Abstract
The olfactory-jump response assay was used to analyze habituation in Drosophila mutants of potassium (K+) channel subunits. As with physiological assays of the giant fiber-mediated escape reflex, mutations at loci that encode K+ channel subunits have distinct effects on habituating the olfactory-jump response. The data for slowpoke and ether à go-go indicate similar effects on habituation of the olfactory-jump response and the giant fiber-mediated escape. Habituation in the olfactory jump assay in Hyperkinetic and Shaker mutants was drastically different from the degree of defect in the giant fiber-mediated escape reflex, indicating differential control mechanisms underlying the two forms of non-associative conditioning.
Keywords: eag, Hk, Slo, Shab and Sh mutants, Escape reflex, Giant fiber pathway, Invertebrate learning, Non-associative conditioning
INTRODUCTION
Potassium (K+) channels are involved in many forms of neural and behavioral plasticity (Cowan & Siegel, 1984, 1986; Deschaux & Bizot, 1997; Engel & Wu, 1998; Misonou et al., 2004; Murphy et al., 2004). K+ channel subunits have distinct and profound effects on habituation in Drosophila (Engel & Wu, 1998). In the giant fiber escape circuit, habituation is induced by repetitive visual stimuli or by a 5-Hz electric stimulus train applied across the brain to recruit the afferent neurons of the giant fiber, bypassing the photoreceptors of a tethered fly (Engel & Wu, 1996; 1998). The olfactory-jump response, used in this study, is thought to involve a different set of interneurons to activate the flight and jump muscles from the giant fiber escape circuit (Trimarchi & Schneiderman, 1995). Response to a strong odor is unlike the response to visual stimuli. The visual pathway has a fast reaction of jump-and-tumble, whereas the olfactory-induced jump is slower and followed by flight (Trimarchi & Schneiderman, 1995; Sun & Wyman, 1997; Allen et al., 2006). While the giant fiber-mediated escape reflex results from stimulating the giant fiber pathway (Levine & Tracey, 1973; Tanouye & Wyman, 1980; Thomas & Wyman, 1984), Trimarchi and Schneiderman (1995) deduce that an olfactory- induced jump-and-flight response is conducted to the mesothoracic leg and wing muscles via an alternate neural route.
Plasticity in the giant fiber escape circuit likely is localized to neurons afferent to the giant fiber in the central brain and, in this habituation paradigm, does not involve sensory adaptation or motor fatigue (Engel & Wu, 1996). The physiological assay of the giant fiber-mediated escape circuit differs from the olfactory jump response assay in that flies are immobilized in the former assay (Engel & Wu, 1998), whereas in the latter assay flies move freely and feedback from their jump and flight or tumble response are potentially involved in their habituation to the odor pulse (Figure 1).
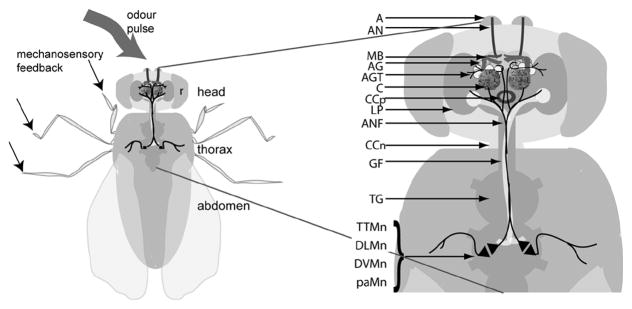
Figure 1.
Neural pathway for habituation in response to odors. Flies tested in the olfactory-jump habituation receive an odor stimulus through the third antennal segment (A). Sensory neurons project via the antennal nerve (AN) to the antennal glomeruli (AG) (for a review of the olfactory system see Jefferis & Hummel, 2006). The antennal glomeruli tract (AGT) projects from the AG, however, the specific neural tracts downstream of this are not well defined. The mushroom bodies (MB) are involved in olfactory learning and receive olfactory information via the AGT that projects to the calyx (C) (Jefferis et al., 2002; Tanaka et al., 2004; Jefferis & Hummel, 2006). The AGT sends dendrites into the lateral protocerebrum (LP). Further, cross-modal connections occur between the olfactory system and visual inputs, which may interact in the LP (Guo and Guo, 2005). The olfactory jump response uses an alternate neural fiber (ANF) to the giant fiber (GF) for the neural pathway downstream of the central brain (Allen et al., 1999). Chemical synapses between the ANF and interacting motor neurons (Mn) are indicated by opposing triangles. The TTMn project to the muscle affecting the mesothoracic legs, while the DLMn, DVMn and paMn are involved in different aspects of wing muscle depression and lift (Allen et al., 2006). r = retina, CCp = central complex, CCn = cervical connective.
