The concept of an endogenous descending analgesia system was born of Reynolds’ dramatic discovery that electric stimulation was capable of producing profound analgesia [40]. In Reynolds’ study, rats failed to protest to a surgical laparotomy so long as a region in the dorsal midbrain, close to the periaqueductal gray, was electrically stimulated. Even as midbrain stimulation suppressed escape, the normal behavioral reaction to surgical incision, animals continued to startle in response to unexpected auditory or looming visual stimuli. This dichotomy is evidence for sensory suppression rather than motor immobilization. Reynolds’ demonstration of stimulation-produced surgical analgesia was therefore viewed with great excitement as a potentially novel approach to treating patients with intractable pain. In short order, the search for ways to utilize this endogenous system for the betterment of pain patients was launched in multiple laboratories and clinics. Neurosurgeons tried, with some success, to provide pain relief to patients with intractable pain using stimulation in either the periaqueductal or periventricular gray [25;26;31;44;45]. The discovery several years later of endogenous opioid peptides and the distribution of these opioids within the brainstem regions implicated in endogenous analgesia led to the idea that morphine’s efficacy derives from its ability to engage the endogenous analgesia system. Fueled by a concern for the tolerance and side effects caused by morphine, a search for compounds that specifically and cleanly engage endogenous analgesia pathways began. That quest, which has led to incremental improvements in pain management but has not produced a revolutionary breakthrough, continues today. Unfortunately, as I argue in this review, it may be that analgesia simply cannot be divorced from so-called side effects.
The fulcrum of the canonical endogenous analgesia pathway is the medullary raphe magnus
During the 1970s and early ‘80s, the research of Allan Basbaum, Jean-Marie Besson, Howard Fields, John Liebeskind, Barry Sessle, Linda Watkins and several others revealed the basic anatomy and physiology of the analgesia-producing circuitry engaged by brainstem stimulation and supraspinal opioids [4;11]. The resulting canonical pathway involves projections from neurons in the midbrain periaqueductal gray to neurons of the medullary raphe magnus which in turn project to the spinal and trigeminal dorsal horn. Activation of neurons in both the periaqueductal gray and raphe magnus suppresses both the responses of dorsal horn cells to noxious stimulation and nocifensive motor reactions. Fundamentally, it is important to note that this pathway descends to contact nociceptive laminae of the dorsal horn where neurons receive direct nociceptor input from the periphery. Thus, the output from raphe magnus modulates sensory input at the initial central synapses carrying nociceptive information.
Howard Fields propelled our understanding of the cellular basis of descending analgesia forward when he described the on and off cell classes of the raphe magnus and proposed a basic functional circuit involving these cell types [4;12;13]. Not only does Fields’ fundamental proposal endure in its nearly original form to this day, more importantly, the framework of on and off cells has proved spectacularly heuristic over the years. Indeed, experiments aimed at selectively lesioning on cells have revealed that this cell population within raphe magnus is necessary for the development and maintenance of certain chronic pain conditions including the neuropathic pain that results from spinal nerve ligation [38;39]. Thus, raphe magnus is capable of facilitating as well as suppressing nociceptive transmission. The recognition that nociceptive transmission is bi-directionally modulated by raphe magnus led to the renaming of endogenous analgesia to the more neutral term, endogenous pain modulation.
