Abstract
We report a powerful method to replace wild type genes on the chromosome of Escherichia coli. Employing a unique form of PCR, we generate easily constructible gene fusions bearing single point mutations. Used in conjunction with homologous recombination, this method eliminates cloning procedures previously used for this purpose.
Keywords: SOE PCR, Transformation, E. coli, dinB, Lambda Red, Point Mutation
Methods, Results, Discussion
There has been an effort by many labs to develop a quick and easy protocol for gene replacement on the bacterial chromosome (Datsenko and Wanner, 2000; Murphy et al., 2000; Heermann et al., 2008; Link et al., 1997; Gordon et al., 1997). Though a rapid method for chromosomal gene replacement was recently reported by Heermann et al. (2008), it includes two major limitations: gene replacement requires two sequential recombination events and must be done in strains bearing the rpsL150 mutation in the ribosomal protein S12. We present here an efficient and streamlined method, which relies on a single recombination event and could be carried out in any bacterial strain expressing the Lambda red proteins (Datsenko and Wanner, 2000).
We report for the first time the use of Splicing by Overlap Extension (SOE) polymerase chain reaction (PCR) to join noncontiguous DNA sequences with the purpose of constructing a fusion product to replace wild type genes with ones bearing point mutations. We call this method SOE-LRed to indicate the merger of SOE PCR (Horton et al., 1989) and lambda Red (Datsenko and Wanner, 2000) methodologies to localize genes with point mutations onto the chromosome. SOE PCR is a variation of traditional PCR that since its inception has been extremely valuable in joining noncontiguous DNA sequences from different sources (Horton et al., 1989). Notably, it eliminates traditional construction of recombinant DNA molecules, which is not only laborious, but also generally introduces unnecessary DNA sequences to the final product (Murphy et al., 2000).
Fusion of sequences via SOE PCR relies on two sequential PCR amplification reactions and is directed by primers that introduce a common linker region (CLR). In the first PCR reaction, the CLR is incorporated separately into each gene. Amplification of the full-length fusion sequence draws on the CLR for effective annealing of the two noncontiguous sequences, as well as the forward primer of sequence 1 and the reverse primer of sequence 2 (Figure 1).
Figure 1. Schematic of products generated by SOE PCR.
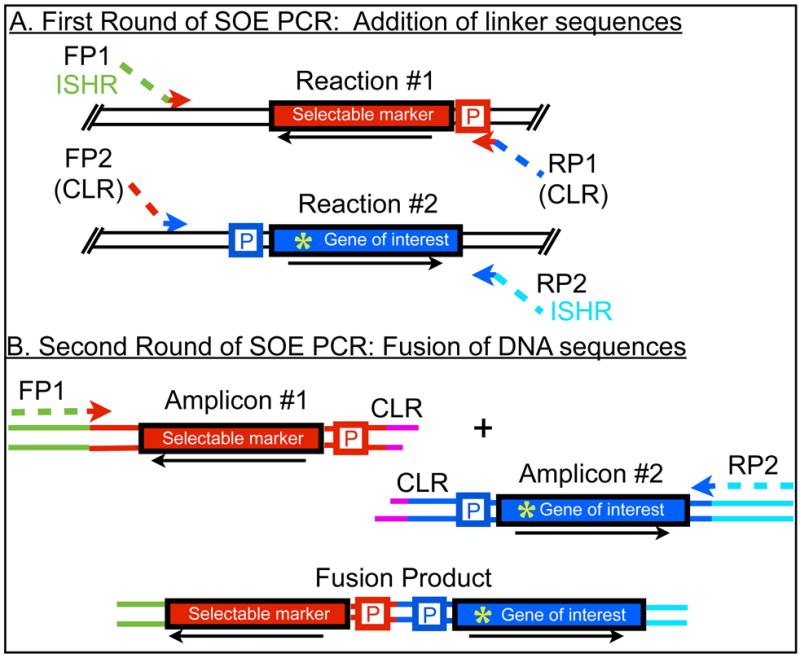
A. First round of SOE PCR: addition of linker sequences. In these reactions (#1 and #2), primers add a common linker region (CLR) to the genes to be fused. An insertion site homology region (ISHR) is also added to the amplicons of the first reaction; this sequence will later direct the insertion of the gene fusion at a specific locus on the bacterial chromosome. B. Second round of SOE PCR: fusion of DNA sequences. In this reaction, the fusion product is generated, relying on the CLR to anneal the two originally noncontiguous sequences. Genes are depicted as red or blue rectangles and flanking sequences as lines. The CLR is shown in pink lines and is common to both amplicons to be fused. P indicates the promoters of each gene and the arrow the orientation of transcription. FP stands for forward primer and RP stands for reverse primer. Green asterisk indicates a point mutation in the gene of interest.
