Abstract
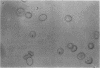
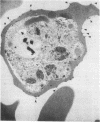
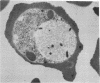
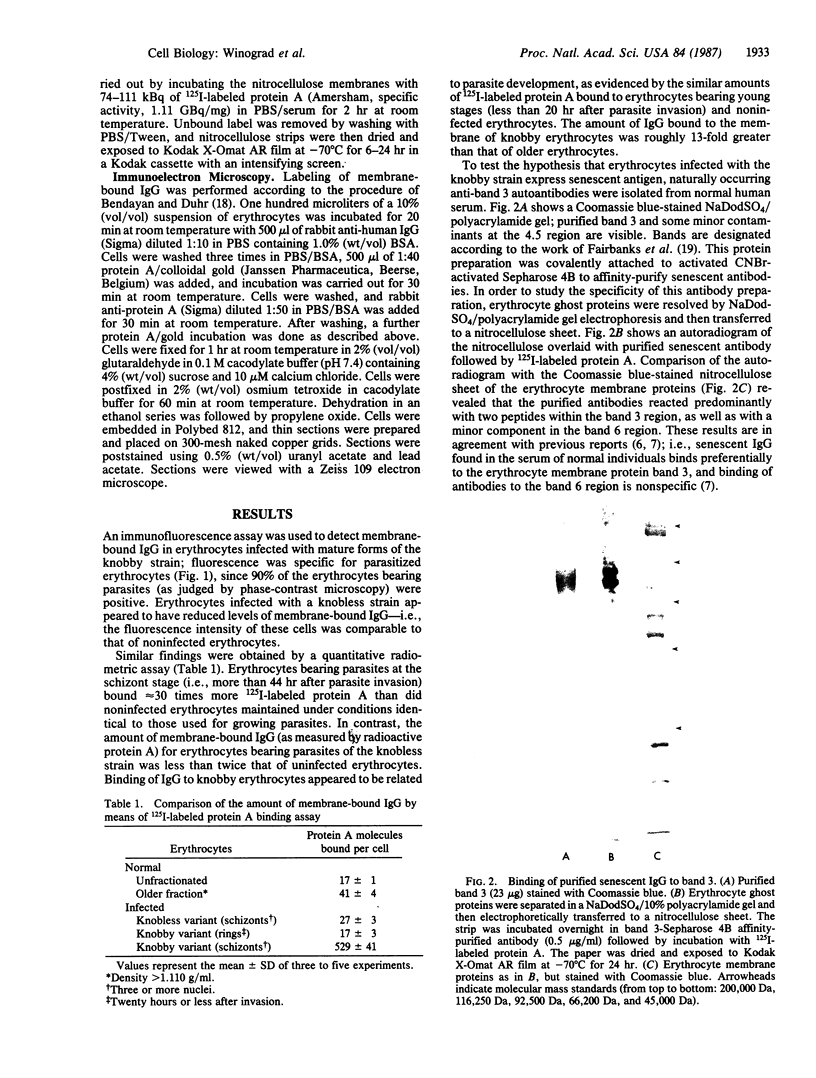
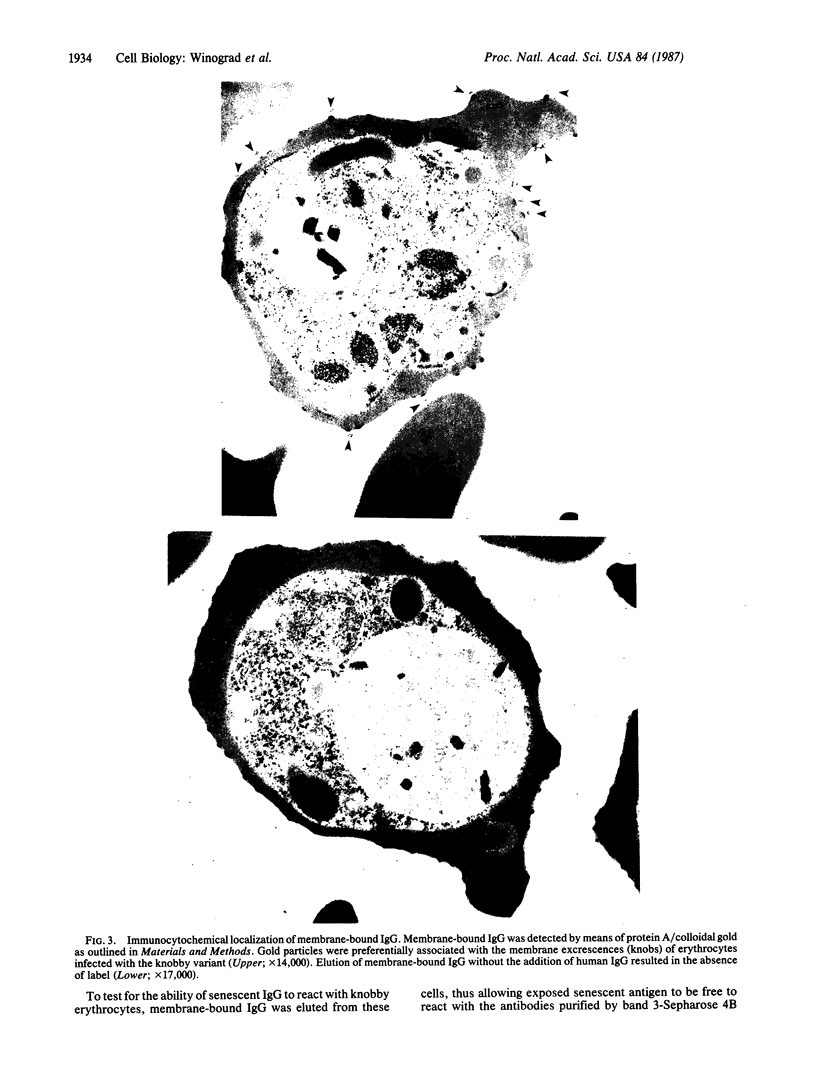
Erythrocytes infected with a knobby variant of Plasmodium falciparum selectively bind IgG autoantibodies in normal human serum. Quantification of membrane-bound IgG, by use of 125I-labeled protein A, revealed that erythrocytes infected with the knobby variant bound 30 times more protein A than did noninfected erythrocytes; infection with a knobless variant resulted in less than a 2-fold difference compared with noninfected erythrocytes. IgG binding to knobby erythrocytes appeared to be related to parasite development, since binding of 125I-labeled protein A to cells bearing young trophozoites (less than 20 hr after parasite invasion) was similar to binding to uninfected erythrocytes. By immunoelectron microscopy, the membrane-bound IgG on erythrocytes infected with the knobby variant was found to be preferentially associated with the protuberances (knobs) of the plasma membrane. The removal of aged or senescent erythrocytes from the peripheral circulation is reported to involve the binding of specific antibodies to an antigen (senescent antigen) related to the major erythrocyte membrane protein band 3. Since affinity-purified autoantibodies against band 3 specifically bound to the plasma membrane of erythrocytes infected with the knobby variant of P. falciparum, it is clear that the malaria parasite induces expression of senescent antigen.