To examine whether the involvement of some K+ channel families in the giant fiber-mediated escape generalizes to other habituating protocols, we examined K+ channel subunit mutant strains in the olfactory-jump response (McKenna et al., 1989; Sharma et al., 2005). The mutants used in this study affect identified K+ channel subunits with different functional attributes (Wu & Ganetzky, 1992). Shaker (Sh) encodes the pore forming subunit of the inactivating current (IA) voltage-gated K+ channel, which is affected differently by two mutant alleles. The allele Sh5, a neomorph, alters current properties and induces repetitive firing in the giant fiber pathway, while the Sh133 null mutation affects the pore forming region, eliminates the current and prolongs action potentials in the giant fiber (Tanouye & Ferrus, 1985; Wu & Haugland, 1985; Kamb et al., 1987; Lichtinghagen et al., 1990). Hyperkinetic (Hk) encodes the β subunit of Sh potassium channels (Chouinard et al., 1995; Wang & Wu, 1996; Stern & Ganetzky, 1998; Wilson et al., 1998; Yao & Wu, 1999). ether à go-go (eag) codes for a pore-forming subunit of voltage-gated K+ ion channel complexes (Warmke et al., 1991; Wilson et al., 1998) that can functionally interact with Sh subunits (Ganetzky & Wu, 1983; Zhong & Wu, 1991; Chen et al., 1996). The eag1 allele produces a COOH-terminus truncated protein (Warmke et al., 1991), and no molecular data are available for eag4pm. Another pore-forming, voltage-gated channel is encoded by Shab (Covarrubias et al., 1991). Shab9g is likely a null allele and removes part of the slowly inactivating K+ current (Hegde et al., 1999; Singh & Singh, 1999; Peng & Wu, 2007). Finally, slowpoke (slo) encodes a Ca2+-activated K+ channel (Atkinson et al., 1991; Adelman et al., 1992) and slo98 is a functional null allele (Elkins et al., 1986; Komatsu et al., 1990). Using mutations in genes of five K+ channel sub-units we show that separate K+ channels have distinct roles in habituation of the olfactory-jump response circuit. The resulting habituating properties, notably those of the Shaker mutants, differ from that described in the giant fiber-mediated escape circuit.
MATERIALS AND METHODS
Drosophila Strains
A wild type stock of Canton-S (de Belle & Heisenberg, 1994; Sharma et al., 2005) was used as a control strain. Flies of the genotypes used: eag4pm, eag1, Shab9g, Hk1, Hk2, Sh133, Sh5 and slo98 are described on www.flybase.org. Two lines carry a body- or an eye-color mutation: Shab is marked with e and slo with st (www.flybase.org). We cannot rule out a contribution of these morphological markers to the results obtained, as they were not separately tested. Flies were raised in glass bottles on a cornmeal, sucrose and yeast medium. Test males were aged in groups of 10 in 8 cm plastic tubes with cornmeal-based medium.
Olfactory-Jump Response Assay
Habituation testing was carried out as described in Sharma et al. (2005). That is, testing was done in an environmentally controlled room (25°C and 65–75% relative humidity) with 2-day-old male flies in individual chambers. A 4 s odor pulse stimulus was given with a 60 s intertrial interval. A response was recorded if the fly jumped within 4 s of the odor pulse. Habituation occurred when a criterion of 4 failures to jump in response to 4 consecutive odor pulses was reached. Habituation is expressed as a habituation index defined as the number of trials to criterion (TTC). Immediately following habituation about half of the flies were tested for dishabituation and the remainder for spontaneous recovery. Flies were dishabituated by 75 s vortex in their chambers followed by a 45 s wait. Spontaneous recovery was tested by a 120 s wait. The time allowed for both dishabituation and spontaneous recovery was the same (120 s) before testing for a jump response to an odor pulse. The odor pulse consisted of 5% high-grade benzaldehyde (Sigma-Aldrich, Fluka) dissolved in heavy mineral oil (Fisher Scientific, UK). Separate operators collected data at two different locations, but with the same set up. In the earlier phase, fewer mutants were involved and 10% benzaldehyde was used without testing dishabituation. Only flies that responded to the initial odor pulse were included in the habituation data. Of all flies tested only 2 individuals did not show an initial jump to the first odor pulse (data not shown), with the exception of eag4PM mutant flies. Only 3 of 24 eag4PM flies tested reacted to the first odor pulse, indicating that this mutation may reduce benzaldehyde sensitivity. Figure 1 shows the conjectural pathway for sensory input through the CNS to motor output in this assay, based on a number of studies (see the figure legend).
