Abstract
This paper examines whether a telephone-based, automated maintenance enhancement program can help to reduce opioid and NSAID analgesics use in patients with chronic pain.
Following 11 weeks of group CBT, fifty-one subjects with chronic musculoskeletal pain were randomized to one of two study groups. Twenty-six subjects participated in 4 months of a Therapeutic Interactive Voice Response (TIVR) program in addition to standard follow-up care, while a control group of twenty-five subjects received standard follow-up care only. TIVR is an automated, telephone-based tool developed for the maintenance and enhancement of CBT skills.
Opioid analgesic use decreased in the experimental group in both follow-ups: 4- and 8-months post-CBT. In addition, at 8-month follow up, 21% of the TIVR subjects had discontinued the use of opioid analgesics, 23% had discontinued NSAIDS, and 10% had discontinued antidepressant medications. In contrast, the control group showed increases in opioid and NSAIDS use. Analysis of covariance (ANCOVA) revealed significant between-group differences in opioid analgesic use at 8-month follow up (p=0.004).
We have previously demonstrated the efficacy of Therapeutic IVR to decrease pain and improve coping; this analysis demonstrates that the use of TIVR may also result in concurrent reductions in opioid analgesic and NSAID medications use.
Keywords: Chronic Pain, Coping, Opioid Analgesics, IVR, Cognitive Behavioral Therapy
1. INTRODUCTION
Persistent musculoskeletal pain is common but difficult to treat effectively. Opioid analgesics are frequently used in the management of chronic musculoskeletal pain. However, the use of opioids in the management of chronic pain remains controversial due to concerns about efficacy, safety, and the possibility of abuse or addiction33. Mortality rates from prescription-type opioids have risen steadily since 1997 and the National Institute on Drug Abuse estimated that over 2 million Americans used pain-relievers non-medically for the first time in that same year29 While most prescription opioid use is not associated with abuse or addiction20, opioid analgesics remain an important and preventable risk factor for addictive behavior and outright substance abuse.
Nonsteroidal anti-inflammatory drugs (NSAIDS) are also considered a mainstay of therapy for musculoskeletal pain and provide useful symptomatic relief. While there is little risk of addiction, NSAIDS pose special hazards to many patients due to untoward effects such as gastrointestinal bleeding and compromised renal blood flow 7,25. Therefore, careful consideration must be given when prescribing opioid and NSAID analgesics to be certain that their intended benefit merits their use despite potential adverse effects.
There is now substantial evidence that cognitive-behavioral therapy (CBT) is a particularly valuable addition to chronic pain management1, 2, 4, 5, 12, 32. Its advantages include low cost, minimal side effects and demonstrated clinical efficacy in reducing chronic pain12. However, there is also evidence of relapse to chronic pain and pain behavior after the completion of CBT 1, 13, 30. If such relapse could be reduced, it could also reduce the need for ongoing use of potentially high-risk medications.
In two recently published studies we demonstrated the efficacy of a new tool, Therapeutic Interactive Voice Response (TIVR), to reduce pain and relapse into pain behavior following group CBT18, 19. Although developed to prevent relapse into pain behavior, we found that subjects continued to improve while using TIVR. To our knowledge, TIVR is the only empirically supported strategy for further enhancing outcomes after CBT for chronic pain. Given the high abuse, diversion, and dependence potential of opioid analgesics, and untoward effects of NSAIDS, our goal in this report is to test whether TIVR can also be used as a strategy to decrease opioid analgesic and NSAIDS use in patients with chronic musculoskeletal pain.
2. MATERIALS and METHODS
2.1. Overview of the design
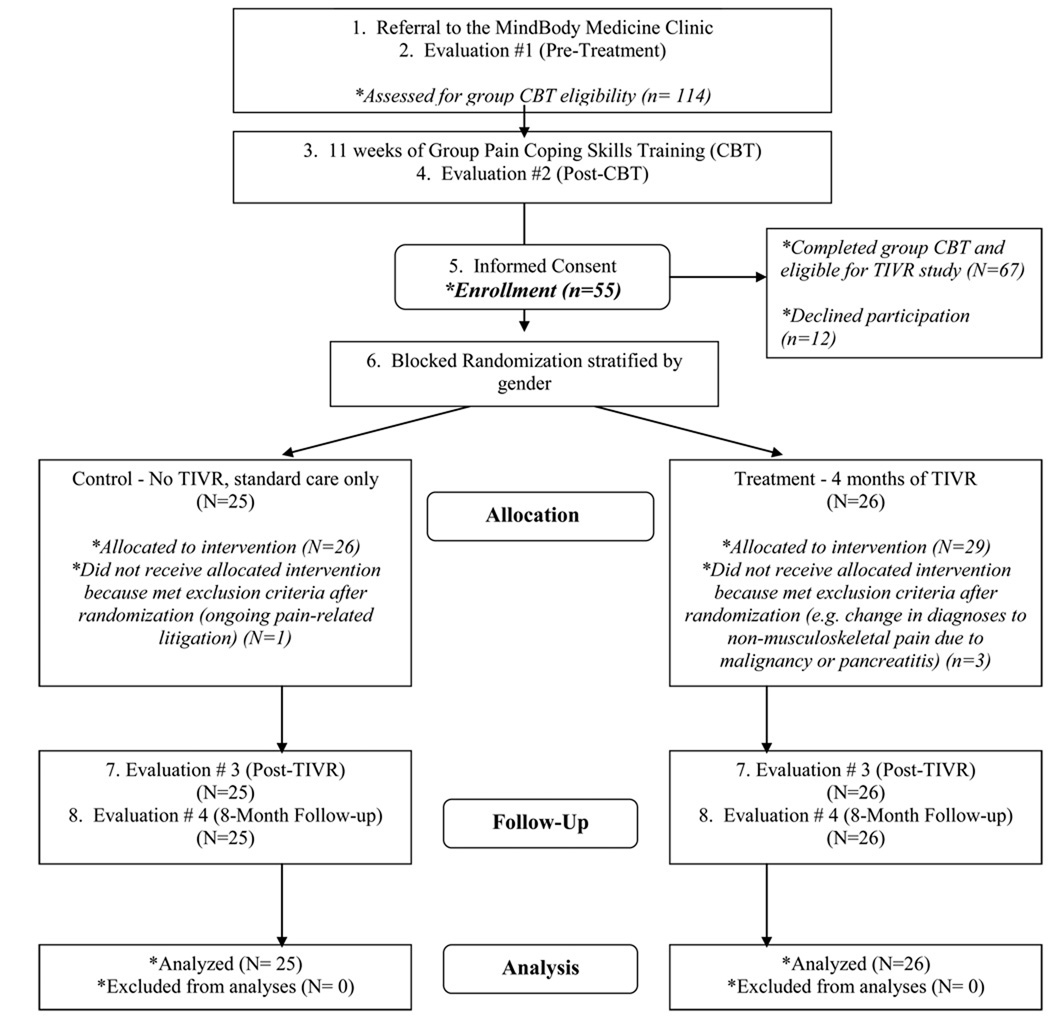
This study was designed as a two-group, prospective, randomized trial to examine whether Therapeutic Interactive Voice Response (TIVR) is an effective relapse prevention intervention for patients with chronic musculoskeletal pain. The primary aim of the analysis presented in the current paper was to test whether TIVR can be used as a strategy to decrease opioid analgesic and NSAIDS use in patients with chronic musculoskeletal pain. The overall study design is briefly described below and illustrated in Figure 1. For further details please see the description in Naylor et al., 200819.
