods to measure cardiac output are available (see Table 2). None of the methods studied fulfil the criteria of accuracy, precision, operator independence, fast responding, non-invasiveness, continuous measurement, ease of use, low cost and without complications. The Fick for O2, for instance, is labour intensive and invasive but highly accurate and precise. The continuous thermodilution method does not have a fast response, needs skilled physicians to introduce the PAC and is invasive. The pulse contour methods are non-invasiveness, give beat-to-beat cardiac output and are easy to use. The ultrasound methods have large inter- and intra-observer variability. The transpulmonary indicator dilution methods score better in accuracy and precision. The ultrasound methods are limited by large inter- and intra-observer variability. With respect to precision and accuracy, all methods can replace single thermodilution estimates with a 2 SD-precision of 30%, most can replace the averaged result of three randomly applied intermittent thermodilution measurements but none can replace the averaged results of three estimates equally distributed over the ventilatory cycle.
Table 2.
Overview of characteristics for different methods to measure cardiac output
| CO method | Invasiveness | Response | Accuracy | Precision | Limitations |
|---|---|---|---|---|---|
| Fick O2 | +++ | Intermittent | High | Moderate | Requires a PAC for venous O2 and spirometer or mechanical ventilator. Labour intensive technique |
| Fick CO2 | + | Slow | Low | Low | Subject must be on ventilator Errors due to shunts |
| PAC Td bolus | +++ | Intermittent | High | High | Special precaution during mechanical ventilation Requires a PAC and triplicate measurements |
| PAC CCO | +++ | Continuous | Moderate | Moderate | Requires a PAC and triplicate measurements |
| TP Td bolus | ++ | Intermittent | High | High | Requires a PAC and triplicate measurements |
| TP Li bolus | ++ | Intermittent | Moderate | Moderate | Requires only arterial catheter but needs triplicate measurement for sufficient agreement with reference methods |
| PiCCO | ++ | Beat-to-beat | Moderate | Moderate | Requires frequent calibration with independent (other) method |
| LiDCO | ++ | Beat-to-beat | Moderate | Moderate | Requires frequent calibration with independent (other) method or lithium indicator method |
| Vigileo | ++ | Beat-to-beat | Moderate | High | Needs specific sensor |
| Modelflow | ++ | Beat-to-beat | High | High | Needs femoral or radial arterial catheter |
| TOD | + | Continuous | High | Low | Not well tolerated in awake subjects and transducer position difficult |
| TTE | − | Continuous | Moderate | Low | Large inter-operator variability |
| Bioimpedance | − | Continuous | Low | Low | Artifacts due to anatomic variations, shunt, movement, electrical noise |
CO cardiac output, CCO continuous cardiac output, Li Lithium, PAC pulmonary artery catheter, Td thermodilution, TOD transoesophageal Doppler, TP transpulmonary, TTE transthoracic echography.
Competing interests
J J was involved in the development of the modelflow method. However, he has no financial interests related to the modelflow method.
There was no financial support for this review.