Full text
PDF
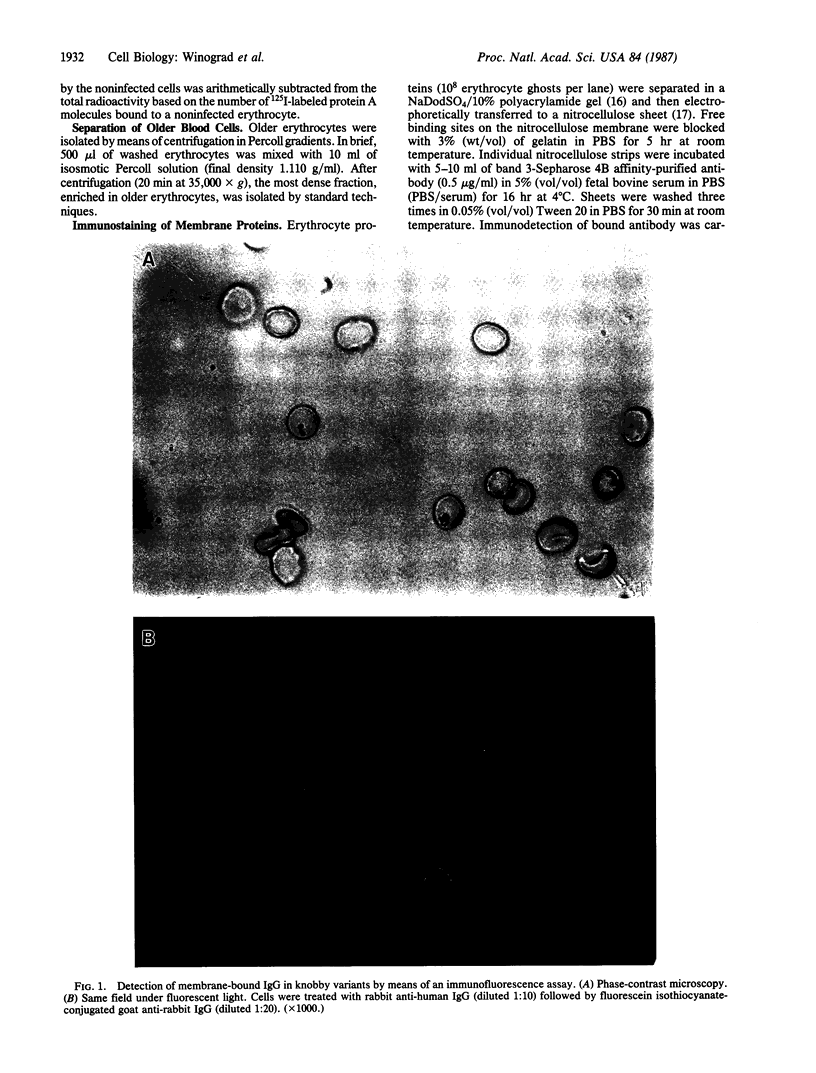

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendayan M., Duhr M. A. Modification of the protein A-gold immunocytochemical technique for the enhancement of its efficiency. J Histochem Cytochem. 1986 May;34(5):569–575. doi: 10.1177/34.5.2422247. [DOI] [PubMed] [Google Scholar]
- Celada A., Cruchaud A., Perrin L. H. Opsonic activity of human immune serum on in vitro phagocytosis of Plasmodium falciparum infected red blood cells by monocytes. Clin Exp Immunol. 1982 Mar;47(3):635–644. [PMC free article] [PubMed] [Google Scholar]
- David P. H., Hommel M., Oligino L. D. Interactions of Plasmodium falciparum infected erythrocytes with ligand-coated agarose beads. Mol Biochem Parasitol. 1981 Dec;4(3-4):195–204. doi: 10.1016/0166-6851(81)90018-9. [DOI] [PubMed] [Google Scholar]
- Dufton M. J., Cherry R. J., Coleman J. W., Stanworth D. R. The capacity of basic peptides to trigger exocytosis from mast cells correlates with their capacity to immobilize band 3 proteins in erythrocyte membranes. Biochem J. 1984 Oct 1;223(1):67–71. doi: 10.1042/bj2230067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufton M. J., Hider R. C., Cherry R. J. The influence of melittin on the rotation of band 3 protein in the human erythrocyte membrane. Eur Biophys J. 1984;11(1):17–24. doi: 10.1007/BF00253854. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Eshdat Y., Tarone G., Marchesi V. T. Isolation and characterization of peptides derived from the cytoplasmic segment of band 3, the predominant intrinsic membrane protein of the human erythrocyte. J Biol Chem. 1978 Apr 10;253(7):2419–2428. [PubMed] [Google Scholar]
- Green G. A., Rehn M. M., Kalra V. K. Cell-bound autologous immunoglobulin in erythrocyte subpopulations from patients with sickle cell disease. Blood. 1985 May;65(5):1127–1133. [PubMed] [Google Scholar]
- Gruenberg J., Sherman I. W. Isolation and characterization of the plasma membrane of human erythrocytes infected with the malarial parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1087–1091. doi: 10.1073/pnas.80.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. B. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am J Trop Med Hyg. 1978 Nov;27(6):1274–1276. doi: 10.4269/ajtmh.1978.27.1274. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Goodman S. R., Sorensen K., Whitfield C. F., Wong P., Zaki L., Rudloff V. Senescent cell antigen is immunologically related to band 3. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1631–1635. doi: 10.1073/pnas.80.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Mechanism of removal of senescent cells by human macrophages in situ. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3521–3525. doi: 10.1073/pnas.72.9.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilejian A., Abati A., Trager W. Plasmodium falciparum and Plasmodium coatneyi: immunogenicity of "knob-like protrusions" on infected erythrocyte membranes. Exp Parasitol. 1977 Jun;42(1):157–164. doi: 10.1016/0014-4894(77)90073-x. [DOI] [PubMed] [Google Scholar]