Figure 1.
This CONSORT flow diagram details study recruitment (numbers 1–5), randomization and group allocation (number 6), follow-up (numbers 7–8) and analysis.
All study subjects completed group pain coping skills training (CBT) which consisted of 90-minute weekly sessions over 11 weeks. Therapy groups consisted of 7–9 patients. The CBT was delivered using a manualized treatment protocol. During the study, subjects in both study conditions received “standard care” managed by their primary care physicians. After completing 11 weeks of CBT, participants were randomly assigned to one of two study conditions. The experimental group received four months of maintenance program via the TIVR (see Treatment Procedures below). The control group continued receiving standard care only. Subjects in both study conditions were assessed at four time-points: prior to starting CBT, the conclusion of CBT, four months after the completion of CBT (corresponding to completion of the TIVR calls in the experimental group), and at eight months after CBT.
2.2. Procedure
The University of Vermont Institutional Review Board approved the research protocol and informed consent was obtained from each subject. Subjects were a consecutive sample of patients with chronic musculoskeletal pain referred to the MindBody Medicine Clinic (MBMC) at the university medical center from February 2003 through July 2004. All study subjects who successfully completed the standard 11 weeks of group CBT and met the inclusion/exclusion criteria were offered the opportunity to participate in the research project if they signed formal consent after being fully informed of the study details. Inclusion Criteria were as follows: at least 6 months of musculoskeletal pain (such as back pain, osteoarthritis, or fibromyalgia); met study threshold for severity of pain “over the past four weeks” of 4 or more on a 10-point scale measured at baseline on the McGill Pain Questionnaire; able to perform usual self-care; had ongoing health care from a physician; age 18 or older; had a touch-tone phone in the home. Exclusion Criteria were: patients with malignancy, radiation, or chemotherapy causing or influencing chronic pain; awaiting a pain-related surgical procedure; involved in pain-related litigation; any psychiatric illness that would interfere with participation in group therapy; inability to use the telephone-based TIVR due to cognitive or hearing impairment.
The group therapist and a research assistant were responsible for study enrollment during the final CBT group session. Consenting subjects were stratified by gender and current pain level, and were randomized using a stratified block design. Consecutively numbered, sealed envelopes were prepared for each gender group by the statistician. In order to avoid the risk of differential CBT exposure based on group assignment, randomization by assigning envelopes was done by the study coordinator only after group therapy was completed.
2. 3. Assessment Measures
The following measures of medications used, pain, function/disability, depression, and coping were used. All the clinical measures have been validated by previous research. All were self-administered at each evaluation. More details about the clinical measures can be found in Naylor et al., 200819.
Medication Intake
We assessed the use of opioid analgesics, benzodiazepines, NSAIDS and antidepressants. Subjects were asked to report all medications used in the month preceding each evaluation time point. Baseline medication use (doses and frequencies) was verified with the medical records obtained from the primary care providers and/or referring physicians. At follow-up evaluations the dose and average frequency of medications were collected from self-report study questionnaires and verified via a structured interview with a study psychiatrist. Medications were then classified into one of the following four categories: 1) opioid analgesics, 2) NSAIDS, 3) benzodiazepines, and 4) antidepressants. Standardized doses were calculated within each category. All PRN dosages were calculated using maximal frequency and dose per 24 hours.
Opioid analgesics
Morphine 50 mg/24 hours was used as the reference dose, consistent with guidelines provided by the American Pain Society17. The 24 hour morphine dose equivalencies were as follows: butorphanol 2.0mg (intranasal spray), codeine 200mg, fentanyl 25 mcg/hr (transdermal), hydrocodone 12.5mg, meripidine 300mg, methadone 30mg, oxycodone 35 mg, propoxyphene 140mg.
Non-steroidal anti-inflammatory
Aspirin 2600 mg/24 hours was used as the reference drug10. Combination NSAIDS (e.g. Excedrin) were divided into individual non-steroidal components and converted to aspirin equivalents. The 24 hour aspirin dose equivalencies were as follows: acetaminophen 2600mg, celecoxib 200mg, ibuprofen 800mg, ketorolac 40mg, meloxicam 7.5mg, nabumetone 1000mg, naproxen 1000mg, piroxicam 20mg, rofecoxib 25mg. valdecoxib 40mg.
Benzodiazepines
Diazepam 5 mg/dose was used as the reference drug3, 10. The 24 hour diazepam dose equivalencies were as follows: aprazolam 0.5mg, clonazepam 0.5mg, lorazepam 1mg.
Antidepressants
Fluoxetine 20 mg/24 hours was used as the reference drug consistent with the study by J.B. Weilburg et al.34, who developed these equivalencies using manufacturer’s guidelines and expert opinions. The 24 hour fluoxetine dose equivalencies were as follows: amitriptyline 125mg, buproprion 150mg, citalopram 20mg, escitalopram 20mg, imipramine 125 mg, mirtazapine 15mg, nortriptyline 75mg, paroxetine 20mg, sertraline 50mg, tranylcypromine 40mg, trazodone 300mg, venlafaxine 75mg.
Pain
Two measures were used to assess pain: 1) the short form of the McGill Pain Questionnaire (MPQ)15, and 2) the Pain Symptoms subscale from the Treatment Outcomes in Pain Survey (TOPS)21.
Function/Disability
The TOPS provides three measures of patients' functioning and disability: 1) the SF-36 Mental Function scale 2) the SF-36 Physical Function Scale, 3) The TOPS Total Pain Experience scale21,9, 22..
Depression
All participants completed the Beck Depression Inventory (BDI)16, 6 at all evaluations. The BDI is a 21-item self-report depression screening measure with the score ranges of 0 to 13 corresponding to minimal depression, 14 to 19 mild depression, 20 to 28 moderate depression, and scores of 29 to 63 corresponding to severe depression.