Statistics
Student’s-t test was used to perform all habituation comparisons. Data are expressed as mean ± SEM and were analyzed with JMP software (SAS, Cary, NC). Pair wise comparisons with Canton-S as control by Fisher’s exact test (Prism Software) were performed on the dishabituation and spontaneous recovery data.
RESULTS AND DISCUSSION
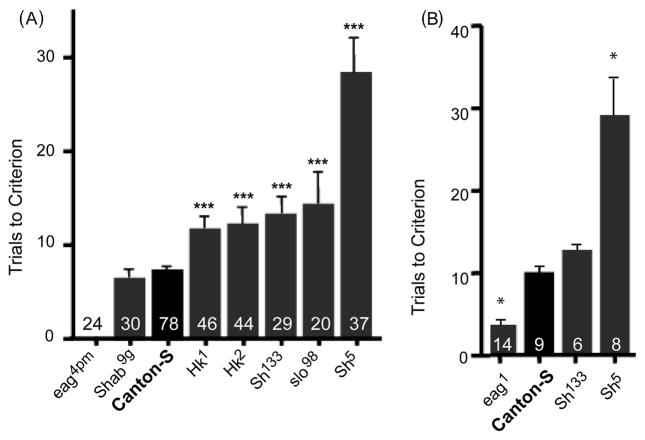
The different mutants involving five K+ channel subunit genes that were tested in the olfactory-jump response for their effect on habituation were eag4pm, eag1, Shab9g, Hk1, Hk2, Sh133, Sh5 and slo98(Figure 2A & B). The Hk and slo mutations caused an increase in the number of trials it took to reach criteria of habituation for these flies and eag1 decreased the number of trials needed to habituate compared to that of Canton-S control flies. Only 1.25 percent of eag4pm flies responded to the first pulse of 5% benzaldehyde. However, eag1 flies consistently showed initial jump in response to 10% benzaldehyde pulses, and the TTC is significantly slower than that of control flies (Figure 2, Table I). The two Sh mutations both increased the time to habituate compared with that of control flies; however, TTC for Sh133 was lower than that for Sh5 mutant animals (Figure 2A). The similar results in Figures 2A and B performed by different operators over an eight year span point out the reproducibility of differences in habituation caused by two Sh mutations. All genotypes tested, including the wild type, showed a low rate of spontaneous recovery at 120 s after TTC (Table I), indicating the enduring quality of the learned response.
Figure 2.
Habituation scores in response to a repeated odor pulse. A. Flies tested with 5% benzaldehyde presented as the mean trials to criterion (TTC * SEM). Habituation occurred when a criterion of 4 consecutive failures to jump in response to odor pulses was reached (described in MATERIALS AND METHODS). ***p < 0.0001. B. Flies tested with 10% benzaldehyde. *p < 0.05. The number of flies tested is written on the bar for each genotype.
Table I.
Spontaneous recovery and dishabituation parameters of habituation in mutants tested
| Genotype | Spontaneous recovery |
Dishabituation |
||
|---|---|---|---|---|
| n | Mean fraction | n | Mean fraction | |
| Tested with 5% benzaldehyde | ||||
| eag4pm | na | – | – | – |
| Shab9g | 15 | 0.0 | 15 | 0.80 |
| Canton-S | 38 | 0.12 | 40 | 0.97 |
| Hk1 | 23 | 0.17 | 23 | 0.60** |
| Hk2 | 22 | 0.0 | 22 | 0.79 |
| Sh133 | 15 | 0.23 | 14 | 0.64** |
| slo98 | 10 | 0.22 | 10 | 0.78 |
| Sh5 | 37 | 0.31* | 18 | 0.83 |
| Tested with 10% benzaldehyde | ||||
| eag1 | 14 | 0.14 | nd | |
| Canton-S | 9 | 0.0 | nd | |
| Sh133 | 6 | 0.67* | nd | |
| Sh5 | 8 | 0.29 | nd | |
Flies are dishabituated by 75 s vortex in their chambers followed by a 45 s wait. Spontaneous recovery is tested by a 120 s wait. Mean fraction refers to the number of flies that showed the response over the total number of flies tested. n, number of flies. na, not applicaple. nd, not determined. eag4pm flies did not respond to 5% benzaldehyde. Assuming binomial distribution, pairwise comparisons with Canton-S indicate statistical differences at the level of **p < 0.01 and *p < 0.05.