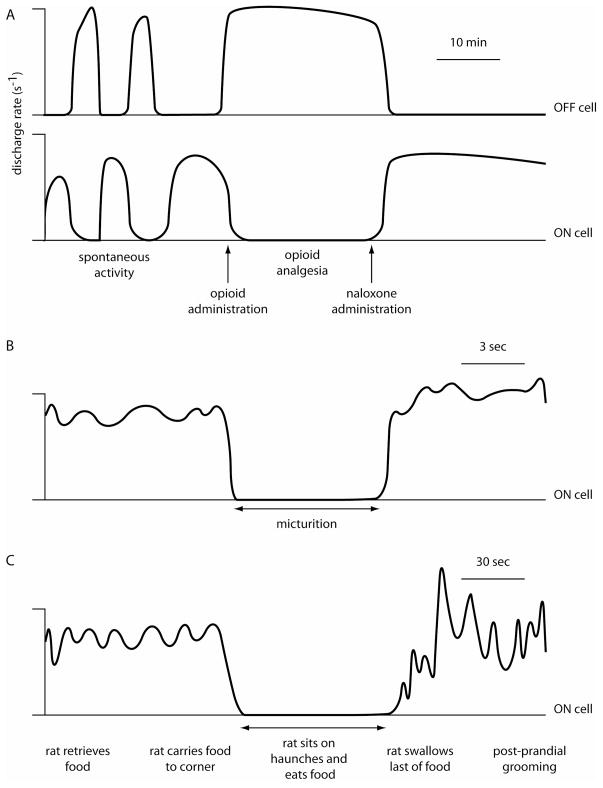
The power of Fields’ model derives from the consistent finding that a cell’s response to noxious stimulation predicts that cell’s response to an analgesic dose of an opioid in the anesthetized rat [3]. Thus, after easily identifying on and off cells characterized by their excitatory and inhibitory responses to noxious stimulation, respectively [12], the experimenter immediately knows that on cells will be inhibited by opioids and off cells excited. Using this physiological shortcut, numerous studies have led to the conclusion that on cell firing is pro-nociceptive and off cell firing is antinociceptive. Under baseline conditions in the anesthetized rat, on cells fire together, off cells fire together, and on and off cells fire in alternating bursts [24]. In response to an analgesic manipulation such as morphine administration, off cells fire continuously and on cells fall silent (Figure 1A) [3;6]. The model holds that it is the continuous discharge of off cells which suppresses nociceptive transmission so that “pain” is suppressed. Conversely, an increase in on cell discharge is thought to be the substrate for the hyperalgesia and allodynia produced by descending pain modulatory pathways.
Figure 1.
Diagrammatic representations of idealized on and off cell firing during opioid analgesia (A) and micturition (B) in the anesthetized rat and during eating in the unanesthetized rat (C). Note that cell activity is more variable in the unanesthetized condition.
Developing an ethological understanding of pain modulatory circuits requires the study of behaving animals
Even a mechanistic understanding of descending pain modulatory circuits begs the question of what the endogenous pain modulatory system “does” for a regular animal living a regular life. By viewing this problem from an ethological and ecological vantage point, the functions served by pain modulation upon which evolution could possibly operate can be identified, or at least postulated. Here I argue that neither chronic pain nor endogenous release of analgesia-producing amounts of endogenous opioid occur with sufficient frequency to be the subject of natural selection.
Selections from The Study of Instinct, by the great naturalist Niko Tinbergen, illustrate the rarity of animals’ experiencing chronic, persistent pain in the wild [41]. First, Tinbergen describes escape behavior in response to remote stimuli that signal a potential but not actual predator. In Tinbergen’s account, visual and chemical stimuli elicit hiding and escape and this escape occurs in the absence of any noxious input and indeed without any physical contact. Second, Tinbergen noted that “‘fighting’ in animals usually consists of threatening or bluff” rather than actual physical engagement and injury. The predominance of posturing over harmful and wound-producing fighting within the animal kingdom is now well accepted in the field of animal behavior. The uncommonness of injurious animal altercations results in a paucity of wild animals with painful disability [23]. In essence, in the rare instances of an intense physical fight, the loser dies after a short interval so that wild animals seldom survive with chronic pain. These considerations suggest that the way in which the nervous system reacts to chronic pain is a by-product of evolution related to other selection pressures rather than the result of targeted evolution.
If an analgesic concentration of endogenous opioids were released under natural conditions then administration of an opioid receptor antagonist such as naloxone would be expected to increase nociceptive responsiveness. However, naloxone administration to normal human volunteers has no effect on pain elicited by ischemia or cold water immersion [18–20]. Although naloxone has no effect on the pain report evoked by ischemia, it does adversely affect mood and alter thermoregulation, hormonal release and resting blood pressure [18]. In support of the idea that physiological stress may facilitate endogenous opioid release, runners showed naloxone-sensitive elevations in the pain report elicited by ischemia or cold water immersion as well as in positive mood following a 6-mile run [27]. In the same individuals, the pain report evoked by a noxious thermal stimulus applied to the skin, similar to the stimulus used in many animal experiments, was unaltered. Thus, while endogenous opioids may be released under natural conditions, they are unlikely to suppress the pain evoked by noxious cutaneous stimulation. Thus, it appears that the two known triggers for raphe magnus engagement – chronic pain and analgesic levels of opioids – are rare events in the wild. In order to discover the natural circumstances that would lead to the activation of descending pain modulatory circuits and the adaptive functions of pain modulation in day-to-day life, cellular recordings in behaving animals are clearly needed.