Pain Coping Strategies
We used the Coping Strategies Questionnaire (CSQ) to measure the degree to which subjects perceive themselves as able to use coping strategies to control and decrease pain and the degree of catastrophizing (Catastrophizing sub-scale)11, 14.
2.3.1 Treatment Procedures
Group Cognitive Behavioral Therapy (CBT) (for further details please see Naylor et al., 200819)
Cognitive behavioral therapy was delivered in eleven, 90-minute weekly group sessions. Our CBT intervention for pain management was designed to: 1) change cognition and decrease catastrophizing and other maladaptive behavior, 2) teach relaxation techniques, 3) enhance patients' ability to use attention diversion, 4) change detrimental activity patterns to better control pain, and 5) manage pain related medication use with special emphases on opioid analgesics use and controlling side effects or withdrawal symptoms.
Standard care
After the completion of group CBT, all subjects were encouraged to continue standard care from their usual care sources. We did not monitor the frequency of doctor visits.
Description of Therapeutic Interactive Voice Response (TIVR) (for further details please see Naylor et al., 200819)
The technology underlying the TIVR is Interactive Voice Response (IVR). When using IVR, an individual interacts with a computer through the medium of a telephone using the touch-tone keypad. We developed the Therapeutic IVR system (TIVR) to help patients maintain treatment gains following their pain coping skills training. The TIVR has four components:
Component 1: Daily Self Monitoring Questionnaire. This 21-item questionnaire assesses daily level of pain and perceived pain control, as well as daily mood, sleep, stress, coping, and pain medication use.
Component 2: Didactic Review of Skills. Participants are able to access a verbal review of eight of the pain management skills they had learned during the 11 weeks of CBT.
Component 3: Guided Behavioral Rehearsal of Pain Coping Skills (Practice Sessions). Patients can access the pre-recorded voice of a therapist guiding them through behavioral rehearsals of eight of the coping skills taught during CBT.
Component 4: Monthly Therapist Feedback Message. Once a month the group therapist records a personalized message for each participant based on participant’s daily reports with insight into possible relationships between use of coping skills, mood, stress and pain levels, suggestions for new pain management coping skills other then medication, reminders about underutilized coping skills learned in CBT, and verbal encouragement.
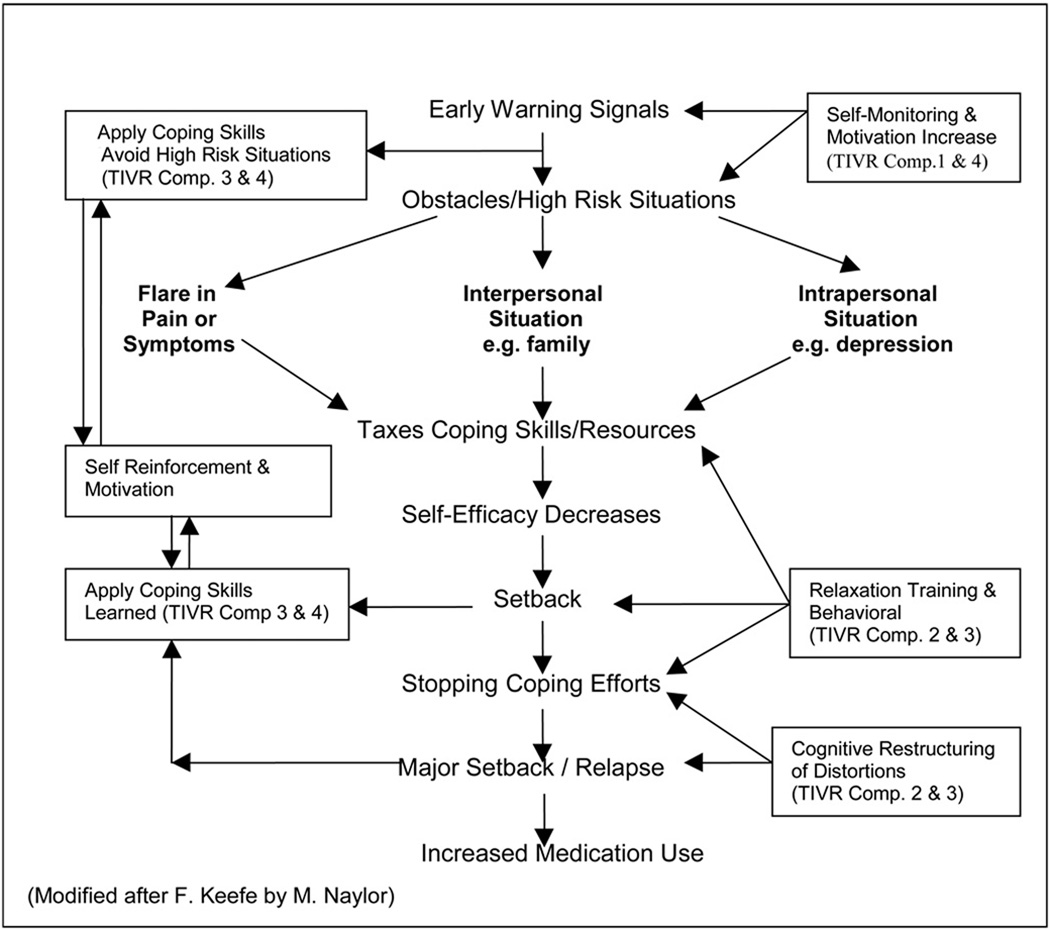
Our clinical and research approach was based on the Gate Control Theory of pain which describes pain as multi-dimensional rather than a single sensory experience. This theory emphasizes the central nervous system as an essential component in nociceptive processing and perception and that cognition, emotions, feeling, and belief systems have an influence on pain experience. Our relapse prevention model shown in Figure 2 shows a typical relapse cascade in the central core of the figure. The boxes in the periphery represent the particular coping skills that are promoted by the TIVR, which can intervene in the relapse cascade at the indicated points. The numerals in the boxes refer to the operative TIVR component(s). The TIVR is operative at many points along the way to this end stage, and works to modify the relapse process before it results in pain recurrence and or increase of medication use. TIVR Component 1 (Daily Questionnaire) is designed to improve self-monitoring; Components 2&3 (Skills Review & Rehearsal) are developed to help master coping skills and increase adherence to practice; Component 4 (Monthly Message) is designed to reinforce consistent coping behavior by enhancing motivation and reward for using coping skills and by improving self-efficacy (see Treatment Procedures for the description of TIVR). For those patients whose goal was to decrease the use of pain-related medication, the monthly messages carried special feedback and encouragements. The goal to decrease or discontinue opioid analgesics were usually made prior to or during the CBT group but some subjects made this goal post-CBT- during the TIVR use.