The results described in this paper are similar to those for mutants when tested in the electrical stimulation of the giant fiber-mediated escape circuit (Tanouye & Wyman, 1980; Engel & Wu, 1998) with two notable exceptions (Figure 3). The rate of habituation for genotypes tested by an electrical stimulation directed across the brain via neurons afferent to the giant fiber (slowest to fastest, Engel & Wu, 1998) is: Hk1&2 > slo98 > Canton-S (control) ~ Sh133 > Sh5&122 > eag4pm and the order for the olfactory jump response is Sh5 > slo98 > Sh133 > Hk1&2 > Canton-S (control) ~ Shab9g > eag4pm&1. Surprisingly, although the Sh5 mutants resist habituation in the olfactory-jump response, in the giant fiber-mediated escape circuit they habituate faster relative to control flies (Engel & Wu, 1998). The typical fly response to a strong odor is a coordinated and normal jump from the side of the vial, followed by flight. The Sh5 mutant flies showed a synchronized jump only in the initial trials. Eventually Sh5 mutant flies showed a tumble response to the odor pulse with no clear jump from the bottom of the vial where they otherwise stayed (not shown).
Figure 3.

Comparison of habituation between the olfactory-jump response and the giant fiber-mediated escape reflex. A. Rate of habituation for genotypes tested by an odor response (Figure 2). B. Rate of habituation for genotypes tested by an electrical stimulation directed across the brain via neurons afferent to the giant fiber (Engel & Wu, 1998). Slowest to fastest is left to right.
The effects of Hk mutations reveal another difference. Whereas Hk mutants tested in the giant fiber-mediated escape circuit show an extreme resistance to habituation (Engel & Wu, 1998), for the olfactory-jump response they took longer than control flies to habituate, but were not as resistant to habituation (Figure 2). These differences suggest that modulation by Hk, a β subunit of Sh channels (Chouinard et al., 1995), is more fundamental to habituation involving the giant fiber pathway (Engel & Wu, 1998) than it is to habituation induced by a strong olfactory stimulus (Figure 3).
The disparity observed between the two tests may result from (1) a difference in mode of sensory input (visual versus olfactory, that recruit separate pathways and may involve a divergence in neural processing), and (2) distinct fiber tracts that conduct the response from the brain to the thoracic ganglion. The giant fiber-mediated escape circuit uses the giant fiber connection between the brain and thorax (Engel & Wu, 1998), while the olfactory-jump response uses an alternate neural pathway (Trimarchi & Schneiderman, 1993, 1994, 1995). These separate pathways may transmit processed information originating in distinct brain regions.
Our data clearly indicate the involvement of separate K+ channel subunits, suggesting a complex control of olfactory jump habituation behaviors. However, the most extreme effect in the habituation tested here was exhibited by flies with mutations in two subunits of the same IA channel subtype, Sh and Hk. One Sh allele took a particularly long period to reach TTC, highlighting the extent of involvement for this channel. The Shaker gene is expressed in the antennal nerve and mushroom bodies (Gasque et al., 2005). The availability of anti-Sh antibodies may provide further insight into the brain centers involved (Rogero et al., 1997). Anti-Sh antibody strongly labels the mushroom body lobes, which are required for olfactory learning (de Belle & Heisenberg, 1994) and the central complex, which directs locomotor activity (Bouhouche et al., 1993; Joiner & Griffith, 1999; Strauss, 2002). Sensory- or motor-neuron feedback from the mesothoracic leg or wing to the central complex may modify Sh channel function in this central neuropil that result in habituation. Even though the leg extension and wing lift and depression are no longer coordinated in some Sh mutant flies, habituation of their uncoordinated response indicates that these Sh flies remain able to discern the odor pulse. That this uncoordinated response can habituate argues that the habituation occurs in neurons afferent to the TTMns, DVMns, DLMns and paMns (Figure 1). The neurons upstream may involve the central brain rather than the neural circuits of the thoracic ganglion.