One additional point is worth mentioning here. The raphe magnus is located in the central core of the medulla. This prime location within “brain real estate” raises the intriguing question, “could pain modulatory circuits, like neighboring circuits that regulate breathing, balance, and blood pressure, serve a daily function fundamental to all animal life?”.
Raphe magnus neurons respond to unexpected sensory stimuli in unanesthetized animals
On and off cells are present, active, and recognizable in the unanesthetized rodent [30;37]. Yet there are differences in the response profiles of on and off cells in anesthetized and unanesthetized animals. In the presence of most general anesthetics, on and off cells only respond consistently to noxious stimulation [29]. In contrast, in the unanesthetized animal, on and off cells respond similarly to innocuous and noxious stimulation alike [30;37]. This somatosensory response profile coupled with responses to unexpected sounds suggest that raphe magnus cells respond to unexpected events signaled by any modality. In other words, the response profile of raphe magnus neurons is coupled more to meaning than to stimulus characteristics. For example, lightly stroking the front paw ventrum with a soft brush excites on cells and inhibits off cells. Yet when the rat grooms himself and essentially presents a very similar – in physical characteristics - brush stimulus to the paw, on and off cells do not respond. A parsimonious interpretation of these findings is that raphe magnus neurons respond to stimuli that signal unexpected events which themselves may be harbingers of future danger. Thus the adequate stimulus for raphe magnus neurons is an unexpected somatosensory input regardless of whether it is innocuous or noxious.
The activity of raphe magnus neurons during critical behaviors mimics patterns observed after opioid administration
In order to understand the role of pain modulation in daily animal life, a useful strategy is to look for naturally-occurring circumstances when the discharge of on and off cells resembles the pattern observed after morphine administration. Put another way, are there behaviors or conditions when on cells are silent and off cells are continuously active as they are after morphine is administered to anesthetized animals? To date, three such behaviors have been identified: sleeping, urine voiding, and ingesting [1;14;30]. During slow wave sleep, off cells fire continuously and on cells are inactive [30]. Every microarousal from slow wave sleep, marked by slow wave desynchronization and the appearance of muscle activity, is accompanied by a pause in off cell activity and a burst in on cell discharge. Similarly, during the seconds – about 6 on average – that a rat requires to empty its bladder, off cells are active and on cells inactive (Figure 1B) [1]. Finally, off cells are active and on cells inactive during eating (Figure 1C) and drinking bouts [14]. Thus, during the three critical behaviors of sleeping, voiding and ingesting, on and off cells appear as though “they just got a shot of morphine”.
Raphe magnus mediates the suppression of pain reactions during ingestion
To investigate whether analgesia occurs during natural behaviors accompanied by off cell activity and on cell inactivity, we examined the motor reactions to noxious stimuli during sleeping, voiding and ingestion [1;14;33]. Here, I focus on findings obtained during ingestion, the behavior for which the roles of on and off cells have been most extensively studied. Yet, findings obtained during sleep and voiding are similar but incomplete.
While eating, rats either do not respond to a noxious thermal stimulus or respond at a significantly delayed latency [14;15]. In addition, rats who normally lick their paw after a noxious thermal stimulus is applied to the hindpaw rarely do so when the stimulus is applied during eating [15]. These results are mirrored by similar findings in a range of animals including cat [7], chicken [43] and mollusk [8;16], suggesting that reduced responsiveness during eating is a phylogenetically conserved phenomenon.
Over the years, we have compared the effects of all manner of stimuli applied to rats either during quiet waking or during eating. As is the case for noxious stimuli, rats are far less responsive to innocuous stimuli while ingesting than when sitting quietly or grooming. For example, an awake rat that is not eating tries to escape the “wind” blown from a miniature fan and can be essentially chased around the cage by moving the fan (Ama Thrasher, unpublished observations). However, when that same rat is eating, he remains in the same spot and shows no sign that he even feels or is bothered by the fan’s effects. The reduced sensory responsiveness during ingestion clearly does not depend on appetite as animals were always fed ad libitum and had access to chow throughout all experiments. Furthermore, the latency to withdraw from a noxious stimulus is significantly lengthened even during ingestion of water that is delivered directly into the oral cavity [15]. Thus, suppression of nocifensive reactions does not require any appetitive behavior on the part of the rat.