In this report we specifically describe the construction of chromosomal dinB alleles encoding derivatives of DNA Polymerase IV, an evolutionarily conserved specialized DNA polymerase (Friedberg et al., 2006). dinB templates containing the point mutations were available on low-copy number plasmids, which were generated by site-specific mutagenesis of the wild-type sequence using the GeneTailor™ Site-Directed Mutagenesis System (Invitrogen). SOE PCR was utilized to fuse the dinB sequences of interest to a selectable marker, the cat gene, enabling selection for chloramphenicol resistant (CmR) recombinants. Flippase Recognition Target (FRT) sites, which permit the excision of the resistance marker by the site-specific recombinase, flippase (FLP), flank the cat gene (Datsenko and Wanner, 2000). Primer design was carried out such that the native cat and dinB promoters, as well as FRT sites upstream and downstream of cat, (Figure 1) were included in the fusion sequences. Replacement of wild-type chromosomal genes with the amplified fusion sequences relied on the lambda Red recombinase system described by Datsenko and Wanner (2000).
Gradient PCR cycle conditions were employed for both amplification reactions (Dieffenbach and Dveksler, 1995) and primer sequences are listed in Table 1. Primers added approximately 50 bp at the 5’ end and 80 bp at the 3’ end of the fusion sequences. These regions, termed insertion site homology regions (ISHR), are homologous to genes located upstream and downstream of chromosomal dinB (mhbA and yafN, respectively) and direct the replacement of the wild-type chromosomal dinB sequence at this locus. Primers also included a 54 bp region of dinB’s native promoter, which served as the CLR. Total volume of the PCR reactions was 25 μL, using GoTaq Master Mix (Promega), and 1 μL each of a forward and reverse primer at 10μM. Template for the first round of amplification was diluted plasmid DNA (~2μg/mL). After the first amplification reaction, PCR products were gel purified to a final concentration of about 20 ng/μL. Two microliters of each amplicon were used as template for the second round of SOE PCR, and the obtained full-length size product was verified by agarose gel electrophoresis. Examples of all PCR products obtained from the first and second amplification reactions are shown in Figure 2.
Table 1.
SOE PCR Primer Sequences
| Details | Sequence | |
|---|---|---|
| FP1 | Adds mhaB ISHR to 5' end; amplifies cat gene | 5'CTGGTGCAAAAGCTGGATAAGCAGCAG |
| GTGCTTTCGCAGCGAACGCGTTAATGAGC | ||
| GATTGTGTAGGCTGG 3' | ||
| RP1 | Adds dinB sequence as common linker; amplifies cat gene | 5'CTGGTAAAGTATACAGTGATTTCAGGGT |
| TTGAGAAATGCGTAAAGATTCAGCATGCC | ||
| ATGGTCCATATGAATATCCTCC 3' | ||
| FP2 | Amplifies dinB with promoter; contains linker sequence | 5'ATGCTGAATCTTTACGCATTTCTCAAACC |
| CTGAAATCACTGTATACTTTACCAGTGT | ||
| TGAGAGGTGAGCAATGC 3' | ||
| RP2 | Amplifies dinB; adds yafN ISHR at to 3' end | 5'GACCGATTTTTCAGCGAGAATTCGATGC |
| ATACAGTGATACCCTCATAATAATGCACAC | ||
| CAGAATATACATAATAGTATAC 3' |
Figure 2. Amplification of DNA sequences using gradient cycling conditions.
