Abstract
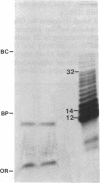
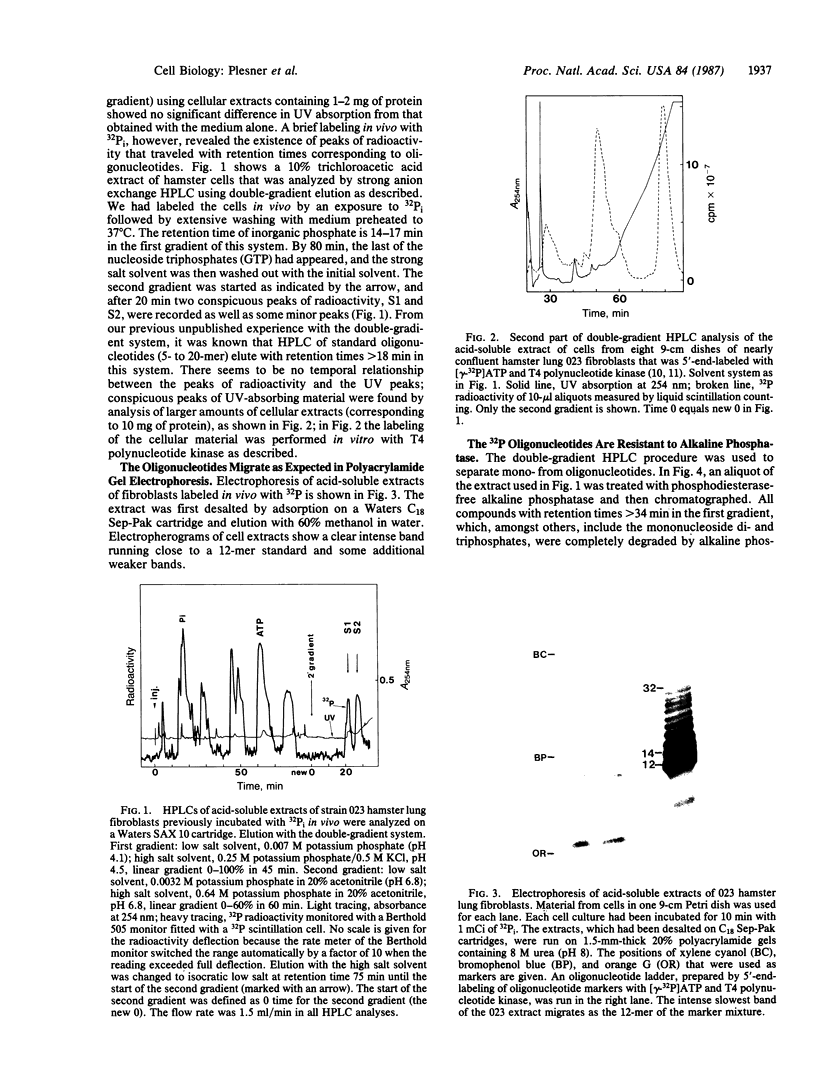
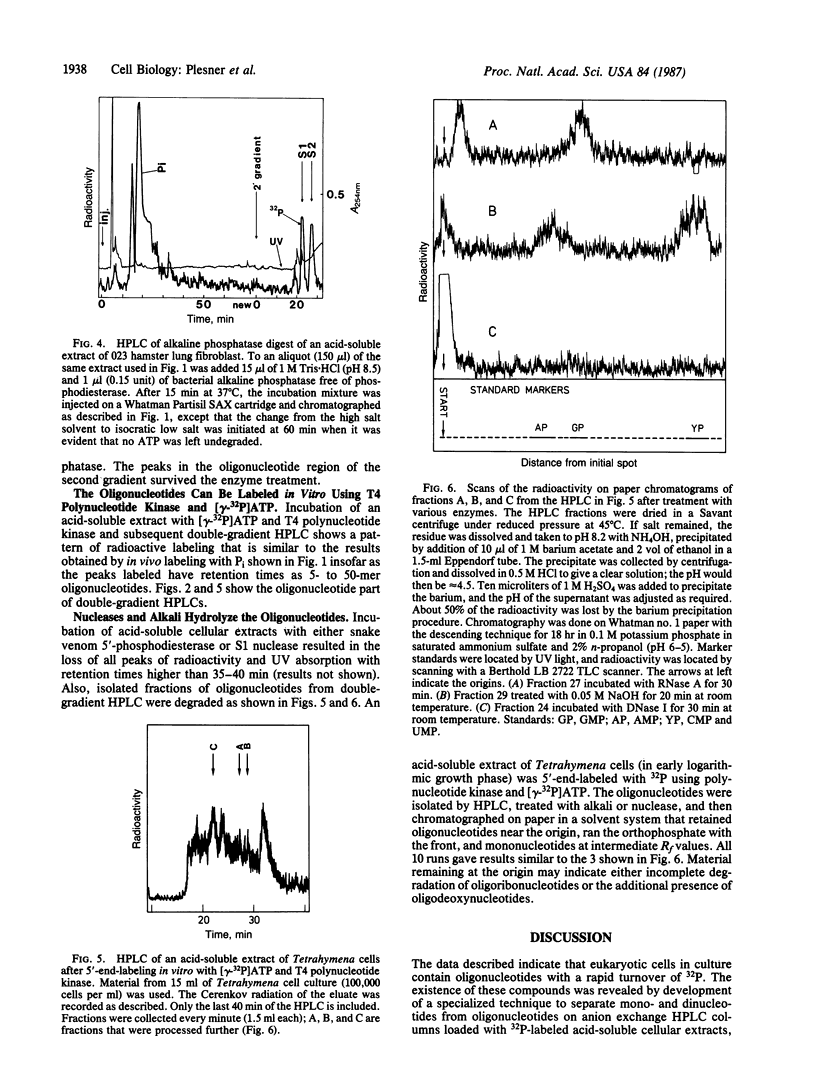
Endogenous oligonucleotides were found in trichloroacetic acid extracts of hamster lung fibroblasts and Tetrahymena cells. Peaks of radioactivity that eluted with retention times similar to oligonucleotide markers (5- to 50-mer) were found by HPLC in cells labeled briefly with 32Pi. Only minute amounts of UV-absorbing material were detected, consistent with a rapid turnover of phosphate groups. The 32P-labeled material also migrated as oligonucleotides on 20% polyacrylamide gels; it was not hydrolyzed by alkaline phosphatase but was digested by snake venom phosphodiesterase, S1 nuclease, and pancreatic RNase and was phosphorylated by T4 polynucleotide kinase. The 32P-labeled material isolated by HPLC was alkali labile and the hydrolyzate ran as nucleotides on paper chromatography. It is concluded that the oligonucleotides are mainly oligoribonucleotides, but it is possible that oligodeoxynucleotides are also present.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkner K. L., Folk W. R. Polynucleotide kinase exchange reaction: quantitave assay for restriction endonuclease-generated 5'-phosphoroyl termini in DNA. J Biol Chem. 1977 May 25;252(10):3176–3184. [PubMed] [Google Scholar]
- Chen S. C., Brown P. R., Rosie D. M. Extraction procedures for use prior to HPLC nucleotide analysis using microparticle chemically bonded packings. J Chromatogr Sci. 1977 Jun;15(6):218–221. doi: 10.1093/chromsci/15.6.218. [DOI] [PubMed] [Google Scholar]
- Hartwick R. A., Brown P. R. The performance of microparticle chemically-bonded anion-exchange resins in the analysis of nucleotides. J Chromatogr. 1975 Oct 29;112:650–662. doi: 10.1016/s0021-9673(00)99994-1. [DOI] [PubMed] [Google Scholar]