Confirmation that nocifensive withdrawal latencies increase during ingestion is consistent with, but not conclusive evidence that raphe magnus neurons are involved in the analgesia that accompanies ingestion. To directly test whether the suppression of nocifensive reactions during ingestion depends upon the raphe magnus, raphe magnus was inactivated by muscimol microinjection during quiet waking and during ingestion of chow, chocolate, or intraorally-delivered water [14;15]. When raphe magnus was inactivated, rats withdrew from noxious stimulation at the same short latencies during quiet waking and during ingestion. In contrast when saline was microinjected into raphe magnus, rats withdrew at longer latencies during ingestion than during quiet waking. This demonstrates that activity within raphe magnus is necessary for the suppression of nocifensive reactions that accompanies ingestion.
Raphe magnus neurons serve to protect food consumption from interruption
Eating consists of two fairly independent phases: the appetitive and consumptive phases [5;42]. The appetitive phase of eating involves seeking and retrieving food under the control of forebrain circuits including those that signal appetite. In contrast, food consumption depends critically on brainstem circuits, albeit ones that are modified by forebrain circuits, and occurs automatically when palatable food is placed in the mouth [21;22]. Changes in on and off cell discharge observed during eating are restricted to the phase of food consumption and do not accompany food retrieval [14].
In trying to understand the adaptive value of sensory suppression during eating, we consider the potential benefits resulting from pairing a reduction in nociceptive sensitivity with food consumption from an ecological and evolutionary perspective. A reduced sensitivity to noxious stimulation during ingestion operationally serves to increase the likelihood that an animal consumes available food at hand. In other words, by suppressing reactions to novel stimuli during ingestion, an animal is less likely to become distracted and not consume food retrieved at some measurable cost to the animal. Indeed, in our experiments once a rat retrieved a chocolate chip, he always consumed it either in one uninterrupted eating bout (n=912.5 chips) or after a brief interruption (n=31). Since rats did not switch midstream from food consumption to other behaviors, we can view sensory suppression during ingestion as a mechanism of enforcing action selection of feeding [34].
Throughout the history of animals on earth, food availability has been unpredictable and labile. In conditions of uncertain food availability, consumption of food which is automatic, brainstem-driven, and independent of appetite helps to protect against starvation. For example, the automaticity of food consumption prevents a carnivore from killing a prey animal and then walking away and “deciding” not to eat it. Unfortunately, in modern urban society where energy-dense food is widely available at low cost, the same biological mechanism that protected against starvation in previous eras now promotes obesity [5].
Pain modulation does not occur in isolation
As mentioned above, neurosurgeons began stimulating in the periaqueductal gray of patients with intractable pain shortly after Reynolds’ demonstration of stimulation-produced analgesia [25;26;31;44;45]. Yet, the utility of such stimulation was limited by a number of unwanted effects that stimulated patients experienced along with pain relief. Examples of the uncomfortable symptoms include persistent headache, incontinence or urinary retention, confusion, hypertension and dyspnea. The conclusion that no brain region promotes analgesia and analgesia alone is supported by an important series of experiments in unanesthetized rats. Jean-Louis Oliveras and colleagues stimulated throughout the large extent of the periqueductal gray in search of a site where stimulation yielded “pure analgesia” [9;10]. Stimulation in one small region within the caudal ventrolateral periqueductal gray came the closest to being a source of analgesia unaccompanied by other obvious behavioral effects. However, since these experiments, we have learned that stimulation within the caudal ventrolateral periqueductal gray produces a number of changes in autonomic variables that were not measured by Oliveras and colleagues [2]. Thus, it appears that the promise of an endogenous circuit dedicated to analgesia, originally raised by Reynolds, is not fulfilled in its purest and most clinically desirable form. Instead, sensory modulation that can tilt towards analgesia is accompanied by changes in behavioral state, movement, and homeostasis.