Figure 2.
A Model of Relapse Prevention Process in Coping with Pain. The central core of Figure 2 depicts a typical relapse cascade with the boxes in the periphery representing the particular TIVR Components and the coping skills promoted to intervene in the relapse cascade. (Modified after F. Keefe by M. Naylor)
2.3.2 Statistical Procedures
A power analysis for the current study was based on data from our pilot study18, 28 to detect an effect size of 0.5 using ANCOVA for the group comparisons of the primary clinical outcomes. An intent-to-treat approach was used. All subjects who were randomized were retained for the primary analyses. Missing medication values were imputed for two subjects both in the TIVR group. For one case with missing data at the third follow-up, the averages of the scores from the second and fourth time points were used. One other subject for whom we had no data for the final medication survey was assumed to have returned to the highest prior doses; thus we were biasing against a positive result for the TIVR group.
Only subjects who reported using the medication of interest at least once at any given time point were retained when calculating change in a specific class of medication. Group differences in medication use were evaluated at each follow-up time point using analysis of covariance (ANCOVA); the covariates used to adjust the outcome included the outcome (medication of interest) baseline dose and change from baseline dose for the other three medication classes. Similarly, within subjects comparisons to baseline were done using repeated measures ANCOVA with dose changes of the other medications as the covariates. A log transformation was used with narcotics, NSAIDS, and benzodiazepines to adjust for these distributions’ skewness. Outlier values were retained in the final analyses. Sensitivity analyses were run omitting these outliers but the results did not change.
In order to increase power and consistency, the impact of changes in medication use on clinical outcomes was estimated using multilevel random effects models, SAS Proc Mixed24, across all subjects and time points simultaneously. The change scores of each clinical measure (eg. MPQ typical pain) were considered individually as outcomes with all four medication dose changes (using actual doses, not log transformed) considered together as the predictors. The subjects, the level 2 units, each had three sets of change scores: post-CBT minus baseline, 4-month follow-up minus post-CBT, and the final 8-month follow-up minus the 4-month follow-up. The clinical raw scores were then adjusted by subtracting the expected change due to the subject’s changes in medication (i.e., the predicted outcome of the multilevel model). For the group comparisons, these adjusted clinical scores were then used in ANCOVA models. At the post-CBT time point the baseline scores were used as the covariate. At all other follow up points the post-CBT scores were used as the covariate. Paired t tests were run for the within-group comparisons to baseline.
Analyses were done using SAS version 924. All tests were two-sided with alpha set at 0.05. Given the small sample size, the alpha was not adjusted for multiple tests.
3. RESULTS
Subjects
Fifty-five subjects met inclusion/exclusion criteria, agreed to participate in the study, and were randomly assigned to one of the two study groups. Four subjects met exclusion criteria soon after randomization (e.g. diagnosed with cancer or became involved in pain-related litigation) and were thus excluded from the analysis. Most of the enrollees were Caucasian (96%), were women (84%), and the mean age of the sample was 46 (Table 1). There were no statistically significant differences in gender, age or mean duration of pain between groups. All 51 subjects took medications from at least one of the four classes at one or more of the study assessment intervals: 46 reported taking NSAIDS, 46 - antidepressants, 32 – opioid analgesics and 22 - benzodiazepines.
Table 1.
Demographics for each group and total sample
| TIVR Group N=26 |
Control Group N=25 |
Total Sample N=51 |
|
|---|---|---|---|
| Age | x̄ = 47 ± 10.42 | x̄ = 46 ± 12.42 | x̄ = 46 ± 11.47 |
| Gender | |||
| • Females | 23 (88%) | 21 (84%) | 44 (86%) |
| Race | |||
| • White/Caucasian | 25 (96%) | 24 (96%) | 48 (96%) |
| Martial Status | |||
| • Never Married | 6 (24%) | 0 | 6 (12%) |
| • Married/Living Together | 17 (64%) | 20 (80%) | 37 (72%) |
| • Divorced/Separated | 3 (12%) | 5 (20%) | 8 (16%) |
| Education in Years | x̄ = 14.12 ± 1.83 | x̄ = 14.29 ± 1.76 | x̄ = 14 ± 1.80 |
| • 9–12 years | 9 (32%) | 6 (24%) | 15 (28%) |
| • 13–16 years | 14 (56%) | 16 (64%) | 30 (60%) |
| • 17+ years | 3 (12%) | 2 (8%) | 5 (10%) |
| • Did not report education | 0 | 1 (4%) | 1 (2%) |
| Employment Status | |||
| ● full time employment | 6 (23%) | 6(24%) | 12 (23%) |
| ● part-time employment | 8 (30%) | 4 (16%) | 12 (23%) |
| ● disability | 9 (35%) | 12 (48%) | 21 (41%) |
| ● unemployed | 2 (8%) | 3(12%) | 5 (10%) |
| ● retired | 1 (4%) | 0 | 1(2%) |
| Living Situation | |||
| • 3+ person household | 5 (16%) | 12 (48%) | 17 (32%) |
| • 2 person household | 13 (52%) | 11 (44%) | 24 (48%) |
| • Living alone | 8 (32%) | 2 (8%) | 10 (20%) |
| Duration of Pain in Years | x̄ = 13.60 ± 9.53 | x̄ = 8.60 ± 8.45 | x̄ = 11.15 ± 9.27 |
| Pain Related Surgery | |||
| Diagnoses (primary) | |||
| ● back pain | 9 (35%) | 11 (44%) | 20 (39%) |
| ● osteoarthritis | 4 (15%) | 4 (16%) | 8 (16%) |
| ● fibromyalgia | 1 (4%) | 3 (12%) | 4 (8%) |
| ● TMJ/jaw pain | 2 (8%) | 2 (8%) | 4 (8%) |
| ● headaches | 5 (19%) | 2 (8%) | 7 (14%) |
| ● post surgical/post trauma muscle pain | 5 (19%) | 3 (12%) | 8 (16%) |
Frequency of TIVR use
Frequency of TIVR use in 26 subjects over the 120 day study is presented in Table 2.
Table 2.