Although eag4PM mutants may have reduced odor perception preventing their initial jump response (Table I), they nevertheless showed clear habituation of giant fiber-mediated escape (Engel & Wu, 1998). In addition eag1 mutants did respond to odor pulses and showed clear habituation in the olfactory jump (Figure 2B). These data indicate that eag1 mutations facilitate habituation relative to that of wild-type flies (Figure 3).
The different genotypes of flies were tested for one or two parameters of this habituation assay to control for two essential criteria for habituation, i.e., dishabituation by a different stimulus and spontaneous recovery (Thompson & Spencer, 1966). To rule out sensory adaptation and motor fatigue, dishabituation was tested by giving the habituated flies a strong novel mechanical stimulus. All flies tested showed the same level of dishabituation as that of wild-type flies apart from the Hk1 and Sh133 strains (Table I). However, our dishabituation data further support the idea that non-associative learning occurs in central neurons. That is, convergence of olfactory and mechanosensory information most likely occurs in higher centers. To test spontaneous recovery, flies are given the same amount of time (120 s) to show recovery without a dishabituating stimulus (Table I). A low spontaneous recovery rate across genotypes indicates the enduring quality of the learned response. The different mutants tested showed a rate of spontaneous recovery that was not significantly higher than that of wild-type flies, with two exceptions (Table I).
Interactions between neuronal excitability and synaptic plasticity play a role in learning (Stackman et al., 2002; Xu et al., 2005). Excitatory synaptic transmission in the insect brain is based mainly on the cholinergic system (Oleskevich, 1999). However, K+ channel activation can in principle interact with muscarinic, nicotinic, NMDA-type glutamate, octopamine or GABA receptors during activity-dependent plasticity (Müller & Connor, 1991; Farooqui et al., 2003; Su & O’Dowd, 2003; Xia et al., 2005). Mushroom bodies are required for olfactory learning (see Heisenberg, 2003) and K+ currents, important for neuronal excitability, may lead to activity-dependent receptor modulation (e.g., nicotinic acetylcholine receptors in mushroom bodies) (Gu & O’Dowd, 2006; Ismail et al., 2006). The current findings indicate that identified K+ channel families play distinct roles controlling different neural circuits of non-associative learning in Drosophila. The information we present provides groundwork for studies on the modulation of neuronal activity in neural circuits relevant to behavior modification conferred by cholinergic and other synaptic transmitter systems in the brain of a fly.
Acknowledgments
We thank Dr. Cahir O’Kane for the use of his lab at Cambridge University, Cambridge England, for part of the behavioral work. This work was supported by the NIH grants HD18577 and NS26528 to CFW and Human Frontier Science Program. MAJ was supported by a Burroughs Welcome Fund grant. TT was supported by NIH grant MH 066242 and by Dart Neuroscience, Inc.
Footnotes
This article not subject to United States copyright law.
References
- Adelman J, Shen K, Kavanaugh M, Warren R, Wu Y, Lagrutta A, Bond C, North R. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. doi: 10.1016/0896-6273(92)90160-f. [DOI] [PubMed] [Google Scholar]
- Allen M, Godenschwege T, Tanouye M, Phelan P. Making an escape: development and the function of the Drosophila giant fiber system. Semin Cell Devel Biol. 2006;17:31–41. doi: 10.1016/j.semcdb.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Allen M, Shan X, Caruccio P, Froggett S, Moffat K, Murphey R. Targeted expression of truncated glued disrupts giant fiber synapse formation in Drosophila. J Neurosci. 1999;19:9374–9384. doi: 10.1523/JNEUROSCI.19-21-09374.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson N, Robertson G, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. doi: 10.1126/science.1857984. [DOI] [PubMed] [Google Scholar]