The inevitable weave of homeostasis and pain is beautifully illustrated by a recently described pain syndrome called familial episodic pain syndrome [28]. Familial episodic pain syndrome results from a point mutation in the gene encoding the TRPA1 receptor. Individuals with familial episodic pain syndrome experience roughly 90 min-long episodes of unbearable pain that are triggered by hunger, cold, exercise, or illness. Several aspects of familial episodic pain syndrome, found in four generations of a single Columbian family, are noteworthy as they dramatically illustrate the integrative nature of pain. First, the triggers for painful attacks are not somatic but rather challenges to homeostasis. Second, the pain is not described in sensory terms such as aching, lancinating, burning but in largely emotional terms such as making life bitter (far more poetic sounding in the original Spanish: que amarga la vida), unbearable (terriblemente molesto), heavy and terrifying. Third, homeostatic changes including alterations in vasomotion, sweating, and nausea accompany the emotional and affective experience of pain. The threads of homeostasis and of the pain experience knit together the triggers, perception, and manifestations of familial episodic pain syndrome.
Homeostatic challenges change the pain experience. In familial episodic pain syndrome, attacks of pain are brought on by hunger and staved off by eating. This is a particularly dramatic example of pain facilitated by a homeostatic challenge. A more common example of this same phenomenon is the now often replicated finding that sleep deprivation or poor sleep exacerbate the experience of chronic pain on the following day [35;36].
Unlike other sensory modalities, pain is never experienced as emotionally neutral. Pain invariably has emotional content. This truism has been codified in the modern formulation that pain consists of sensory-discriminative and emotional affective components. The affective component of pain not only is experienced emotionally but it is this component that people “care” about, which drives sufferers to seek medical help, and which is the target of analgesic pharmaceuticals.
Homeostatic challenges can be experienced similarly to pain. Clear examples of the blurry distinction between pain and a homeostatic challenge abound. Dyspnea is experienced very similarly to how pain is experienced [17]. Further, similar regions of the brain receive increased blood flow after dyspnea and somatic pain. Insufficient perfusion of cardiac vessels produces a strongly uncomfortable perception of heavy chest pressure, typically known as angina, and is experienced as painful and emotionally aversive. Extreme hunger or thirst or deep chill are challenges to homeostasis that produce a curiously imperative, to borrow from Sherrington’s phraseology for pain, unpleasant reaction, much as pain does.
Homeostatic changes invariably accompany pain
Virtually every pain-evoking stimulus also evokes homeostatic adjustments. Even simple cuts and bruises elicit changes in vasomotion. Vascular headache is marked by the presence of nausea and anorexia. Superficial pain typically elicits a pressor response, tachycardia and tachypnea while the response to deep pain is more varied but often dominated by parasympathetic activation. Some pains are dominated by feelings that are not signaled by nociceptors such as nausea, photophobia, air hunger, and a racing heartbeat.
It is instructive to consider what life would be like without the link between pain and homeostasis. Consider the famous lion attack on the explorer and missionary David Livingstone. Livingstone shot a lion and then, not surprisingly, was attacked by a very angry lion [32]. In his account from 1857, Livingstone describes one of the most pure examples of analgesia, “a sort of dreaminess in which there was no sense of pain nor feeling of terror, although quite conscious of all that was happening”. As Livingstone does not describe any feeling of great panic or arousal, it is surprising that he lives to tell his tale. Without sympathoexcitatory and motor arousal, how could Livingstone escape his predicament? The answer is that he did not; rather he survived because another’s actions saved him. It is likely that, without the actions of his companion, dreamy Livingstone would have died, feeling no pain. This story highlights the importance of integrating motor and autonomic arousal with sensory suppression so that complex escape and protective behaviors can be accomplished without overwhelming pain and affect. In sum, throughout evolutionary history, it has been highly advantageous to couple physiological changes in breathing, blood pressure, posture, and so on with both pain and pain relief. The unfortunate corollary of this coupling is that the quest for pure pain relief may be in vain.