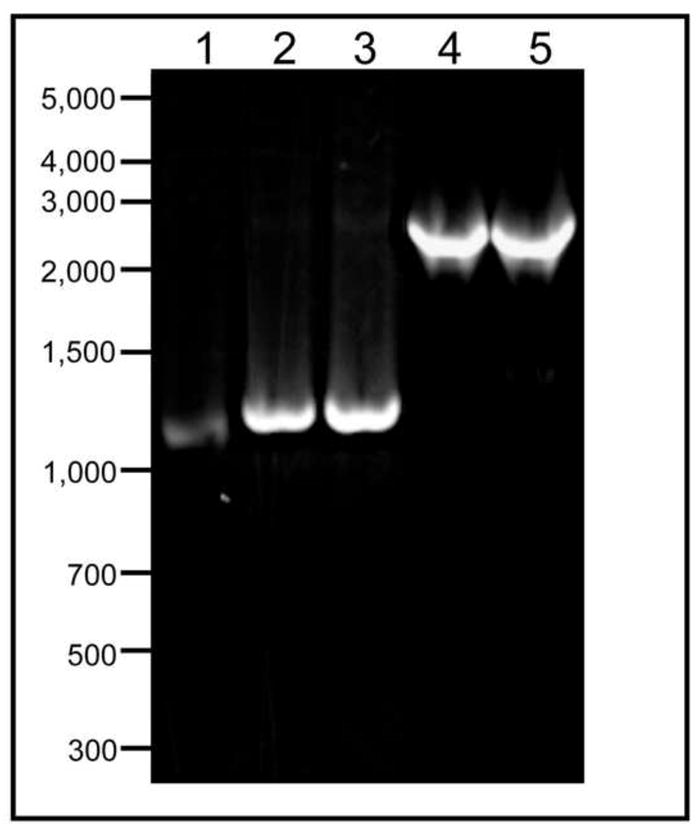
Amplification of cat-CLR, lane 1; dinB(D103N)-CLR, lane 2; dinB(Y79A)-CLR, lane 3; dinB(D103N)-cat fusion sequence, lane 4;, and dinB(Y79A)-cat gene fusion, lane 5. A gradient cycle was used in both phases of SOE PCR. Products were separated in a 1% agarose gel in Tris Acetate Buffer (Ausubel et al., 2001). Lanes 1–5 contain 3μL of DNA.
The obtained fusion sequences were introduced into ΔdinB::KanR JW0221 cells (Baba et al., 2006) by electroporation at 1.8 mV for 5 msec following standard protocols (Ausubel et al., 2001). Cells contained the plasmid expressing lambda Red proteins (pKD46) and were made electrocompetent as previously described (Datsenko and Wanner, 2000). After incubation for 1 to 1.5 hours at 37°C to permit cat gene expression, cells were plated on LB agar containing 20 μg/mL of chloramphenicol (Cm), and incubated for 20 hours at 37°C. Cm resistant (CmR) colonies were purified on the same plates and screened for sensitivity to kanamycin (KanS), the marker replacing dinB in JW0221. Since the dinB-cat fusion products contained sequences homologous to regions upstream of the dinB promoter and downstream of the dinB open reading frame (ORF), recombination into the chromosome replaces the KanR sequence with the dinB fusion products containing the desired point mutations (Figure 3). Therefore, colonies that were both CmR and KanS were examined for unique junction sequences by standard PCR to confirm that the replacement had taken place at the desired location on the chromosome. DNA sequencing of a PCR amplicon spanning the dinB gene and promoter region confirmed the presence of the desired dinB allele on the chromosome. All three CmR colonies analyzed were also KanS because the KanR gene was replaced with the dinB(D103N) allele in JW0221 (Wagner et al., 1999). However, just 2 of 11 CmR colonies analyzed were also KanS when introducing the dinB(Y79A) allele (Jarosz et al., 2009) on the chromosome. It is possible that the frequency of obtaining the desired point mutation on the chromosome depends on the efficiency of the SOE PCR reactions. Nevertheless, the desired alleles were recombined into the chromosome in the time frame of a week. The dinB-cat fusion was transduced (Miller, 1972) from the JW0221 strain into other E. coli strains with P1 virulent phage.
