Abstract
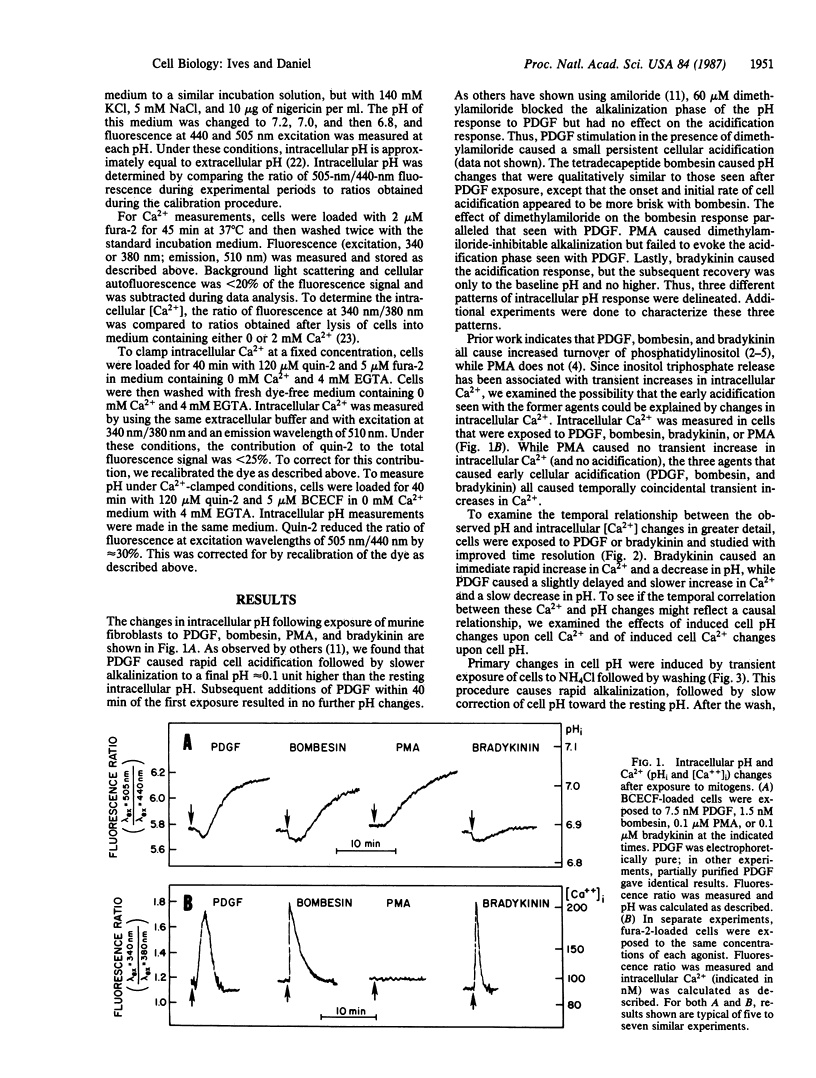
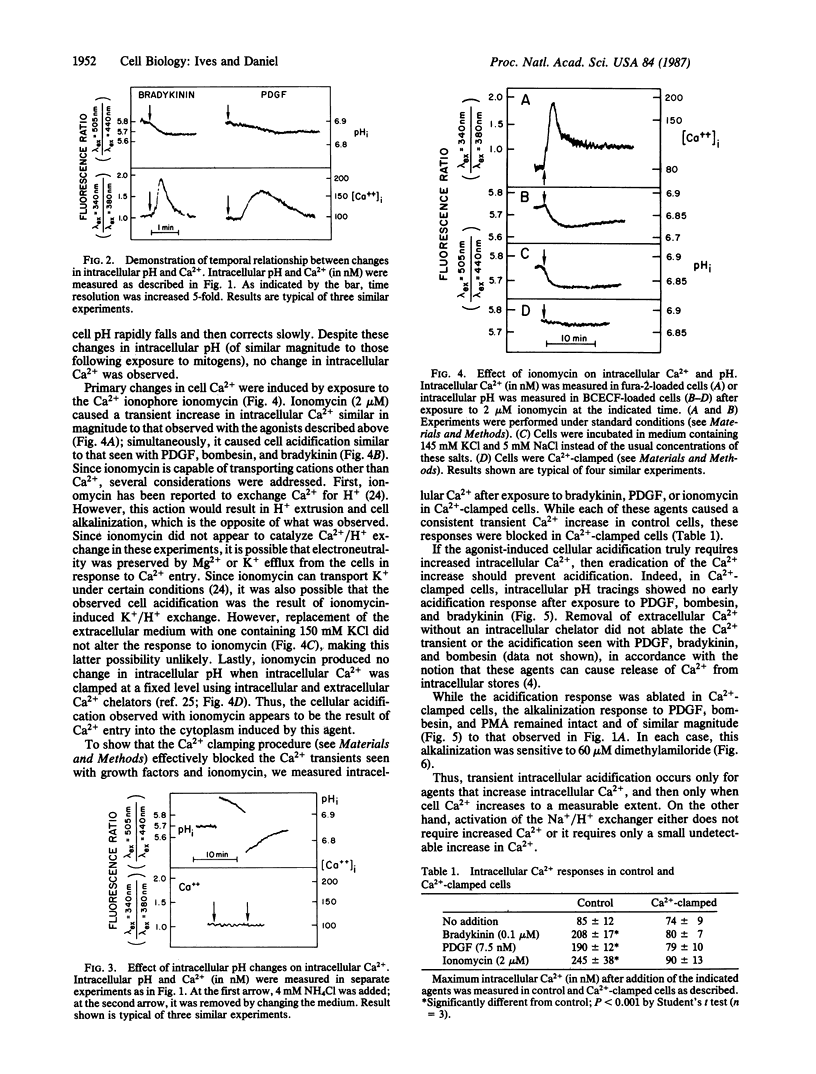
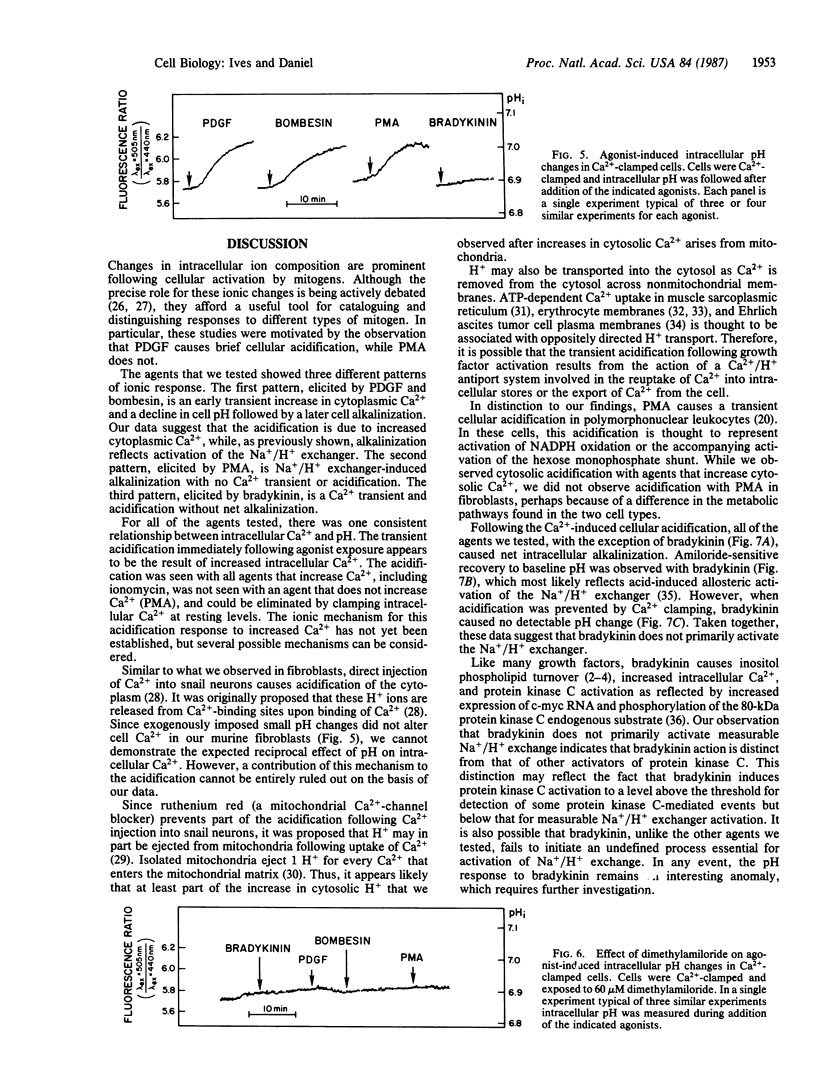
Many mitogens cause rapid changes in intracellular pH and Ca2+. We studied the patterns of pH and Ca2+ changes after exposure of murine fibroblasts to platelet-derived growth factor (PDGF), bombesin, phorbol 12-myristate 13-acetate (PMA), and the vasoactive peptide bradykinin. Intracellular pH and Ca2+ were measured by using the fluorescent dyes 2',7'-bis(carboxyethyl)-5(6)-carboxyfluorescein and fura-2. Three distinct patterns of intracellular pH change were observed. PDGF and bombesin caused a rapid (maximum change, less than 2 min) cytoplasmic acidification of 0.03 pH unit followed by a slower (5-10 min) alkalinization of approximately 0.11 pH unit above the resting pH of 6.88. PMA caused alkalinization without causing the early acidification. Bradykinin caused rapid acidification without the slower net alkalinization. Ionomycin also caused acidification without subsequent alkalinization. All acidification responses were amiloride resistant. Patterns of intracellular Ca2+ response were also determined for each agent. PDGF and bombesin caused a transient increase in cytoplasmic Ca2+ from a resting level of 85 +/- 12 nM to 190 +/- 12 nM within 2 min and return to baseline within 5 min. PMA caused no change in intracellular Ca2+. Bradykinin caused the most rapid (maximum response, less than 20 sec) increase in intracellular Ca2+. For each agonist, the Ca2+ transient could be blocked by buffering intracellular Ca2+ with quin-2. In Ca2+-buffered cells, PDGF, bombesin, bradykinin, and ionomycin failed to induce cellular acidification, but alkalinization responses to PDGF, bombesin, and PMA persisted. We propose that the transient acidification seen with PDGF, bombesin, and other agents is the result of increased intracellular Ca2+. However, growth factor-induced alkalinization via the Na+/H+ exchanger is independent of changes in Ca2+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., Tyrey S. J., Cragoe E. J., Jr, Cuatrecasas P. Inhibition of epidermal growth factor-induced mitogenesis by amiloride and an analog: evidence against a requirement for Na+/H+ exchange. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6762–6766. doi: 10.1073/pnas.81.21.6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F. Cell activation. The 'basic' connection. Nature. 1984 Nov 22;312(5992):312–312. doi: 10.1038/312312a0. [DOI] [PubMed] [Google Scholar]