Acknowledgments
The author thanks Dr. Kevin Hellman for helpful discussions and comments on the manuscript. The author has no conflicts of interest related to this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Baez MA, Brink TS, Mason P. Roles for pain modulatory cells during micturition and continence. J Neurosci. 2005;25:384–394. doi: 10.1523/JNEUROSCI.3536-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandler R, Carrive P, Zhang SP. Integration of somatic and autonomic reactions within the midbrain periaqueductal grey: viscerotopic, somatotopic and functional organization. Prog Brain Res. 1991;87:269–305. doi: 10.1016/s0079-6123(08)63056-3. [DOI] [PubMed] [Google Scholar]
- 3.Barbaro NM, Heinricher MM, Fields HL. Putative pain modulating neurons in the rostral ventral medulla: reflex-related activity predicts effects of morphine. Brain Res. 1986;366:203–210. doi: 10.1016/0006-8993(86)91296-5. [DOI] [PubMed] [Google Scholar]
- 4.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 5.Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 6.Brink TS, Hellman KM, Lambert AM, Mason P. Raphe magnus neurons help protect reactions to visceral pain from interruption by cutaneous pain. J Neurophysiol. 2006;96:3423–3432. doi: 10.1152/jn.00793.2006. [DOI] [PubMed] [Google Scholar]
- 7.Casey KL, Morrow TJ. Nocifensive responses to cutaneous thermal stimuli in the cat: stimulus-response profiles, latencies, and afferent activity. J Neurophysiol. 1983;50:1497–1515. doi: 10.1152/jn.1983.50.6.1497. [DOI] [PubMed] [Google Scholar]
- 8.Davis WJ, MGJPJM The behavioral hierarchy of the mollusk Pleurobranchaea. I. The dominant position of the feeding behavior. Journal of Comparative Physiology. 1974;90:207–224. [Google Scholar]
- 9.Fardin V, Oliveras JL, Besson JM. A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. I. The production of behavioral side effects together with analgesia. Brain Res. 1984;306:105–123. doi: 10.1016/0006-8993(84)90360-3. [DOI] [PubMed] [Google Scholar]
- 10.Fardin V, Oliveras JL, Besson JM. A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. II. Differential characteristics of the analgesia induced by ventral and dorsal PAG stimulation. Brain Res. 1984;306:125–139. doi: 10.1016/0006-8993(84)90361-5. [DOI] [PubMed] [Google Scholar]
- 11.Fields HL, Basbaum AI. Brainstem control of spinal pain-transmission neurons. Annu Rev Physiol. 1978;40:217–248. doi: 10.1146/annurev.ph.40.030178.001245. [DOI] [PubMed] [Google Scholar]
- 12.Fields HL, Bry J, Hentall I, Zorman G. The activity of neurons in the rostral medulla of the rat during withdrawal from noxious heat. J Neurosci. 1983;3:2545–2552. doi: 10.1523/JNEUROSCI.03-12-02545.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields HL, Vanegas H, Hentall ID, Zorman G. Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature. 1983;306:684–686. doi: 10.1038/306684a0. [DOI] [PubMed] [Google Scholar]
- 14.Foo H, Mason P. Sensory suppression during feeding. Proc Natl Acad Sci U S A. 2005;102:16865–16869. doi: 10.1073/pnas.0506226102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foo H, Mason P. Analgesia accompanying food consumption requires ingestion of hedonic foods. J Neurosci. 2009;29:13053–13062. doi: 10.1523/JNEUROSCI.3514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillette R, Huang RC, Hatcher N, Moroz LL. Cost-benefit analysis potential in feeding behavior of a predatory snail by integration of hunger, taste, and pain. Proc Natl Acad Sci U S A. 2000;97:3585–3590. doi: 10.1073/pnas.97.7.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gracely RH, Undem BJ, Banzett RB. Cough, pain and dyspnoea: similarities and differences. Pulm Pharmacol Ther. 2007;20:433–437. doi: 10.1016/j.pupt.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grevert P, Albert LH, Inturrisi CE, Goldstein A. Effects of eight-hour naloxone infusions on human subjects. Biol Psychiatry. 1983;18:1375–1392. [PubMed] [Google Scholar]
- 19.Grevert P, Goldstein A. Effects of naloxone on experimentally induced ischemic pain and on mood in human subjects. Proc Natl Acad Sci U S A. 1977;74:1291–1294. doi: 10.1073/pnas.74.3.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grevert P, Goldstein A. Endorphins: naloxone fails to alter experimental pain or mood in humans. Science. 1978;199:1093–1095. doi: 10.1126/science.343250. [DOI] [PubMed] [Google Scholar]