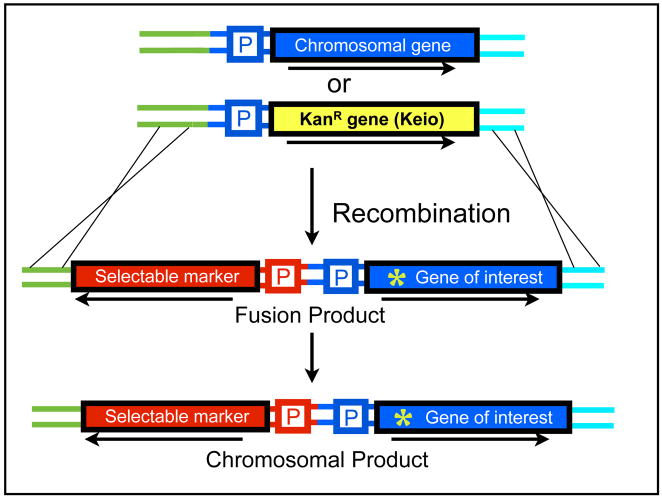
Figure 3. Schematic of the recombination event that replaces the chromosomal gene with the fusion construct.
The reaction relies on homologous sequences in the fusion constructs and chromosomal regions flanking the gene to be deleted, depicted as a blue rectangle. Gene deletions of the Keio collection may also be used (shown as a yellow rectangle), since the flanking regions of deleted genes are still present and can be used as the ISHR. Green asterisk indicates a point mutation in the fusion construct. Cells in which the recombination has occurred are resistant to the selectable marker and bear the point mutation of interest. If the Keio strain is used, recombinants would also be resistant to kanamycin.
Here we have shown, through use of SOE-LRed, that chromosomal genes may be replaced with sequences encoding variant gene products of interest. Although we have used chloramphenicol, any selectable marker may be chosen. Furthermore, use of the readily available KanR Keio collection (Baba et al., 2006), allows for positive selection throughout the process. Gene replacement permits the removal of the KanR marker sequence and insertion of the selectable marker linked to the gene of interest. Although we used dinB, the same experiment can be carried out with essential genes. Depending on the efficiency of the SOE PCR reactions, it may be necessary to screen a larger number of recombinant colonies for appropriate junctions with PCR.
This technique is very powerful because of its simplicity and time efficiency. It permits the in vivo study of strains encoding genes with a variety of point mutations, and allows for their expression at physiologically relevant concentrations. The expression of genes of interest at chromosomal levels facilitates genetic analyses such as structure-function, gene dominance, alteration of activity, and studies of gene expression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. J. Wiley; New York: 2001. [Google Scholar]
- Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2:2006 0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieffenbach CW, Dveksler GS. PCR primer: a laboratory manual. Cold Spring Harbor Laboratory Press; Plainview, N.Y: 1995. [Google Scholar]
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA repair and mutagenesis. ASM Press; Washington, D.C: 2006. [Google Scholar]
- Gordon GS, Sitnikov D, Webb CD, Teleman A, Straight A, Losick R, Murray AW, Wright A. Chromosome and low copy plasmid segregation in E. coli: visual evidence for distinct mechanisms. Cell. 1997;90:1113–1121. doi: 10.1016/s0092-8674(00)80377-3. [DOI] [PubMed] [Google Scholar]
- Heermann R, Zeppenfeld T, Jung K. Simple generation of site-directed point mutations in the Escherichia coli chromosome using Red®/ET® Recombination. Microbial Cell Factories. 2008;7:14. doi: 10.1186/1475-2859-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Jarosz DF, Cohen SE, Delaney JC, Essigmann JM, Walker GC. A DinB variant reveals diverse physiological consequences of incomplete TLS extension by a Y-family DNA polymerase. Proc Natl Acad Sci U S A. 2009;106:21137–21142. doi: 10.1073/pnas.0907257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link AJ, Phillips D, Church GM. Methods for generating precise deletion and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J Bacteriol. 1997;179:6228–6237. doi: 10.1128/jb.179.20.6228-6237.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. Experiments in molecular genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y: 1972. [Google Scholar]
- Murphy KC, Campellone KG, Poteete AR. PCR-mediated gene replacement in Escherichia coli. Gene. 2000;246:321–330. doi: 10.1016/s0378-1119(00)00071-8. [DOI] [PubMed] [Google Scholar]
- Wagner J, Gruz P, Kim SR, Yamada M, Matsui K, Fuchs RP, Nohmi T. The dinB gene encodes a novel E. coli DNA polymerase, DNA pol IV, involved in mutagenesis. Mol Cell. 1999;4:281–286. doi: 10.1016/s1097-2765(00)80376-7. [DOI] [PubMed] [Google Scholar]