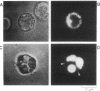
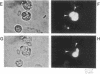
Abstract
Secretion by single mast cells was studied in normal and beige mice, a mutant with grossly enlarged secretory vesicles or granules. During degranulation, the membrane capacitance increased in steps, as single secretory vesicles fused with the cell membrane. The average step size was 10 times larger in beige than in normal mice, in agreement with the different granule sizes measured microscopically in the two preparations. Following individual capacitance steps in beige mice, individual granules of the appropriate size were observed to swell rapidly. Capacitance steps are frequently followed by the stepwise loss of a fluorescent dye loaded into the vesicles. Stepwise capacitance increases were occasionally intermittent before they became permanent, indicating the existence of an early, reversible, and incomplete state of vesicle fusion. During such "capacitance flicker," loss of fluorescent dye from vesicles did not occur, suggesting that the earliest aqueous connection between vesicle interior and cell exterior is a narrow channel. Our results support the view that the reversible formation of such a channel, which we term the fusion pore, is an early step in exocytosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caulfield J. P., Lewis R. A., Hein A., Austen K. F. Secretion in dissociated human pulmonary mast cells. Evidence for solubilization of granule contents before discharge. J Cell Biol. 1980 May;85(2):299–312. doi: 10.1083/jcb.85.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi E. Y., Lagunoff D. Abnormal mast cell granules in the beige (Chédiak-Higashi syndrome) mouse. J Histochem Cytochem. 1975 Feb;23(2):117–122. doi: 10.1177/23.2.46876. [DOI] [PubMed] [Google Scholar]
- Cohen F. S., Akabas M. H., Finkelstein A. Osmotic swelling of phospholipid vesicles causes them to fuse with a planar phospholipid bilayer membrane. Science. 1982 Jul 30;217(4558):458–460. doi: 10.1126/science.6283637. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Finkelstein A., Zimmerberg J., Cohen F. S. Osmotic swelling of vesicles: its role in the fusion of vesicles with planar phospholipid bilayer membranes and its possible role in exocytosis. Annu Rev Physiol. 1986;48:163–174. doi: 10.1146/annurev.ph.48.030186.001115. [DOI] [PubMed] [Google Scholar]
- Gillespie J. I. The effect of repetitive stimulation on the passive electrical properties of the presynaptic terminal of the squid giant synapse. Proc R Soc Lond B Biol Sci. 1979 Dec 31;206(1164):293–306. doi: 10.1098/rspb.1979.0106. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Holz R. W., Senter R. A. Effects of osmolality and ionic strength on secretion from adrenal chromaffin cells permeabilized with digitonin. J Neurochem. 1986 Jun;46(6):1835–1842. doi: 10.1111/j.1471-4159.1986.tb08502.x. [DOI] [PubMed] [Google Scholar]
- Holz R. W., Senter R. A., Sharp R. R. Evidence that the H+ electrochemical gradient across membranes of chromaffin granules is not involved in exocytosis. J Biol Chem. 1983 Jun 25;258(12):7506–7513. [PubMed] [Google Scholar]
- Jaffe L. A., Hagiwara S., Kado R. T. The time course of cortical vesicle fusion in sea urchin eggs observed as membrane capacitance changes. Dev Biol. 1978 Nov;67(1):243–248. doi: 10.1016/0012-1606(78)90314-7. [DOI] [PubMed] [Google Scholar]
- Johnson R. G., Carty S. E., Fingerhood B. J., Scarpa A. The internal pH of mast cell granules. FEBS Lett. 1980 Oct 20;120(1):75–79. doi: 10.1016/0014-5793(80)81050-7. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. The chromaffin granule proton pump and calcium-dependent exocytosis in bovine adrenal medullary cells. J Membr Biol. 1985;83(1-2):147–156. doi: 10.1007/BF01868746. [DOI] [PubMed] [Google Scholar]
- Lawson D., Raff M. C., Gomperts B., Fewtrell C., Gilula N. B. Molecular events during membrane fusion. A study of exocytosis in rat peritoneal mast cells. J Cell Biol. 1977 Feb;72(2):242–259. doi: 10.1083/jcb.72.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornberg R. L., Reese T. S. Beginning of exocytosis captured by rapid-freezing of Limulus amebocytes. J Cell Biol. 1981 Jul;90(1):40–54. doi: 10.1083/jcb.90.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon K. C., Liu P. I., Spicer S. S. Mast cell degranulation in beige mice with the Chédiak-Higashi defect. Am J Pathol. 1981 Aug;104(2):142–149. [PMC free article] [PubMed] [Google Scholar]
- Satir B., Schooley C., Satir P. Membrane fusion in a model system. Mucocyst secretion in Tetrahymena. J Cell Biol. 1973 Jan;56(1):153–176. doi: 10.1083/jcb.56.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Whitaker M. Irreversible swelling of secretory granules during exocytosis caused by calcium. Nature. 1985 Jun 13;315(6020):581–584. doi: 10.1038/315581a0. [DOI] [PubMed] [Google Scholar]