- 21.Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 22.Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 1978;143:281–297. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- 23.Hall KRL. Aggression in monkey and ape societies. In: Carthy JD, Ebling FJ, editors. The natural history of aggression. London: Academic Press; 1964. [Google Scholar]
- 24.Heinricher MM, Barbaro NM, Fields HL. Putative nociceptive modulating neurons in the rostral ventromedial medulla of the rat: firing of on- and off-cells is related to nociceptive responsiveness. Somatosens Mot Res. 1989;6:427–439. doi: 10.3109/08990228909144685. [DOI] [PubMed] [Google Scholar]
- 25.Hosobuchi Y. The current status of analgesic brain stimulation. Acta Neurochir Suppl (Wien ) 1980;30:219–227. doi: 10.1007/978-3-7091-8592-6_27. [DOI] [PubMed] [Google Scholar]
- 26.Hosobuchi Y, Adams JE, Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science. 1977;197:183–186. doi: 10.1126/science.301658. [DOI] [PubMed] [Google Scholar]
- 27.Janal MN, Colt EW, Clark WC, Glusman M. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: effects of naloxone. Pain. 1984;19:13–25. doi: 10.1016/0304-3959(84)90061-7. [DOI] [PubMed] [Google Scholar]
- 28.Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, Marsh S, Woods CG, Jones NG, Paterson KJ, Fricker FR, Villegas A, Acosta N, Pineda-Trujillo NG, Ramirez JD, Zea J, Burley MW, Bedoya G, Bennett DL, Wood JN, Ruiz-Linares A. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron. 2010;66:671–680. doi: 10.1016/j.neuron.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung CG, Mason P. Physiological survey of medullary raphe and magnocellular reticular neurons in the anesthetized rat. J Neurophysiol. 1998;80:1630–1646. doi: 10.1152/jn.1998.80.4.1630. [DOI] [PubMed] [Google Scholar]
- 30.Leung CG, Mason P. Physiological properties of raphe magnus neurons during sleep and waking. J Neurophysiol. 1999;81:584–595. doi: 10.1152/jn.1999.81.2.584. [DOI] [PubMed] [Google Scholar]
- 31.Levy RM, Lamb S, Adams JE. Treatment of chronic pain by deep brain stimulation: long term follow-up and review of the literature. Neurosurgery. 1987;21:885–893. doi: 10.1227/00006123-198712000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Livingstone D. Missionary travels and researches in South Africa. New York: Harper & Brothers Publishers; 1857. [Google Scholar]
- 33.Mason P, Escobedo I, Burgin C, Bergan J, Lee JH, Last EJ, Holub AL. Nociceptive responsiveness during slow-wave sleep and waking in the rat. Sleep. 2001;24:32–38. doi: 10.1093/sleep/24.1.32. [DOI] [PubMed] [Google Scholar]
- 34.McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P. Subcortical loops through the basal ganglia. Trends Neurosci. 2005;28:401–407. doi: 10.1016/j.tins.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Moldofsky H. Sleep influences on regional and diffuse pain syndromes associated with osteoarthritis. Semin Arthritis Rheum. 1989;18:18–21. doi: 10.1016/0049-0172(89)90011-5. [DOI] [PubMed] [Google Scholar]
- 36.Moldofsky H. The significance of the sleeping-waking brain for the understanding of widespread musculoskeletal pain and fatigue in fibromyalgia syndrome and allied syndromes. Joint Bone Spine. 2008;75:397–402. doi: 10.1016/j.jbspin.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Oliveras JL, Martin G, Montagne J, Vos B. Single unit activity at ventromedial medulla level in the awake, freely moving rat: effects of noxious heat and light tactile stimuli onto convergent neurons. Brain Res. 1990;506:19–30. doi: 10.1016/0006-8993(90)91194-l. [DOI] [PubMed] [Google Scholar]
- 38.Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Jr, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- 41.Tinbergen N. The study of instinct. Clarendon Press; Oxford: 1989. [Google Scholar]
- 42.Watts AG. Understanding the neural control of ingestive behaviors: helping to separate cause from effect with dehydration-associated anorexia. Horm Behav. 2000;37:261–283. doi: 10.1006/hbeh.2000.1581. [DOI] [PubMed] [Google Scholar]
- 43.Wylie LM, Gentle MJ. Feeding-induced tonic pain suppression in the chicken: reversal by naloxone. Physiol Behav. 1998;64:27–30. doi: 10.1016/s0031-9384(98)00020-1. [DOI] [PubMed] [Google Scholar]
- 44.Young RF. Brain stimulation. Neurosurg Clin N Am. 1990;1:865–879. [PubMed] [Google Scholar]
- 45.Young RF, Kroening R, Fulton W, Feldman RA, Chambi I. Electrical stimulation of the brain in treatment of chronic pain. Experience over 5 years. J Neurosurg. 1985;62:389–396. doi: 10.3171/jns.1985.62.3.0389. [DOI] [PubMed] [Google Scholar]