REFERENCES
- 1.Prys-Roberts C. The measurement of cardiac output. Br J Anaesth. 1969;41:751–60. doi: 10.1093/bja/41.9.751. [DOI] [PubMed] [Google Scholar]
- 2.Ultman JS, Bursztein S. Analysis of error in the determination of respiratory gas exchange at varying FIO2. J Appl Physiol. 1981;50:210–6. doi: 10.1152/jappl.1981.50.1.210. [DOI] [PubMed] [Google Scholar]
- 3.Tachibana K, Imanaka H, Takeuchi M, Takauchi Y, Miyano H, Nishimura M. Noninvasive cardiac output measurement using partial carbon dioxide rebreathing is less accurate at settings of reduced minute ventilation and when spontaneous breathing is present. Anesthesiology. 2003;98:830–7. doi: 10.1097/00000542-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Gueret G, Kiss G, Rossignol B, Bezon E, Wargnier JP, Miossec A, Corre O, Arvieux CC. Cardiac output measurements in off-pump coronary surgery: comparison between NICO and the Swan-Ganz catheter. Eur J Anaesthesiol. 2006;23:848–54. doi: 10.1017/S0265021506000573. [DOI] [PubMed] [Google Scholar]
- 5.Jansen JR. The thermodilution method for the clinical assessment of cardiac output. Intensive Care Med. 1995;21:691–7. doi: 10.1007/BF01711553. [DOI] [PubMed] [Google Scholar]
- 6.Breukers RB, Jansen JR. Pulmonary artery thermodilution cardiac output vs. transpulmonary thermodilution cardiac output in two patients with intrathoracic pathology. Acta Anaesthesiol Scand. 2004;48:658–61. doi: 10.1111/j.1399-6576.2004.00375.x. [DOI] [PubMed] [Google Scholar]
- 7.Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med. 1970;283:447–51. doi: 10.1056/NEJM197008272830902. [DOI] [PubMed] [Google Scholar]
- 8.Jansen JR, Schreuder JJ, Settels JJ, Kornet L, Penn OC, Mulder PG, Versprille A, Wesseling KH. Single injection thermodilution. A flow-corrected method. Anesthesiology. 1996;85:481–90. doi: 10.1097/00000542-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Jansen JR, Schreuder JJ, Punt KD, van den Berg PC, Alfieri O. Mean cardiac output by thermodilution with a single controlled injection. Crit Care Med. 2001;29:1868–73. doi: 10.1097/00003246-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Jansen JR, Schreuder JJ, Bogaard JM, van Rooyen W, Versprille A. Thermodilution technique for measurement of cardiac output during artificial ventilation. J Appl Physiol. 1981;51:584–91. doi: 10.1152/jappl.1981.51.3.584. [DOI] [PubMed] [Google Scholar]
- 11.Jansen JR, Versprille A. Improvement of cardiac output estimation by the thermodilution method during mechanical ventilation. Intensive Care Med. 1986;12:71–9. doi: 10.1007/BF00254515. [DOI] [PubMed] [Google Scholar]
- 12.Jansen JR, Schreuder JJ, Settels JJ, Kloek JJ, Versprille A. An adequate strategy for the thermodilution technique in patients during mechanical ventilation. Intensive Care Med. 1990;16:422–5. doi: 10.1007/BF01711218. [DOI] [PubMed] [Google Scholar]
- 13.Yelderman M. Continuous measurement of cardiac output with the use of stochastic system identification techniques. J Clin Monit. 1990;6:322–32. doi: 10.1007/BF02842492. [DOI] [PubMed] [Google Scholar]
- 14.Aranda M, Mihm FG, Garrett S, Mihm MN, Pearl RG. Continuous cardiac output catheters: delay in in vitro response time after controlled flow changes. Anesthesiology. 1998;89:1592–5. doi: 10.1097/00000542-199812000-00047. [DOI] [PubMed] [Google Scholar]
- 15.Vincent JL, Pinsky MR, Sprung CL, Levy M, Marini JJ, Payen D, Rhodes A, Takala J. The pulmonary artery catheter: in medio virtus. Crit Care Med. 2008;36:3093–6. doi: 10.1097/CCM.0b013e31818c10c7. [DOI] [PubMed] [Google Scholar]
- 16.Harvey S, Harrison DA, Singer M, Ashcroft J, Jones CM, Elbourne D, Brampton W, Williams D, Young D, Rowan K. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet. 2005;366:472–7. doi: 10.1016/S0140-6736(05)67061-4. [DOI] [PubMed] [Google Scholar]