- Kilejian A. Characterization of a protein correlated with the production of knob-like protrusions on membranes of erythrocytes infected with Plasmodium falciparum. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4650–4653. doi: 10.1073/pnas.76.9.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Langreth S. G., Reese R. T. Antigenicity of the infected-erythrocyte and merozoite surfaces in Falciparum malaria. J Exp Med. 1979 Nov 1;150(5):1241–1254. doi: 10.1084/jem.150.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech J. H., Barnwell J. W., Aikawa M., Miller L. H., Howard R. J. Plasmodium falciparum malaria: association of knobs on the surface of infected erythrocytes with a histidine-rich protein and the erythrocyte skeleton. J Cell Biol. 1984 Apr;98(4):1256–1264. doi: 10.1083/jcb.98.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low P. S., Waugh S. M., Zinke K., Drenckhahn D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science. 1985 Feb 1;227(4686):531–533. doi: 10.1126/science.2578228. [DOI] [PubMed] [Google Scholar]
- Lutz H. U., Flepp R., Stringaro-Wipf G. Naturally occurring autoantibodies to exoplasmic and cryptic regions of band 3 protein, the major integral membrane protein of human red blood cells. J Immunol. 1984 Nov;133(5):2610–2618. [PubMed] [Google Scholar]
- Mendis K. N., David P. H., Hommel M., Carter R., Miller L. H. Immunity to malarial antigens on the surface of Plasmodium falciparum-infected erythrocytes. Am J Trop Med Hyg. 1983 Sep;32(5):926–930. doi: 10.4269/ajtmh.1983.32.926. [DOI] [PubMed] [Google Scholar]
- Miller L. H. Distribution of mature trophozoites and schizonts of Plasmodium falciparum in the organs of Aotus trivirgatus, the night monkey. Am J Trop Med Hyg. 1969 Nov;18(6):860–865. doi: 10.4269/ajtmh.1969.18.860. [DOI] [PubMed] [Google Scholar]
- Müller H., Lutz H. U. Binding of autologous IgG to human red blood cells before and after ATP-depletion. Selective exposure of binding sites (autoantigens) on spectrin-free vesicles. Biochim Biophys Acta. 1983 Apr 6;729(2):249–257. doi: 10.1016/0005-2736(83)90491-1. [DOI] [PubMed] [Google Scholar]
- Rekvig O. P., Hannestad K. Acid elution of blood group antibodies from intact erythrocytes. Vox Sang. 1977;33(5):280–285. doi: 10.1111/j.1423-0410.1977.tb04476.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Vernot-Hernandez J. P., Heidrich H. G. Time-course of synthesis, transport and incorporation of a protein identified in purified membranes of host erythrocytes infected with a knob-forming strain of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Jul;12(3):337–350. doi: 10.1016/0166-6851(84)90090-2. [DOI] [PubMed] [Google Scholar]
- Wyler D. J. Malaria--resurgence, resistance, and research (second of two parts). N Engl J Med. 1983 Apr 21;308(16):934–940. doi: 10.1056/NEJM198304213081605. [DOI] [PubMed] [Google Scholar]