Frequency of TIVR use 26 participants over the 120 day study
| Daily Questionnaire | |
| • Expected number of calls | 3168 |
| • Calls actually made | 2176 (69%) |
| • mean number of calls per person | 84 (SD = 32) |
| • more than 80% of the calls | 9 participants |
| • less than 50% of the daily calls | 7 participants |
| Daily Coping Strategies used | |
| • average number of coping strategies reported per call for all participants | 5.6 (SD = 1.7). |
| • number of participants reported using an average 5 or more coping strategies per day | 70% |
| Review of Skills | Average of 4 times per subject over the study (from 0–42 reviews) |
| Practice sessions | Average of 11 times per subject over the study (from 0–34 practices) |
Patient Feedback about the TIVR use
Our empirical results to-date suggest that TIVR can be used to improve coping skills adherence, decrease relapse into pain behavior and help to decrease pain related medication use. At the conclusion of the study, we interviewed each subject in the TIVR condition about their experience using this telephone based intervention and patient feedback tends to confirm these empirical findings. Patients reported that the TIVR helped them to improve their motivation for both self-examination and self-awareness. They also strongly felt the TIVR provided a helpful structure for practicing what they had learned in the group CBT so that the coping skills became second nature. All of the subjects who used TIVR 50% or more felt the TIVR was useful and only one felt that four months was too long. In fact several expressed the desire to have continued beyond the four months of calling and one subject has continued to call daily for last 3 years since the study ended. Some subjects specifically mentioned TIVR’s impact on their medication use, for instance: “The TIVR reinforced all that I learned in the group. It was good motivation to continue practicing the new skills I learned to decrease reliance on medication use”; or: “TIVR gave me new lease on life without dependency on pain medication. I can think clearly again.”
Changes in Medication Use Within-Group Analysis
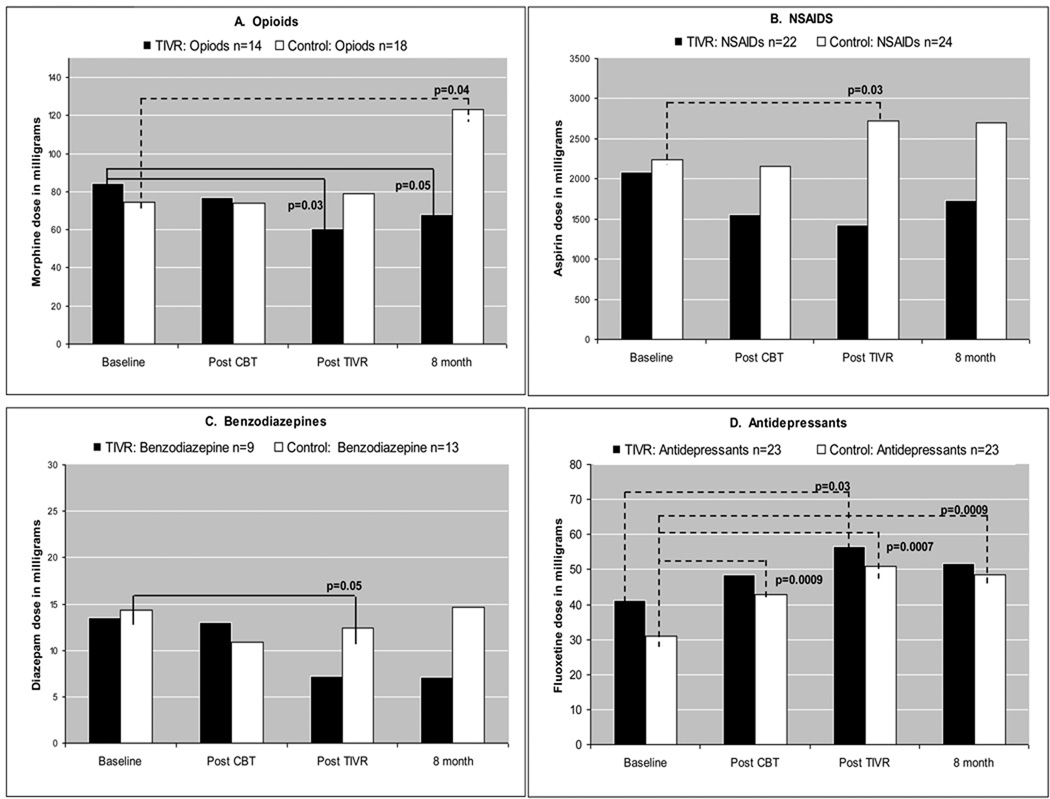
Within-group analysis ANCOVA revealed statistically significant changes in medication doses from baseline. The 4 medication classes were considered separately as outcomes, with changes in the other three medication classes included as covariates. Only subjects reporting using the medication of interest at least once at any given time point were retained for the analysis (Figure 3).
Figure 3.
Figure 3 depicts the changes in medications dose used while retaining for analysis all subjects who used the medication at least once. A) opioids – equivalent of mg morphine sulfate, B) NSAIDS – equivalent of mg aspirin, C) benzodiazepines - equivalent of mg diazepam, D) antidepressants - equivalent of mg fluoxetine. There was one outlier in control group in opioids at the 8th month follow-up.
Note: black bars represent TIVR group
white bars represent Control group
dotted line (----) represents statistically significant dose increase
solid line (—) represents statistically significant dose decrease
For opioid analgesics and NSAIDS dose change, the experimental and control groups had opposite trends: there was a significant decrease in the mean opioid analgesic dose (p=0.03 at 4-month follow-up, p=0.05 at 8-month follow-up) in the experimental group, but a significant increase in opioid analgesics mean dose at 8-month follow-up in the control group (p=0.045). The control group also had a significant increase (p=0.03) in NSAIDS dose at 4-month follow-up. Mean benzodiazepine dose was significantly decreased at 4-month follow-up in the control group but reverted back to baseline level by the last follow-up. Mean antidepressant dose increased in both groups, but the increase in the experimental group was not significant at 8-month follow-up (p=0.19) whereas there was a significant increase in comparison to the baseline for the control group (p<0.001).
In addition to the mean medication dose changes, there were changes from baseline in the number of subjects taking specific medications at 8-month follow-up (Table 3). In the TIVR group three subjects stopped taking opioid analgesics, five subjects stopped taking NSAIDS, and two discontinued antidepressants. In the control group at 8-month follow-up the number of subjects taking opioid analgesics increased by three, and the number of subjects taking NSAIDS increased by three. The number of patients taking antidepressants was unchanged. The number of control subjects taking benzodiazepines decreased by three while the number in the TIVR group increased by one subject.
Table 3.