- 17.Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, Laporta DP, Viner S, Passerini L, Devitt H, Kirby A, Jacka M. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348:5–14. doi: 10.1056/NEJMoa021108. [DOI] [PubMed] [Google Scholar]
- 18.Richard C, Warszawski J, Anguel N, Deye N, Combes A, Barnoud D, Boulain T, Lefort Y, Fartoukh M, Baud F, Boyer A, Brochard L, Teboul JL. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2003;290:2713–20. doi: 10.1001/jama.290.20.2713. [DOI] [PubMed] [Google Scholar]
- 19.Squara P, Bennett D, Perret C. Pulmonary artery catheter: does the problem lie in the users? Chest. 2002;121:2009–15. doi: 10.1378/chest.121.6.2009. [DOI] [PubMed] [Google Scholar]
- 20.Fowler RA, Cook DJ. The arc of the pulmonary artery catheter. JAMA. 2003;290:2732–4. doi: 10.1001/jama.290.20.2732. [DOI] [PubMed] [Google Scholar]
- 21.Tibby SM, Hatherill M, Marsh MJ, Morrison G, Anderson D, Murdoch IA. Clinical validation of cardiac output measurements using femoral artery thermodilution with direct Fick in ventilated children and infants. Intensive Care Med. 1997;23:987–91. doi: 10.1007/s001340050443. [DOI] [PubMed] [Google Scholar]
- 22.Sakka SG, Bredle DL, Reinhart K, Meier-Hellmann A. Comparison between intrathoracic blood volume and cardiac filling pressures in the early phase of hemodynamic instability of patients with sepsis or septic shock. J Crit Care. 1999;14:78–83. doi: 10.1016/s0883-9441(99)90018-7. [DOI] [PubMed] [Google Scholar]
- 23.Pauli C, Fakler U, Genz T, Hennig M, Lorenz HP, Hess J. Cardiac output determination in children: equivalence of the transpulmonary thermodilution method to the direct Fick principle. Intensive Care Med. 2002;28:947–52. doi: 10.1007/s00134-002-1334-2. [DOI] [PubMed] [Google Scholar]
- 24.Band DM, Linton RA, O'Brien TK, Jonas MM, Linton NW. The shape of indicator dilution curves used for cardiac output measurement in man. J Physiol. 1997;498:225–9. doi: 10.1113/jphysiol.1997.sp021853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonas MM, Lint RAF, O'Brein TK, Band DM, Linton NW, Kelly F, Burden TJ, Chevalier SFA, Thompson RPH, Birch NJ, Powell JJ. The pharmacokinetics of intravenous lithium chloride in patients and normal volunteers. J Trace Microprobe Techn. 2001;19:313–20. [Google Scholar]
- 26.Cecconi M, Fawcett J, Grounds RM, Rhodes A. A prospective study to evaluate the accuracy of pulse power analysis to monitor cardiac output in critically ill patients. BMC Anesthesiol. 2008;8:3. doi: 10.1186/1471-2253-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank O. Die Gründform des arterielen Pulses erste Abhandlung: mathematische Analyse. Z Biol. 1899:483–526. [Google Scholar]
- 28.Jansen JR, Wesseling KH, Settels JJ, Schreuder JJ. Continuous cardiac output monitoring by pulse contour during cardiac surgery. Eur Heart J. 1990;11(Suppl. I):26–32. doi: 10.1093/eurheartj/11.suppl_i.26. [DOI] [PubMed] [Google Scholar]
- 29.Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol. 1993;74:2566–73. doi: 10.1152/jappl.1993.74.5.2566. [DOI] [PubMed] [Google Scholar]
- 30.Halvorsen PS, Sokolov A, Cvancarova M, Hol PK, Lundblad R, Tonnessen TI. Continuous cardiac output during off-pump coronary artery bypass surgery: pulse-contour analyses vs pulmonary artery thermodilution. Br J Anaesth. 2007;99:484–92. doi: 10.1093/bja/aem199. [DOI] [PubMed] [Google Scholar]
- 31.Johansson A, Chew M. Reliability of continuous pulse contour cardiac output measurement during hemodynamic instability. J Clin Monit Comput. 2007;21:237–42. doi: 10.1007/s10877-007-9079-7. [DOI] [PubMed] [Google Scholar]