- Bouhouche A, Vaysse G, Corbiere M. Immunocytochemical and learning studies of a Drosophila melanogaster neurological mutant, no-bridgeKS49 as an approach to the possible role of altering cell fates and generating dominant phenotypes. J Neurogenet. 1993;9:105–121. doi: 10.3109/01677069309083453. [DOI] [PubMed] [Google Scholar]
- Chen ML, Hoshi T, Wu CF. Heteromultimeric interactions among K+ channel subunits from Shaker and eag families in Xenopus oocytes. Neuron. 1996;17:535–542. doi: 10.1016/s0896-6273(00)80185-3. [DOI] [PubMed] [Google Scholar]
- Chouinard S, Wilson G, Schlimgen A, Ganetzky B. A potassium channel beta subunit related to the aldo-keto reductase superfamily is encoded by the Drosophila Hyperkinetic locus. Proc Natl Acad Sci US. 1995;92:6763–6767. doi: 10.1073/pnas.92.15.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias M, Wei A, Salkoff L. Shaker, Shal, Shab, and Shaw express independent K+ current systems. Neuron. 1991;7:763–773. doi: 10.1016/0896-6273(91)90279-9. [DOI] [PubMed] [Google Scholar]
- Cowan T, Siegel R. Mutational and pharmacological alterations of neuronal membrane function disrupt conditioning in Drosophila. J Neurogenet. 1984;4:333–444. doi: 10.3109/01677068409107095. [DOI] [PubMed] [Google Scholar]
- Cowan T, Siegel R. Drosophila mutations that alter ionic conduction disrupt acquisition and retention of a conditioned odor avoidance response. J Neurogenet. 1986;4:187–201. doi: 10.3109/01677068609106849. [DOI] [PubMed] [Google Scholar]
- de Belle J, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- Deschaux O, Bizot J. Effect of apamin, a selective blocker of Ca2+-activated K+-channel, on habituation and passive avoidance in rats. Neurosci Lett. 1997;227:57–60. doi: 10.1016/s0304-3940(97)00301-7. [DOI] [PubMed] [Google Scholar]
- Elkins T, Ganetzky B, Wu CF. A Drosophila mutation that eliminates a calcium-dependent potassium current. Proc Natl Acad Sci US. 1986;83:8415–8419. doi: 10.1073/pnas.83.21.8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Wu CF. Altered habituation of an identified escape circuit in Drosophila memory mutants. J Neurosci. 1996;16:3486–3499. doi: 10.1523/JNEUROSCI.16-10-03486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J, Wu CF. Genetic dissection of functional contributions of specific potassium channel subunits in habituation of an escape circuit in Drosophila. J Neurosci. 1998;18:2254–2267. doi: 10.1523/JNEUROSCI.18-06-02254.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui T, Robinson K, Vaessin H, Smith B. Modulation of early olfactory processing by an octopaminergic reinforcement pathway in the honeybee. J Neurosci. 2003;23:5370–5380. doi: 10.1523/JNEUROSCI.23-12-05370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganetzky B, Wu CF. Neurogenetic analysis of potassium currents in Drosophila: Synergistic effects on neuromuscular transmission in double mutants. J Neurogenet. 1983;1:17–28. doi: 10.3109/01677068309107069. [DOI] [PubMed] [Google Scholar]
- Gasque G, Labarca P, Reynaud E, Darszon A. Shal and Shaker differential contribution to the K+ currents in the Drosophila mushroom body neurons. J Neurosci. 2005;25:2348–2358. doi: 10.1523/JNEUROSCI.4384-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, O’Dowd D. Cholinergic synaptic transmission in adult Drosophila kenyon cells in situ. J Neurosci. 2006;26:265–272. doi: 10.1523/JNEUROSCI.4109-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Guo A. Crossmodal interactions between olfactory and visual learning in Drosophila. Science. 2005;309:307–309. doi: 10.1126/science.1111280. [DOI] [PubMed] [Google Scholar]
- Hegde P, Gu GG, Chen D, Free S, Singh S. Mutational analysis of the Shab-encoded delayed rectifier K1 channels in Drosophila. J Biol Chem. 1999;247:22109–22113. doi: 10.1074/jbc.274.31.22109. [DOI] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nature Rev Neurosci. 2003;4:266–267. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- Ismail N, Robinson G, Fahrbach S. Stimulation of muscarinic receptors mimics experience-dependent plasticity in the honey bee brain. Proc Natl Acad Sci US. 2006;103:207–211. doi: 10.1073/pnas.0508318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis G, Hummel T. Wiring specificity in the olfactory system. Semin Cell Devel Biol. 2006;17:50–65. doi: 10.1016/j.semcdb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Jefferis G, Marin E, Watts R, Luo L. Development of neuronal connectivity in Drosophila antennal lobes and mushroom bodies. Curr Opin Neurobiol. 2002;12:80–86. doi: 10.1016/s0959-4388(02)00293-3. [DOI] [PubMed] [Google Scholar]