- Kalckar H. M., Ullrey D. B. Further clues concerning the vectors essential to regulation of hexose transport, as studied in fibroblast cultures from a metabolic mutant. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1126–1129. doi: 10.1073/pnas.81.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Franchi A., Salomon J. C., Silvestre P. Isolation of a Chinese hamster fibroblast mutant defective in hexose transport and aerobic glycolysis: its use to dissect the malignant phenotype. Proc Natl Acad Sci U S A. 1980 May;77(5):2698–2701. doi: 10.1073/pnas.77.5.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randerath K., Gupta R. C., Randerath E. 3H and 32P derivative methods for base composition and sequence analysis of RNA. Methods Enzymol. 1980;65(1):638–680. doi: 10.1016/s0076-6879(80)65065-4. [DOI] [PubMed] [Google Scholar]
- Stephenson M. L., Zamecnik P. C. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):285–288. doi: 10.1073/pnas.75.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhr-Jessen P. B., Orias E. Mutants of Tetrahymena thermophila with temperature sensitive food vacuole formation. II. Physiological and morphological studies. Exp Cell Res. 1979 Dec;124(2):317–327. doi: 10.1016/0014-4827(79)90207-6. [DOI] [PubMed] [Google Scholar]
- Ullrey D. B., Franchi A., Pouyssegur J., Kalckar H. M. Down-regulation of the hexose transport system: metabolic basis studied with a fibroblast mutant lacking phosphoglucose isomerase. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3777–3779. doi: 10.1073/pnas.79.12.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Goodchild J., Taguchi Y., Sarin P. S. Inhibition of replication and expression of human T-cell lymphotropic virus type III in cultured cells by exogenous synthetic oligonucleotides complementary to viral RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamecnik P. C., Stephenson M. L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc Natl Acad Sci U S A. 1978 Jan;75(1):280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]