- 32.Hamzaoui O, Monnet X, Richard C, Osman D, Chemla D, Teboul JL. Effects of changes in vascular tone on the agreement between pulse contour and transpulmonary thermodilution cardiac output measurements within an up to 6-hour calibration-free period. Crit Care Med. 2008;36:434–40. doi: 10.1097/01.CCM.OB013E318161FEC4. [DOI] [PubMed] [Google Scholar]
- 33.de Wilde RB, Breukers RB, van den Berg PC, Jansen JR. Monitoring cardiac output using the femoral and radial arterial pressure waveform. Anaesthesia. 2006;61:743–6. doi: 10.1111/j.1365-2044.2006.04712.x. [DOI] [PubMed] [Google Scholar]
- 34.Romano SM, Pistolesi M. Assessment of cardiac output from systemic arterial pressure in humans. Crit Care Med. 2002;30:1834–41. doi: 10.1097/00003246-200208000-00027. [DOI] [PubMed] [Google Scholar]
- 35.Langewouters GJ, Wesseling KH, Goedhard WJ. The static elastic properties of 45 human thoracic and 20 abdominal aortas in vitro and the parameters of a new model. J Biomech. 1984;17:425–35. doi: 10.1016/0021-9290(84)90034-4. [DOI] [PubMed] [Google Scholar]
- 36.Remington JW, Noback CR. Volume elasticity characteristics of the human aorta and prediction of the stroke volume from the pressure pulse. Am J Physiol. 1948;153:298–308. doi: 10.1152/ajplegacy.1948.153.2.298. [DOI] [PubMed] [Google Scholar]
- 37.Cecconi M, Dawson D, Grounds RM, Rhodes A. Lithium dilution cardiac output measurement in the critically ill patient: determination of precision of the technique. Intensive Care Med. 2009;35:498–504. doi: 10.1007/s00134-008-1292-4. [DOI] [PubMed] [Google Scholar]
- 38.Jansen JR, Schreuder JJ, Mulier JP, Smith NT, Settels JJ, Wesseling KH. A comparison of cardiac output derived from the arterial pressure wave against thermodilution in cardiac surgery patients. Br J Anaesth. 2001;87:212–22. doi: 10.1093/bja/87.2.212. [DOI] [PubMed] [Google Scholar]
- 39.de Vaal JB, de Wilde RB, van den Berg PC, Schreuder JJ, Jansen JR. Less invasive determination of cardiac output from the arterial pressure by aortic diameter-calibrated pulse contour. Br J Anaesth. 2005;95:326–31. doi: 10.1093/bja/aei189. [DOI] [PubMed] [Google Scholar]
- 40.Turner MA. Doppler-based hemodynamic monitoring: a minimally invasive alternative. AACN Clin Issues. 2003;14:220–31. doi: 10.1097/00044067-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Cholley BP, Singer M. Esophageal Doppler: noninvasive cardiac output monitor. Echocardiography. 2003;20:763–9. doi: 10.1111/j.0742-2822.2003.03033.x. [DOI] [PubMed] [Google Scholar]
- 42.Spahn DR, Schmid ER, Tornic M, Jenni R, von Segesser L, Turina M, Baetscher A. Noninvasive versus invasive assessment of cardiac output after cardiac surgery: clinical validation. J Cardiothorac Anesth. 1990;4:46–59. doi: 10.1016/0888-6296(90)90447-n. [DOI] [PubMed] [Google Scholar]
- 43.Dark PM, Singer M. The validity of trans-esophageal Doppler ultrasonography as a measure of cardiac output in critically ill adults. Intensive Care Med. 2004;30:2060–6. doi: 10.1007/s00134-004-2430-2. [DOI] [PubMed] [Google Scholar]
- 44.de Waal EE, Konings MK, Kalkman CJ, Buhre WF. Assessment of stroke volume index with three different bioimpedance algorithms: lack of agreement compared to thermodilution. Intensive Care Med. 2008;34:735–9. doi: 10.1007/s00134-007-0938-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raaijmakers E, Faes TJ, Scholten RJ, Goovaerts HG, Heethaar RM. A meta-analysis of three decades of validating thoracic impedance cardiography. Crit Care Med. 1999;27:1203–13. doi: 10.1097/00003246-199906000-00053. [DOI] [PubMed] [Google Scholar]
- 46.Raval NY, Squara P, Cleman M, Yalamanchili K, Winklmaier M, Burkhoff D. Multicenter evaluation of noninvasive cardiac output measurement by bioreactance technique. J Clin Monit Comput. 2008;22:113–9. doi: 10.1007/s10877-008-9112-5. [DOI] [PubMed] [Google Scholar]