- Joiner M, Griffith L. Mapping of the anatomical circuit of CaM kinase-dependent courtship conditioning in Drosophila. Learn Mem. 1999;6:177–192. [PMC free article] [PubMed] [Google Scholar]
- Kamb A, Iverson L, Tanouye M. Molecular characterization of Shaker, a Drosophila gene that encodes a potassium channel. Cell. 1987;50:405–413. doi: 10.1016/0092-8674(87)90494-6. [DOI] [PubMed] [Google Scholar]
- Komatsu A, Singh S, Rathe P, Wu CF. Mutational and gene dosage analysis of calcium-activated potassium channels in Drosophila: correlation of micro- and macroscopic currents. Neuron. 1990;4:313–321. doi: 10.1016/0896-6273(90)90105-o. [DOI] [PubMed] [Google Scholar]
- Levine JD, Tracey D. Stucture and function of the giant motoreneuron of Drosophila melanogaster. J Comp Physiol A. 1973;87:213–235. [Google Scholar]
- Lichtinghagen R, Stocker M, Wittka R, Boheim G, Stuhmer W, Ferrus A, Pongs O. Molecular basis of altered excitability in Shaker mutants of Drosophila melanogaster. EMBO J. 1990;9:4399–4407. doi: 10.1002/j.1460-2075.1990.tb07890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna M, Monte P, Helfand S, Woodard C, Carlson J. A simple chemosensory response in Drosophila and the isolation of acj mutants in which it is affected. Proc Natl Acad Sci US. 1989;86:8118–8122. doi: 10.1073/pnas.86.20.8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Mohapatra D, Park E, Leung V, Zhen D, Misonou K, Anderson A, Trimmer J. Regulation of ion channel localization and phosphorylation by neuronal activity. Nature Neurosci. 2004;7:711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- Müller W, Connor J. Cholinergic input uncouples Ca2+ changes from K+ conductance activation and amplifies intradendritic Ca2+ changes in hippocampal neurons. Neuron. 1991;6:901–905. doi: 10.1016/0896-6273(91)90230-w. [DOI] [PubMed] [Google Scholar]
- Murphy G, Fedorov N, Giese K, Ohno M, Friedman F, Chen R, Silva A. Increased neuronal excitability, synaptic plasticity, and learning in aged Kv1.1 knockout mice. Curr Biol. 2004;14:1907–1915. doi: 10.1016/j.cub.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Oleskevich S. Cholinergic synaptic transmission in insect mushroom bodies in vitro. J Neurophysiol. 1999;82:1091–1099. doi: 10.1152/jn.1999.82.2.1091. [DOI] [PubMed] [Google Scholar]
- Peng IF, Wu CF. Differential contributions of Shaker and Shab K+ currents to neuronal firing paterns in Drosophila. J Neurophysiol. 2007;97:780–794. doi: 10.1152/jn.01012.2006. [DOI] [PubMed] [Google Scholar]
- Rogero O, Hammerle B, Tejedor F. Diverse expression and distribution of Shaker potassium channels during the development of the Drosophila nervous system. J Neurosci. 1997;17:5108–5118. doi: 10.1523/JNEUROSCI.17-13-05108.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Asztalos Z, Ayyub C, de Bruyn M, Dornan A, Gomez-Hernandez A, Keane J, Killeen J, Kramer S, Madhavan M, Roe H, Sherkhane P, Siddiqi K, Silva E, Carlson J, Goodwin S, Heisenberg M, Krishnan K, Kyriacou C, Partridge L, Riesgoescovar J, Rodrigues W, Tully T, O’Kane C. Isogenic autosomes to be applied in optimal screening for novel mutants with viable phenotypes in Drosophila melanogaster. J Neurogenet. 2005;19:57–85. doi: 10.1080/01677060591007155. [DOI] [PubMed] [Google Scholar]
- Singh A, Singh S. Unmasking of a novel potassium current in Drosophila by a mutation and drugs. J Neurosci. 1999;19:6838–6843. doi: 10.1523/JNEUROSCI.19-16-06838.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman R, Hammond R, Linardatos E, Gerlach A, Maylie J, Adelman J, Tzounopoulos T. Small conductance Ca2+ – activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci. 2002;22:10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M, Ganetzky B. Altered synaptic transmission in Drosophila Hyperkinetic mutants. J Neurogenet. 1998;5:215–228. doi: 10.3109/01677068909066209. [DOI] [PubMed] [Google Scholar]