Change(s) in number of subjects taking a medication at follow-up(s) as compared to baseline.
| Drug Class | Baseline | Post CBT follow-up (% change) |
4 month follow-up (% change) |
8 month follow-up (% change) |
|||
|---|---|---|---|---|---|---|---|
| TIVR Group (N=26) | |||||||
| Narcotics | 14 | 13 | (−7) | 10 | (−28) | 11 | (−21) |
| NSAIDS | 20 | 17 | (−15) | 17 | (−15) | 15 | (−25) |
| Benzodiazepines | 8 | 9 | (+12) | 9 | (+12) | 9 | (+12) |
| Antidepressants | 19 | 21 | (+10) | 20 | (+5) | 17 | (−10) |
| Control Group (N=25) | |||||||
| Narcotics | 15 | 16 | (+6) | 16 | (+6) | 18 | (+20) |
| NSAIDS | 18 | 18 | (0) | 22 | (+22) | 21 | (+16) |
| Benzodiazepines | 12 | 11 | (−8) | 10 | (−16) | 9 | (−25) |
| Antidepressants | 19 | 22 | (+15) | 21 | (+10) | 19 | (0) |
Between-Group Analysis
Between-group analysis (ANCOVA) for subjects who reported using the target medication at one or more time points post-CBT revealed differences for adjusted mean opioid dose at the post-group and the 8-month assessments (p=0.04 and p=0.004 respectively), with significantly lower mean opioid dose in the experimental group compared to control subjects. It is important to note that group comparison for opioids at the 8-month follow-up remained significant (p=0.004) in a sensitivity analysis where an extremely high dose in one patient in the control group was set to the prior level in order to see if this outlier was influential. The groups also differed in NSAIDS medication use at the post-TIVR follow-up (4-month follow-up) (p=0.006), with a significantly lower mean NSAIDS dose in the experimental group. However, the group difference for NSAIDS was not significant at 8-month follow-up. There were no statistically significant group differences in benzodiazepine or antidepressant use at either 4-or 8-months follow-up. Table 4 shows the effect sizes for these changes in medication.
Table 4.
Effect sizes of group differences at the three follow-ups after adjusting for baseline value and for changes in dose in other medication
| Medication | Sample size (TIVR/control) |
Post CBT | 4 month | 8 month |
|---|---|---|---|---|
| Narcotics | 14/18 | 0.8* | 0.6 | 1.1* |
| NSAIDS | 22/24 | 0.5 | 0.8* | 0.4 |
| Benzodiazepines | 9/13 | 0.3 | 0.1 | 0.1 |
| Antidepressants | 23/23 | 0.3 | 0.2 | 0.2 |
Only subjects who reported using the medication were retained. Cohen’s effect size h are reported.
Significance: TIVR group showed more improvement, p< 0.05.
Group Comparisons of Adjusted Clinical Outcomes
We had previously reported19 that the TIVR group was significantly better on nearly all clinical outcomes. For this report, we repeated the group comparisons after the clinical scores were adjusted for changes in all four medication classes (See Statistical Procedures). In comparison to the previously published effect sizes, we observed a decrease in the significance of the group comparisons at the 4-month follow-up. In contrast, several outcomes showed greater effect sizes at the 8-month follow-up (both SF-36 composite scores, MPQ typical pain, CSQ control and decrease pain). Table 5 summarizes these findings. At the 8-month follow-up, TIVR group scores remained significantly better for all clinical outcomes other than the CSQ catastrophizing scale. In addition the BDI results presented here show significant group differences at the final 8-month follow-up even after adjusting for changes in medication intake.
Table 5.
Effect sizes of group differences in clinical outcomes adjusted for changes in all four medication classes
| Test | Post CBT (1) | 4 month (2) | 8 month (2) |
|---|---|---|---|
| SF-36 Mental Composite | 0.6* | 0.6* | 0.9* |
| SF-36 Physical Composite | 0 | 0.6 | 1.1** |
| MPQ Pain Now | 0.5 | 0.6* | 1.2** |
| MPQ Pain Typical | 0.3 | 1.1** | 1.3*** |
| CSQ Ability to Control Pain | 0.1 | 0.8* | 1.4*** |
| CSQ Ability to Decrease Pain | 0.2 | 1.0* | 1.0* |
| CSQ Catastrophizing | 0.4 | 0.5 | 0.5 |
| TOPS Total Pain Experience | 0.3 | 1.1** | 1.2*** |
| • Pain Symptoms | 0.3 | 1.0* | 1.2*** |
| Beck Depression Inventory | 0.8* | 0.5 | 0.8* |
Post CBT group means were compared after adjusting for baseline scores. Group differences were not expected to be different.
Group means were compared after adjusting for post-CBT scores and changes in medication use (dose at given time point – baseline dose).
Significance * p<0.05
p<0.001
p<0.0001
Where significant, TIVR group showed more improvement
4. DISCUSSION
In our previously published paper19, we documented that using the TIVR for four months post-group CBT resulted in improvements in measures of pain, mental health, coping, and in physical performance. As we demonstrate in the current report, in addition to maintaining or improving gains in clinical outcomes, patients in the TIVR group also reported a decrease in mean dose of opioid analgesics and NSAID medication use at the post-TIVR assessment compared to the baseline. The decrease in opioid analgesic mean dose persisted at the final 8-month post-CBT follow-up, four months after access to the TIVR was terminated. In addition, some subjects in the TIVR group discontinued using opioids or NSAIDS altogether. In contrast, the control group showed significant increases in mean dose of the opioid analgesics and other medication use by the 8-month follow up. Furthermore, in the control group the number of patients taking opioid and NSAID medications increased. Group differences in medication use were significant for opioid analgesics at the 8-month and NSAIDS at the 4-month follow-ups.
The mean anti-depressant dose increased for both groups from baseline to all follow-ups, although the increase was not significant for the TIVR group by 8-month follow-up. Antidepressants remain a common treatment modality in this population although their efficacy for treating chronic musculoskeletal pain per se remains controversial31. Debate continues over whether there are analgesic effects distinct from antidepressants’ effects on mood8, 23, 27. In our study, the improvements in clinical outcomes were not related to increases in antidepressant usage.
Since individual subjects were treated by different physicians and were not taking the same medications, it was necessary to calculate equivalence doses. To our knowledge this is the first study with calculated equivalence doses for a wide range of opioid analgesics, NSAIDS, benzodiazepines and antidepressants, in order to evaluate an efficacy of pain management. We developed our own equivalency formulas for the first three classes of drugs using guidelines provided by the American Pain Society17 and Goodman & Gilman's The Pharmacological Basis of Therapeutics10.