- 47.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 2 – correlation between subjects. BMJ. 1995;310:633. doi: 10.1136/bmj.310.6980.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 1 – correlation within subjects. BMJ. 1995;310:446. doi: 10.1136/bmj.310.6977.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Critchley LA, Critchley JA. A meta-analysis of studies using bias and precision statistics to compare cardiac output measurement techniques. J Clin Monit Comput. 1999;15:85–91. doi: 10.1023/a:1009982611386. [DOI] [PubMed] [Google Scholar]
- 50.Cecconi M, Rhodes A. Validation of continuous cardiac output technologies: consensus still awaited. Crit Care. 2009;13:159. doi: 10.1186/cc7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmid ER, Schmidlin D, Tornic M, Seifert B. Continuous thermodilution cardiac output: clinical validation against a reference technique of known accuracy. Intensive Care Med. 1999;25:166–72. doi: 10.1007/s001340050811. [DOI] [PubMed] [Google Scholar]
- 52.Jakobsen CJ, Melsen NC, Andresen EB. Continuous cardiac output measurements in the perioperative period. Acta Anaesthesiol Scand. 1995;39:485–8. doi: 10.1111/j.1399-6576.1995.tb04104.x. [DOI] [PubMed] [Google Scholar]
- 53.Haller M, Zollner C, Briegel J, Forst H. Evaluation of a new continuous thermodilution cardiac output monitor in critically ill patients: a prospective criterion standard study. Crit Care Med. 1995;23:860–6. doi: 10.1097/00003246-199505000-00014. [DOI] [PubMed] [Google Scholar]
- 54.Boldt J, Menges T, Wollbruck M, Hammermann H, Hempelmann G. Is continuous cardiac output measurement using thermodilution reliable in the critically ill patient? Crit Care Med. 1994;22:1913–8. [PubMed] [Google Scholar]
- 55.Rauch H, Muller M, Fleischer F, Bauer H, Martin E, Bottiger BW. Pulse contour analysis versus thermodilution in cardiac surgery patients. Acta Anaesthesiol Scand. 2002;46:424–9. doi: 10.1034/j.1399-6576.2002.460416.x. [DOI] [PubMed] [Google Scholar]
- 56.Bottiger BW, Soder M, Rauch H, Bohrer H, Motsch J, Bauer H, Martin E. Semi-continuous versus injectate cardiac output measurement in intensive care patients after cardiac surgery. Intensive Care Med. 1996;22:312–8. doi: 10.1007/BF01700452. [DOI] [PubMed] [Google Scholar]
- 57.Bottiger BW, Rauch H, Bohrer H, Motsch J, Soder M, Fleischer F, Martin E. Continuous versus intermittent cardiac output measurement in cardiac surgical patients undergoing hypothermic cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1995;9:405–11. doi: 10.1016/s1053-0770(05)80095-3. [DOI] [PubMed] [Google Scholar]
- 58.Bottiger BW, Sinner B, Motsch J, Bach A, Bauer H, Martin E. Continuous versus intermittent thermodilution cardiac output measurement during orthotopic liver transplantation. Anaesthesia. 1997;52:207–14. doi: 10.1111/j.1365-2044.1997.079-az0077.x. [DOI] [PubMed] [Google Scholar]
- 59.Hogue CW, Jr, Rosenbloom M, McCawley C, Lappas DG. Comparison of cardiac output measurement by continuous thermodilution with electromagnetometry in adult cardiac surgical patients. J Cardiothorac Vasc Anesth. 1994;8:631–5. doi: 10.1016/1053-0770(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 60.Singh A, Juneja R, Mehta Y, Trehan N. Comparison of continuous, stat, and intermittent cardiac output measurements in patients undergoing minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2002;16:186–90. doi: 10.1053/jcan.2002.31063. [DOI] [PubMed] [Google Scholar]
- 61.Della Rocca RG, Costa MG, Coccia C, Pompei L, Di Marco P, Vilardi V, Pietropaoli P. Cardiac output monitoring: aortic transpulmonary thermodilution and pulse contour analysis agree with standard thermodilution methods in patients undergoing lung transplantation. Can J Anaesth. 2003;50:707–11. doi: 10.1007/BF03018714. [DOI] [PubMed] [Google Scholar]