- Cassel D., Rothenberg P., Zhuang Y. X., Deuel T. F., Glaser L. Platelet-derived growth factor stimulates Na+/H+ exchange and induces cytoplasmic alkalinization in NR6 cells. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6224–6228. doi: 10.1073/pnas.80.20.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Zhuang Y. X., Glaser L. Vanadate stimulates Na+/H+ exchange activity in A431 cells. Biochem Biophys Res Commun. 1984 Jan 30;118(2):675–681. doi: 10.1016/0006-291x(84)91356-1. [DOI] [PubMed] [Google Scholar]
- Chiesi M., Inesi G. Adenosine 5'-triphosphate dependent fluxes of manganese and and hydrogen ions in sarcoplasmic reticulum vesicles. Biochemistry. 1980 Jun 24;19(13):2912–2918. doi: 10.1021/bi00554a015. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Lee W. M., Williams P. W., Giels G. M., Williams L. T. c-myc gene expression is stimulated by agents that activate protein kinase C and does not account for the mitogenic effect of PDGF. Cell. 1985 Nov;43(1):243–251. doi: 10.1016/0092-8674(85)90029-7. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Cohen S. Cytoplasmic [Ca2+] and intracellular pH in lymphocytes. Role of membrane potential and volume-activated Na+/H+ exchange. J Gen Physiol. 1987 Feb;89(2):185–213. doi: 10.1085/jgp.89.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W., Biggar W. D. Cytoplasmic pH regulation in normal and abnormal neutrophils. Role of superoxide generation and Na+/H+ exchange. J Biol Chem. 1986 Jan 15;261(2):512–514. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Hesketh T. R., Moore J. P., Morris J. D., Taylor M. V., Rogers J., Smith G. A., Metcalfe J. C. A common sequence of calcium and pH signals in the mitogenic stimulation of eukaryotic cells. Nature. 1985 Feb 7;313(6002):481–484. doi: 10.1038/313481a0. [DOI] [PubMed] [Google Scholar]
- Hinnen R., Miyamoto H., Racker E. Ca2+ translocation in Ehrlich ascites tumor cells. J Membr Biol. 1979 Sep 14;49(4):309–324. doi: 10.1007/BF01868989. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Epel D. Intracellular pH and activation of sea urchin eggs after fertilisation. Nature. 1976 Aug 19;262(5570):661–664. doi: 10.1038/262661a0. [DOI] [PubMed] [Google Scholar]