Results demonstrate that the telephone based Therapeutic Interactive Voice Response relapse prevention program can be used not only to decrease pain, improve coping, and diminish likelihood of relapse into pain behavior but also concurrently decrease opioid and NSAIDS medication use. As opioid analgesics are frequently used in the management of chronic musculoskeletal pain, Therapeutic IVR might therefore help patients with chronic pain to reduce the risk of adverse events associated with opioid treatment. These include but are not limited to constipation with the increased risk for bowel obstruction, hip fracture related to falls, and cognitive impairment, as well as long-term sequelea including increased opioid tolerance and/or hyperalgesia with decreased opioid efficacy and iatrogenic dependency26. While most prescription opioid use is not associated with abuse or addiction, it remains an important and preventable cause of addictive behavior and frank substance abuse. There is therefore a need for optimized pharmacologic and non-pharmacologic treatment strategies for the management of chronic non-malignant pain and TIVR might be one of the answers.
Study Limitations
There are a few limitations that must be considered when interpreting our results. First is the relatively small sample size. In our study, opioid analgesics use was not an inclusion criterion. While all of our subjects used some form of pharmacotherapy, only 29 out of 51 reported using opioid analgesics and 38 out of 51 reported NSAIDS at any of the assessments. This resulted in fewer subjects for the medication effect analyses. The demographic composition is also skewed since the sample is predominantly female and there are only two minority group members. The latter is reflective of the demographic composition of the state of Vermont. We are aware that some of the findings of this study might be related to the fact that that subjects participating in the CBT groups are self–selected. For instance, we noticed that women were less likely to drop out of the groups and were more inclined than men to comply with the phone-based relapse prevention program.
A current ongoing RCT addresses some of the limitations with a larger sample and a longer follow-up. For instance, we are presently collecting more detailed information related to subjects’ pain treatment including the frequency of doctors’ visits, visits to the emergency department, hospitalizations, and medication side effects. Our present study also tests the effectiveness of TIVR without individually-tailored monthly messages as this component is time consuming and therefore expensive.
Also the TIVR technology could be further refined to customize the user experience. For example while our TIVR system uses a female voice, it might be possible to offer either female or male voices to subjects based on preference. Other improvements might include combining IVR technology with computer-based technology to be able to display customized graphs and figures based on results of daily data. For those who are e less inclined to participate in group CBT and/or are keener to use computers, a free standing computer-based CBT program with relapse prevention follow-up might be an ideal solution to capture this chronic pain population.
Finally, in light of the encouraging results of this study, future research plans include creating combination of phone- with computer- based technology to create special Therapeutic Computer and IVR program (TCIVR) tailored to chronic pain patients treated with opioid analgesics.
Conclusion
To our knowledge there is no other self-directed treatment program that has demonstrated efficacy as a tool for pain coping skills maintenance enhancement and concomitant opioid medication use reduction. As our telephone-based program was designed as a post-CBT pain coping skills maintenance enhancement, we were particularly gratified to see that patients using this tool not only continued to improve 8 months post CBT (four months after the TIVR program was completed) but also that they simultaneously decreased their opioid analgesics and NSAID medication use. If our findings are replicable, we believe that using the TIVR as a CBT based relapse prevention program would also be an efficacious and cost-effective treatment for opioid medication use reduction. In the future, we hope to determine if this combination of CBT with TIVR could enhance long-term treatment outcomes in patients with persistent pain and concomitant opioid dependence. We postulate that the TIVR’s applicability can be extended to the patient population at high risk for developing illicit analgesic medication abuse and dependence.
ACKNOWLEDGEMENTS
Acknowledgement of Support: This research was supported by grants from the National Institute of Drug Addiction (NIDA) R21 DA016115, National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS) R01 AR052131, National Institute on Alcohol Abuse and Alcoholism (NIAAA) R01 014270.
The Authors would like to thank Lari Young, MD, and our Clinical Pharmacist at Fletcher Allen Health Care, Barry A. Comeau, R.Ph. BS. Pharm, for their help in developing our equivalency doses, and G. Michael Krauthamer, MA for the technical support in preparation of this manuscript
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Perspective: This article demonstrates that the Therapeutic Interactive Voice Response maintenance enhancement program can help to reduce opioid analgesics use in patients with chronic pain. This automated maintenance enhancement program could potentially assist patients not only to decrease pain, improve coping, but also diminish likelihood of opioid dependence.
Disclosure: There are no competing interests, either scientific or financial, for the authors involved.
REFERENCES
- 1.Basler H. Group treatment for pain and discomfort. Patient Educ Couns. 1993;20:167–175. doi: 10.1016/0738-3991(93)90130-o. [DOI] [PubMed] [Google Scholar]
- 2.Basler H, Jakle C, Kroner-Herwig B. Incorporation of cognitive-behavioral treatment into the medical care of chronic low back patients: A controlled randomized study in German pain treatment centers. Patient Educ Couns. 1997;31:113–124. doi: 10.1016/s0738-3991(97)00996-8. [DOI] [PubMed] [Google Scholar]
- 3.Bezchlibnyk-Butler K, Jeffries J, Martin B, editors. Clinical handbook of psychotropic drugs. Toronto: Hogrefe & Huber Publications; 1997. [Accessed March 12, 2010]. Available at: www.mental-health-today.com/rx/benzo.htm. [Google Scholar]
- 4.Compas B, Haaga D, Keefe F, Leitenberg H, Williams D. A sampling of empirically supported psychological treatments for health psychology: Smoking, chronic pain, cancer, & bulimia nervosa. J Consult Clin Psychol. 1998;66:89–112. doi: 10.1037//0022-006x.66.1.89. [DOI] [PubMed] [Google Scholar]