- 62.Della Rocca G, Costa MG, Pompei L, Coccia C, Pietropaoli P. Continuous and intermittent cardiac output measurement: pulmonary artery catheter versus aortic transpulmonary technique. Br J Anaesth. 2002;88:350–6. doi: 10.1093/bja/88.3.350. [DOI] [PubMed] [Google Scholar]
- 63.Holm C, Melcer B, Horbrand F, Henckel von Donnersmarck G, Muhlbauer W. Arterial thermodilution: an alternative to pulmonary artery catheter for cardiac output assessment in burn patients. Burns. 2001;27:161–6. doi: 10.1016/s0305-4179(00)00088-7. [DOI] [PubMed] [Google Scholar]
- 64.Felbinger TW, Reuter DA, Eltzschig HK, Bayerlein J, Goetz AE. Cardiac index measurements during rapid preload changes: a comparison of pulmonary artery thermodilution with arterial pulse contour analysis. J Clin Anesth. 2005;17:241–8. doi: 10.1016/j.jclinane.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 65.Friesecke S, Heinrich A, Abel P, Felix SB. Comparison of pulmonary artery and aortic transpulmonary thermodilution for monitoring of cardiac output in patients with severe heart failure: validation of a novel method. Crit Care Med. 2009;37:119–23. doi: 10.1097/CCM.0b013e31819290d5. [DOI] [PubMed] [Google Scholar]
- 66.Wiesenack C, Prasser C, Keyl C, Rodig G. Assessment of intrathoracic blood volume as an indicator of cardiac preload: single transpulmonary thermodilution technique versus assessment of pressure preload parameters derived from a pulmonary artery catheter. J Cardiothorac Vasc Anesth. 2001;15:584–8. doi: 10.1053/jcan.2001.26536. [DOI] [PubMed] [Google Scholar]
- 67.Linton RA, Jonas MM, Tibby SM, Murdoch IA, O'Brien TK, Linton NW, Band DM. Cardiac output measured by lithium dilution and transpulmonary thermodilution in patients in a paediatric intensive care unit. Intensive Care Med. 2000;26:1507–11. doi: 10.1007/s001340051347. [DOI] [PubMed] [Google Scholar]
- 68.Linton RA, Band DM, Haire KM. A new method of measuring cardiac output in man using lithium dilution. Br J Anaesth. 1993;71:262–6. doi: 10.1093/bja/71.2.262. [DOI] [PubMed] [Google Scholar]
- 69.Costa MG, Della Rocca G, Chiarandini P, Mattelig S, Pompei L, Barriga MS, Reynolds T, Cecconi M, Pietropaoli P. Continuous and intermittent cardiac output measurement in hyperdynamic conditions: pulmonary artery catheter vs. lithium dilution technique. Intensive Care Med. 2008;34:257–63. doi: 10.1007/s00134-007-0878-6. [DOI] [PubMed] [Google Scholar]
- 70.Garcia-Rodriguez C, Pittman J, Cassell CH, Sum-Ping J, El Moalem H, Young C, Mark JB. Lithium dilution cardiac output measurement: a clinical assessment of central venous and peripheral venous indicator injection. Crit Care Med. 2002;30:2199–204. doi: 10.1097/00003246-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Nilsson LB, Eldrup N, Berthelsen PG. Lack of agreement between thermodilution and carbon dioxide-rebreathing cardiac output. Acta Anaesthesiol Scand. 2001;45:680–5. doi: 10.1034/j.1399-6576.2001.045006680.x. [DOI] [PubMed] [Google Scholar]
- 72.Kotake Y, Moriyama K, Innami Y, Shimizu H, Ueda T, Morisaki H, Takeda J. Performance of noninvasive partial CO2 rebreathing cardiac output and continuous thermodilution cardiac output in patients undergoing aortic reconstruction surgery. Anesthesiology. 2003;99:283–8. doi: 10.1097/00000542-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 73.Odenstedt H, Stenqvist O, Lundin S. Clinical evaluation of a partial CO2 rebreathing technique for cardiac output monitoring in critically ill patients. Acta Anaesthesiol Scand. 2002;46:152–9. [PubMed] [Google Scholar]
- 74.Rocco M, Spadetta G, Morelli A, Dell'Utri D, Porzi P, Conti G, Pietropaoli P. A comparative evaluation of thermodilution and partial CO2 rebreathing techniques for cardiac output assessment in critically ill patients during assisted ventilation. Intensive Care Med. 2004;30:82–7. doi: 10.1007/s00134-003-2069-4. [DOI] [PubMed] [Google Scholar]