- Kauffman R. F., Taylor R. W., Pfeiffer D. R. Cation transport and specificity of ionomycin. Comparison with ionophore A23187 in rat liver mitochondria. J Biol Chem. 1980 Apr 10;255(7):2735–2739. [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Growth factor action and intracellular pH regulation in fibroblasts. Evidence for a major role of the Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5809–5815. [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. Effect of measured calcium chloride injections on the membrane potential and internal pH of snail neurones. J Physiol. 1980 Jan;298:111–129. doi: 10.1113/jphysiol.1980.sp013070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. The effect of calcium injection on the intracellular sodium and pH of snail neurones. J Physiol. 1977 Mar;265(3):867–879. doi: 10.1113/jphysiol.1977.sp011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Aerts R. J., Tertoolen L. G., de Laat S. W. The epidermal growth factor-induced calcium signal in A431 cells. J Biol Chem. 1986 Jan 5;261(1):279–284. [PubMed] [Google Scholar]
- Moolenaar W. H., Mummery C. L., van der Saag P. T., de Laat S. W. Rapid ionic events and the initiation of growth in serum-stimulated neuroblastoma cells. Cell. 1981 Mar;23(3):789–798. doi: 10.1016/0092-8674(81)90443-8. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Growth factors immediately raise cytoplasmic free Ca2+ in human fibroblasts. J Biol Chem. 1984 Jul 10;259(13):8066–8069. [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Phorbol ester and diacylglycerol mimic growth factors in raising cytoplasmic pH. Nature. 1984 Nov 22;312(5992):371–374. doi: 10.1038/312371a0. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Tsien R. Y., van der Saag P. T., de Laat S. W. Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature. 1983 Aug 18;304(5927):645–648. doi: 10.1038/304645a0. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Yarden Y., de Laat S. W., Schlessinger J. Epidermal growth factor induces electrically silent Na+ influx in human fibroblasts. J Biol Chem. 1982 Jul 25;257(14):8502–8506. [PubMed] [Google Scholar]