- 5.Connally G, Sanders S. Predicting low back pain patients' response to lumbar sympathetic nerve blocks and interdisciplinary rehabilitation: The role of pretreatment overt pain behavior and cognitive coping strategies. Pain. 1991;44:139–146. doi: 10.1016/0304-3959(91)90127-J. [DOI] [PubMed] [Google Scholar]
- 6.Dozois D, Covin R. The Beck Depression Inventory-II (BDI-II), Beck Hopelessness Scale (BHS), and Beck Scale for Suicide Ideation (BSS) In: Hersen M, Hilsenroth M, Segal D, editors. Comprehensive handbook of psychological assessment: personality assessment. New York: John Wiley and Sons, Inc.; 2004. pp. 50–69. [Google Scholar]
- 7.Ehrlich G. Future directions in therapy of pain and osteoarthritis. Semin Arthritis Rheum. 1989;18 Suppl 2:100–104. doi: 10.1016/0049-0172(89)90026-7. [DOI] [PubMed] [Google Scholar]
- 8.Fishbain D, Cutler R, Rosomoff H, Rosomoff R. Chronic pain-associated depression: Antecedent or consequence of chronic pain? A review. The Clinical Journal of Pain. 1997;13:116–137. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Gandek B, Ware J., Jr Translating functional health and well-being: International Quality Of Life Assessment (IQOLA) Project studies of the SF-36 health survey. J Clin Epidemiol. 1998;51:891–1214. [PubMed] [Google Scholar]
- 10.Brunton LB, Lazo JS, Parker KL, editors. Goodman & Gilman's the pharmacological basis of therapeutics. 11th edition. New York: McGraw-Hill; 2005. [Google Scholar]
- 11.Keefe F, Caldwell D, Martinez S, Nunley J, Williams D. Analyzing pain in rheumatoid arthritis patients: Pain coping strategies in patients who have had knee replacement surgery. Pain. 1991;46:153–160. doi: 10.1016/0304-3959(91)90070-E. [DOI] [PubMed] [Google Scholar]
- 12.Keefe F, Van Horn Y. Cognitive-behavioral treatment of rheumatoid arthritis pain: Understanding and enhancing maintenance of treatment gains. Arthritis Care Res. 1993;6:213–222. doi: 10.1002/art.1790060408. [DOI] [PubMed] [Google Scholar]
- 13.Lanes T, Gauron E, Spratt K, Wernimont T, Found E, Weinstein J. Long-term follow-up of patients with chronic back pain treated in a multidisciplinary rehabilitation program. Spine. 1995;20:801–806. doi: 10.1097/00007632-199504000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Lawson K, Reesor K, Keefe F, Turner J. Dimensions of pain-related cognitive coping: Cross-validation of the factor structure of the coping strategy questionnaire. Pain. 1990;43:195–204. doi: 10.1016/0304-3959(90)91073-R. [DOI] [PubMed] [Google Scholar]
- 15.Melzack R. The McGill pain questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 16.Beck A, Steer R, Brown G, editors. Manual for the Beck Depression Inventory (BDI-II) 2nd edition. San Antonio: Psychological Corporation; 1996. [Google Scholar]
- 17.Miakowski C, Bair M, Chou C, D'arcy Y, Hartwick C, Huffman, Maleki J, Manwarren R, editors. Principles of analgesic use in the treatment of acute pain and cancer pain, 5th edition. Glenview: American Pain Society; 2005. [Google Scholar]
- 18.Naylor M, Helzer E, Naud S, Keefe F. Automated telephone as an adjunct for the treatment of chronic pain: A pilot study. The Journal of Pain. 2002;3:429–438. doi: 10.1054/jpai.2002.129563. [DOI] [PubMed] [Google Scholar]
- 19.Naylor M, Keefe F, Brigidi B, Naud S, Helzer E. Therapeutic interactive voice response for chronic pain reduction and relapse prevention. Pain. 2008;134:335–345. doi: 10.1016/j.pain.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter J, Jick H. Addiction rare in patients treated with narcotics. N Engl J Med. 1981;302:125. doi: 10.1056/nejm198001103020221. [DOI] [PubMed] [Google Scholar]
- 21.Rogers W, Wittink H, Ashburn M, Cynn D, Carr D. Using the TOPS as an outcomes instrument for multidisciplinary outpatient pain treatment by means of an augmented SF-36. Pain Medicine. 2000;1:55–67. doi: 10.1046/j.1526-4637.2000.99101.x. [DOI] [PubMed] [Google Scholar]
- 22.Rogers W, Wittink H, Wagner A, Cynn D, Carr D. Assessing individual outcomes during outpatient multidisciplinary chronic pain treatment by means of an augmented SF-36. Pain Medicine. 2000;1:44–54. doi: 10.1046/j.1526-4637.2000.99102.x. [DOI] [PubMed] [Google Scholar]
- 23.Salerno SM, Browning R, Jackson Jl. The effect of antidepressant treatment on chronic back pain: A meta-analysis. Arch Intern Med. 2002;162:19–24. doi: 10.1001/archinte.162.1.19. [DOI] [PubMed] [Google Scholar]
- 24.SAS Version 9.1 (Software) Cary, NC: SAS Institute Inc.; 2007. [Google Scholar]
- 25.Schnitzer T. Osteoarthritis treatment update. Postgrad Med. 1993;93:89–95. doi: 10.1080/00325481.1993.11701575. [DOI] [PubMed] [Google Scholar]
- 26.Simmonnet G, Le Moal M. Vulneability to opioid tolerance, dependence, and addiction: an individual-centered versus drug-centered paradigm analysis. In: Beaulieu P, Lussier D, Porreca F, Dickenson AH, editors. Pharmacology of Pain. Seattle: IASP; 2010. pp. 405–429. [Google Scholar]
- 27.Staiger T, Gaster B, Sullivan M, Deyo R. Systematic review of antidepressants in the treatment of low back pain. Spine. 2003;28:2540–2545. doi: 10.1097/01.BRS.0000092372.73527.BA. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J, editor. Statistical power analyses for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1988. [Google Scholar]
- 29.Substance Abuse and Mental Health Services Administration. (2009) Results from the 2008 National Survey on Drug Use and Health: National Findings. Rockville, MD: 2009 (Office of Applied Studies), NSDUH Series H-36, HHS Publication No. SMA 09-4443)
- 30.Turk D, Rudy T. Neglected topics in the treatment of chronic pain patients- relapse, noncompliance, and adherence enhancement. Pain. 1991;44:5–28. doi: 10.1016/0304-3959(91)90142-K. [DOI] [PubMed] [Google Scholar]
- 31.Urquhart DM, Hoving JL, Assendelft WJJ, Roland M, van Tulder MW. Antidepressants for non-specific low back pain. Cochrane Database of Systematic Reviews. 2008;(Issue 1) doi: 10.1002/14651858.CD001703.pub3. Art. No.: CD001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vlaeyen J, Haazen I, Schuerman J, Kole-Snijders A, van Eek H. Behavioral rehabilitation of chronic low back pain: Comparison of an operant treatment, an operant-cognitive treatment and an operant-respondent treatment. British Journal of Clinical Psychology. 1995;34:95–118. doi: 10.1111/j.2044-8260.1995.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 33.Wartenbert A. The opiate and opiate abuse. In: Herrington G, Jacobson G, Benzer D, editors. Alcohol and drug abuse. St. Louis: Warren H. Green, Inc.; 1987. [Google Scholar]
- 34.Weilburg J, O'Leary K, Meigs J, Hennen J, Stafford R. Evaluation of the adequacy of outpatient antidepressant treatment. Psychiatr Serv. 2003;54:1233–1238. doi: 10.1176/appi.ps.54.9.1233. [DOI] [PubMed] [Google Scholar]