- 75.Tachibana K, Imanaka H, Takeuchi M, Nishida T, Takauchi Y, Nishimura M. Effects of reduced rebreathing time, in spontaneously breathing patients, on respiratory effort and accuracy in cardiac output measurement when using a partial carbon dioxide rebreathing technique: a prospective observational study. Crit Care. 2005;9:R569–R574. doi: 10.1186/cc3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Wilde RB, Schreuder JJ, van den Berg PC, Jansen JR. An evaluation of cardiac output by five arterial pulse contour techniques during cardiac surgery. Anaesthesia. 2007;62:760–8. doi: 10.1111/j.1365-2044.2007.05135.x. [DOI] [PubMed] [Google Scholar]
- 77.Jellema WT, Wesseling KH, Groeneveld AB, Stoutenbeek CP, Thijs LG, van Lieshout JJ. Continuous cardiac output in septic shock by simulating a model of the aortic input impedance: a comparison with bolus injection thermodilution. Anesthesiology. 1999;90:1317–28. doi: 10.1097/00000542-199905000-00016. [DOI] [PubMed] [Google Scholar]
- 78.Harms MP, Wesseling KH, Pott F, Jenstrup M, Van Goudoever J, Secher NH, van Lieshout JJ. Continuous stroke volume monitoring by modelling flow from non-invasive measurement of arterial pressure in humans under orthostatic stress. Clin Sci (Lond) 1999;97:291–301. [PubMed] [Google Scholar]
- 79.de Wilde RB, Geerts BF, Cui J, van den Berg PC, Jansen JR. Performance of three minimally invasive cardiac output monitoring systems. Anaesthesia. 2009;64:762–9. doi: 10.1111/j.1365-2044.2009.05934.x. [DOI] [PubMed] [Google Scholar]
- 80.Hirschl MM, Binder M, Gwechenberger M, Herkner H, Bur A, Kittler H, Laggner AN. Noninvasive assessment of cardiac output in critically ill patients by analysis of the finger blood pressure waveform. Crit Care Med. 1997;25:1909–14. doi: 10.1097/00003246-199711000-00033. [DOI] [PubMed] [Google Scholar]
- 81.Mielck F, Buhre W, Hanekop G, Tirilomis T, Hilgers R, Sonntag H. Comparison of continuous cardiac output measurements in patients after cardiac surgery. J Cardiothorac Vasc Anesth. 2003;17:211–6. doi: 10.1053/jcan.2003.49. [DOI] [PubMed] [Google Scholar]
- 82.Godje O, Hoke K, Goetz AE, Felbinger TW, Reuter DA, Reichart B, Friedl R, Hannekum A, Pfeiffer UJ. Reliability of a new algorithm for continuous cardiac output determination by pulse-contour analysis during hemodynamic instability. Crit Care Med. 2002;30:52–8. doi: 10.1097/00003246-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 83.Felbinger TW, Reuter DA, Eltzschig HK, Moerstedt K, Goedje O, Goetz AE. Comparison of pulmonary arterial thermodilution and arterial pulse contour analysis: evaluation of a new algorithm. J Clin Anesth. 2002;14:296–301. doi: 10.1016/s0952-8180(02)00363-x. [DOI] [PubMed] [Google Scholar]
- 84.Della Rocca G, Costa MG, Chiarandini P, Bertossi G, Lugano M, Pompei L, Coccia C, Sainz-Barriga M, Pietropaoli P. Arterial pulse cardiac output agreement with thermodilution in patients in hyperdynamic conditions. J Cardiothorac Vasc Anesth. 2008;22:681–7. doi: 10.1053/j.jvca.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 85.Jonas MM, Tanser SJ. Lithium dilution measurement of cardiac output and arterial pulse waveform analysis: an indicator dilution calibrated beat-by-beat system for continuous estimation of cardiac output. Curr Opin Crit Care. 2002;8:257–61. doi: 10.1097/00075198-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 86.Hamilton TT, Huber LM, Jessen ME. PulseCO: a less-invasive method to monitor cardiac output from arterial pressure after cardiac surgery. Ann Thorac Surg. 2002;74:S1408–S1412. doi: 10.1016/s0003-4975(02)04059-6. [DOI] [PubMed] [Google Scholar]