- Strauss R. The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol. 2002;12:633–638. doi: 10.1016/s0959-4388(02)00385-9. [DOI] [PubMed] [Google Scholar]
- Su H, O’Dowd D. Fast synaptic currents in Drosophila mushroom body kenyon cells are mediated by alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors and picrotoxin-sensitive GABA receptors. J Neurosci. 2003;23:9246–9253. doi: 10.1523/JNEUROSCI.23-27-09246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wyman R. Neurons of the Drosophila giant fiber system: I. Dorsal longitudinal motor neurons. J Comp Neurol. 1997;387:157–166. [PubMed] [Google Scholar]
- Tanaka N, Awasaki T, Shimada T, Ito K. Integration of chemosensory pathways in the Drosophila second-order olfactory centers. Curr Biol. 2004;14:449–457. doi: 10.1016/j.cub.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Tanouye M, Ferrus A. Action potentials in normal and Shaker mutant Drosophila. J Neurogenet. 1985;2:253–271. doi: 10.3109/01677068509102322. [DOI] [PubMed] [Google Scholar]
- Tanouye M, Wyman R. Motor outputs of a giant nerve fiber in Drosophila. J Neurophysiol. 1980;44:405–421. doi: 10.1152/jn.1980.44.2.405. [DOI] [PubMed] [Google Scholar]
- Thomas J, Wyman R. Mutations altering synaptic connectivity between identified neurons in Drosophila. J Neurosci. 1984;4:530–538. doi: 10.1523/JNEUROSCI.04-02-00530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R, Spencer W. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Trimarchi J, Schneiderman A. Giant fiber activation of an intrinsic muscle in the mesothoracic leg of Drosophila melanogaster. J Exp Biol. 1993;177:149–167. doi: 10.1242/jeb.177.1.149. [DOI] [PubMed] [Google Scholar]
- Trimarchi J, Schneiderman A. The motor neurons innervating the direct flight muscles of Drosophila melanogaster are morphologically specialized. J Comp Neurol. 1994;240:427–443. doi: 10.1002/cne.903400311. [DOI] [PubMed] [Google Scholar]
- Trimarchi J, Schneiderman A. Different neural pathways coordinate Drosophila flight initiations evoked by visual and olfactory stimuli. J Exp Biol. 1995;198:1099–1104. doi: 10.1242/jeb.198.5.1099. [DOI] [PubMed] [Google Scholar]
- Wang J, Wu CF. In vivo functional role of the Drosophila hyperkinetic beta subunit in gating and inactivation of Shaker K+ channels. Biophys J. 1996;71:3167–3176. doi: 10.1016/S0006-3495(96)79510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmke J, Drysdale R, Ganetzky B. A distinct potassium channel polypeptide encoded by the Drosophila eag locus. Science. 1991;252:1560–1562. doi: 10.1126/science.1840699. [DOI] [PubMed] [Google Scholar]
- Wilson G, Wang Z, Chouinard S, Griffith L, Ganetzky B. Interaction of the K+ channel β subunit, hyperkinetic, with eag family members. J Biol Chem. 1998;273:6389–6394. doi: 10.1074/jbc.273.11.6389. [DOI] [PubMed] [Google Scholar]
- Wu CF, Ganetzky B. Neurogenetic studies of ion channels in Drosophila. Ion Channels. 1992;3:261–314. doi: 10.1007/978-1-4615-3328-3_9. [DOI] [PubMed] [Google Scholar]
- Wu CF, Haugland F. Voltage clamp analysis of membrane currents in larval muscle fibers of Drosophila: alteration of potassium currents in Shaker mutants. J Neurosci. 1985;5:2626–2640. doi: 10.1523/JNEUROSCI.05-10-02626.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Miyashita T, Fu TF, Lin WY, Wu C-l, Pyzocha L, Lin IR, Saitoe M, Tully T, Chiang AS. NMDA Receptors mediate olfactory learning and memory in Drosophila. Curr Biol. 2005;15:603–615. doi: 10.1016/j.cub.2005.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kang N, Jiang L, Nedergaard M, Kang J. Activity-dependent long-term potentiation of intrinsic excitability in hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:1750–1760. doi: 10.1523/JNEUROSCI.4217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao WD, Wu CF. Auxiliary hyperkinetic beta subunit of K+ channels: regulation of firing properties and K+ currents in Drosophila neurons. J Neurophysiol. 1999;81:2472–2484. doi: 10.1152/jn.1999.81.5.2472. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wu CF. Alteration of four identified K+ currents in Drosophila muscle by mutations in eag. Science. 1991;252:1562–1564. doi: 10.1126/science.2047864. [DOI] [PubMed] [Google Scholar]