- Niggli V., Sigel E., Carafoli E. The purified Ca2+ pump of human erythrocyte membranes catalyzes an electroneutral Ca2+-H+ exchange in reconstituted liposomal systems. J Biol Chem. 1982 Mar 10;257(5):2350–2356. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Owen N. E., Villereal M. L. Evidence for a role of calmodulin in serum stimulation of Na+ influx in human fibroblasts. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3537–3541. doi: 10.1073/pnas.79.11.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh M., Nanri H., Takeshige K., Minakami S. Pertussis toxin inhibits intracellular pH changes in human neutrophils stimulated by N-formyl-methionyl-leucyl-phenylalanine. Biochem Biophys Res Commun. 1985 Aug 30;131(1):64–69. doi: 10.1016/0006-291x(85)91770-x. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Rozengurt E. Na+/H+ antiport in Swiss 3T3 cells: mitogenic stimulation leads to cytoplasmic alkalinization. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7778–7782. doi: 10.1073/pnas.79.24.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Oades Z. G. Signal transduction and ligand-receptor dynamics in the neutrophil. Ca2+ modulation and restoration. J Biol Chem. 1985 Sep 25;260(21):11468–11475. [PubMed] [Google Scholar]
- Smallwood J. I., Waisman D. M., Lafreniere D., Rasmussen H. Evidence that the erythrocyte calcium pump catalyzes a Ca2+:nH+ exchange. J Biol Chem. 1983 Sep 25;258(18):11092–11097. [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Vicentini L. M., Villereal M. L. Serum, bradykinin and vasopressin stimulate release of inositol phosphates from human fibroblasts. Biochem Biophys Res Commun. 1984 Sep 17;123(2):663–670. doi: 10.1016/0006-291x(84)90280-8. [DOI] [PubMed] [Google Scholar]
- Wiener E., Dubyak G., Scarpa A. Na+/H+ exchange in Ehrlich ascites tumor cells. Regulation by extracellular ATP and 12-O-tetradecanoylphorbol 13-acetate. J Biol Chem. 1986 Apr 5;261(10):4529–4534. [PubMed] [Google Scholar]
- Yano K., Higashida H., Inoue R., Nozawa Y. Bradykinin-induced rapid breakdown of phosphatidylinositol 4,5-bisphosphate in neuroblastoma X glioma hybrid NG108-15 cells. J Biol Chem. 1984 Aug 25;259(16):10201–10207. [PubMed] [Google Scholar]