- 87.Linton R, Band D, O'Brien T, Jonas M, Leach R. Lithium dilution cardiac output measurement: a comparison with thermodilution. Crit Care Med. 1997;25:1796–800. doi: 10.1097/00003246-199711000-00015. [DOI] [PubMed] [Google Scholar]
- 88.Zangrillo A, Maj G, Monaco F, Scandroglio AM, Nuzzi M, Plumari V, Virzo I, Bignami E, Casiraghi G, Landoni G. Cardiac index validation using the pressure recording analytic method in unstable patients. J Cardiothorac Vasc Anesth. 2010;24:265–9. doi: 10.1053/j.jvca.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 89.Romagnoli S, Romano SM, Bevilacqua S, Ciappi F, Lazzeri C, Peris A, Dini D, Gelsomino S. Cardiac output by arterial pulse contour: reliability under hemodynamic derangements. Interact Cardiovasc Thorac Surg. 2009;8:642–6. doi: 10.1510/icvts.2008.200451. [DOI] [PubMed] [Google Scholar]
- 90.Button D, Weibel L, Reuthebuch O, Genoni M, Zollinger A, Hofer CK. Clinical evaluation of the FloTrac/Vigileo system and two established continuous cardiac output monitoring devices in patients undergoing cardiac surgery. Br J Anaesth. 2007;99:329–36. doi: 10.1093/bja/aem188. [DOI] [PubMed] [Google Scholar]
- 91.McGee WT, Horswell JL, Calderon J, Janvier G, Van Severen T, Van BG, Kozikowski L. Validation of a continuous, arterial pressure-based cardiac output measurement: a multicenter, prospective clinical trial. Crit Care. 2007;11:R105. doi: 10.1186/cc6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mayer J, Boldt J, Beschmann R, Stephan A, Suttner S. Uncalibrated arterial pressure waveform analysis for less-invasive cardiac output determination in obese patients undergoing cardiac surgery. Br J Anaesth. 2009;103:185–90. doi: 10.1093/bja/aep133. [DOI] [PubMed] [Google Scholar]
- 93.Mayer J, Boldt J, Wolf MW, Lang J, Suttner S. Cardiac output derived from arterial pressure waveform analysis in patients undergoing cardiac surgery: validity of a second generation device. Anesth Analg. 2008;106:867–72. doi: 10.1213/ane.0b013e318161964d. [DOI] [PubMed] [Google Scholar]
- 94.Breukers RM, Sepehrkhouy S, Spiegelenberg SR, Groeneveld AB. Cardiac output measured by a new arterial pressure waveform analysis method without calibration compared with thermodilution after cardiac surgery. J Cardiothorac Vasc Anesth. 2007;21:632–5. doi: 10.1053/j.jvca.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 95.Prasser C, Bele S, Keyl C, Schweiger S, Trabold B, Amann M, Welnhofer J, Wiesenack C. Evaluation of a new arterial pressure-based cardiac output device requiring no external calibration. BMC Anesthesiol. 2007;7:9. doi: 10.1186/1471-2253-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mehta Y, Chand RK, Sawhney R, Bhise M, Singh A, Trehan N. Cardiac output monitoring: comparison of a new arterial pressure waveform analysis to the bolus thermodilution technique in patients undergoing off-pump coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2008;22:394–9. doi: 10.1053/j.jvca.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 97.Moxon D, Pinder M, van Heerden PV, Parsons RW. Clinical evaluation of the HemoSonic monitor in cardiac surgical patients in the ICU. Anaesth Intensive Care. 2003;31:408–11. doi: 10.1177/0310057X0303100410. [DOI] [PubMed] [Google Scholar]
- 98.Lafanechere A, Albaladejo P, Raux M, Geeraerts T, Bocquet R, Wernet A, Castier Y, Marty J. Cardiac output measurement during infrarenal aortic surgery: echo-esophageal Doppler versus thermodilution catheter. J Cardiothorac Vasc Anesth. 2006;20:26–30. doi: 10.1053/j.jvca.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 99.Jaeggi P, Hofer CK, Klaghofer R, Fodor P, Genoni M, Zollinger A. Measurement of cardiac output after cardiac surgery by a new transesophageal Doppler device. J Cardiothorac Vasc Anesth. 2003;17:217–20. doi: 10.1053/jcan.2003.50. [DOI] [PubMed] [Google Scholar]


