Abstract
Trace eyeblink conditioning in which a conditioned stimulus and unconditioned stimulus are separated by a gap, is hippocampal dependent and can rescue new neurons in the adult dentate gyrus from death (e.g., Beylin et al., 2001; Gould et al., 1999). Tasks requiring more training trials for reliable expression of the conditioned response are most effective in enhancing survival of neurons (Waddell & Shors, 2008). To dissociate hippocampal-dependence from acquisition rate, we facilitated hippocampal-dependent trace eyeblink conditioning in two ways: a shorter trace interval and signaling the intertrial interval with a post-US cue. Trace conditioning with a shorter trace interval (250 msec) requires an intact hippocampus, and acquisition is faster relative to rats trained with a 500 msec trace interval (e.g., Weiss, et al., 1999). Using excitotoxic hippocampal lesions, we confirmed that eyeblink conditioning with the 250 or 500 msec trace interval is hippocampal dependent. However, training with the post-US cue was not hippocampal dependent. The majority of lesion rats in this condition reached criterion of conditioned responding. To determine whether hippocampal dependence is sufficient to rescue adult generated neurons in the dentate gyrus, rats were injected with BrdU and trained in one of the three trace eyeblink arrangements one week later. Of these training procedures, only the 500 msec trace interval enhanced survival of new cells; acquisition of this task proceeded slowly relative to the 250 msec and post-US cue conditions. These data demonstrate that rate of acquisition and not hippocampal dependence determines the impact of learning on adult neurogenesis.
Keywords: dentate gyrus, neurogenesis, trace eyeblink conditioning, learning
Introduction
The dentate gyrus of the hippocampus produces thousands of new neurons each day (Altman & Das, 1965; Cameron & McKay, 2001; Cameron, et al., 1993; Hastings & Gould, 1999; Kaplan & Hinds, 1977; Markakis & Gage, 1999; van Praag, et al., 2002). Many die within weeks, but survival can be enhanced by exposure to some learning tasks (e.g., Dayer, et al., 2003; Döbrössy et al., 2003; Gould, et al., 1999). Using the eyeblink procedure, our laboratory has demonstrated a relationship between the rate of learning and the survival of new cells (Waddell & Shors, 2008; Dalla et al., 2009). Tasks which require many trials are more effective in enhancing survival (Waddell & Shors, 2008). Similarly, animals that learn relatively slowly exhibit the largest increase in cell survival when the task is effective (Dalla et al., 2009; Döbrössy et al., 2003; Epp et al., 2007; Waddell & Shors, 2008). This pattern has also been noted in the hippocampal-dependent water maze procedure (Döbrössy et al., 2003; Epp et al., 2007).
The majority of learning procedures that enhance survival, such as trace eyeblink conditioning, also require an intact hippocampus for acquisition (Beylin et al., 2001; Gould et al., 1999; Leurner et al., 2006; Snyder et al., 2005; Waddell & Shors, 2008; Weiss et al., 1999). Ablation of adult neurogenesis does not disrupt all tasks that require an intact hippocampus (Jaholkowski et al., 2009; Shors et al., 2002) and hippocampal-dependent tasks do not always enhance survival (Epp et al., 2007; van Praag et al., 1999). We consistently observe enhanced survival following conditioning with a trace eyeblink procedure using a 500 msec gap between the CS and US. This task is both dependent on the hippocampus, and is slowly acquired relative to hippocampal-independent eyeblink conditioning tasks. The experiments presented here sought to facilitate trace conditioning while maintaining hippocampal dependence. To do this, we used two variants of trace eyeblink conditioning that produce faster conditioning than observed using our typical 500 msec trace conditioning procedure. First, rats were trained with a shorter, but hippocampal-dependent trace interval (i.e., 250 msec; Weiss et al., 1999). Second, a cue was presented after delivery of the US to signal the onset of the intertrial interval (Bolles et al., 1978; Mowrer & Lamoreaux, 1951). Both manipulations facilitated acquisition, and surprisingly, hippocampal-lesioned rats trained with the post-US cue did exhibit acquisition of the eyeblink CR. Permanent excitotoxic lesions of the hippocampus abolished acquisition of trace eyeblink conditioning using either the 500 msec or 250 msec trace interval as has been demonstrated (e.g., Walker & Steinmetz, 2008; Weiss et al., 1999). Only training with the 500 msec interval enhanced survival of adult generated cells. Together, these results suggest only tasks requiring many trials, or are more difficult, enhance survival of adult-generated cells. Further, insertion of a post-US cue attenuated the lesion-induced deficit. Precisely how this cue attenuates the lesion deficit in trace conditioning is not known. We hypothesize that the post-US cue reduces fear to the context, and therefore facilitates a discrimination between the trace interval and intertrial interval (Bolles et al., 1978; Kaplan & Hearst, 1982; Mowrer & Lamoreaux, 1951).
Methods
Subjects
Rats were obtained from a breeding facility at Rutgers University. Male Sprague-Dawley rats, between 60–70 days old at the time of BrdU administration or hippocampectomy were used. Rats were housed in groups of 3 until surgery. Following surgery, rats were housed alone in standard plastic ‘shoebox’ cages (44.5 cm long, 21.59 cm wide and 23.32 cm high). Rats had ad lib access to rat chow and water and were maintained on a 12 hr light-dark cycle. All experiments were conducted with full compliance to the rules and regulations specified by the PHS Policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals.
Surgery
Rats were anesthetized with sodium pentobarbital (50 mg/kg) and atropine (0.04 mg/kg). After being placed in the stereotaxic instrument, the scalp was cleaned with Betadine, and an incision was made. For hippocampal lesions, NMDA was infused at 6 sites per hemisphere for a total of 12 infusions were made at the following coordinates relative to bregma: AP: −2.5 mm, ML: ±1.6, DV: −3.8; AP: −4.2 mm, ML: ±2.6 mm, DV: −3.1; AP: −5.3 mm, ML: ±5.0 mm, DV: −5.9 mm; AP: −5.3 mm, ML: ±4.2 mm, DV: −3.4 mm; AP: −5.8 mm, ML: ±4.6 mm, DV: −6.1 mm; AP: −6.0 mm, ML: ± 5.6 mm, DV: −4.1 mm. The DV coordinate was calculated relative to the surface of the brain. A concentration of 20 μg/μl was used and at each injection site, and 0.35 μl of NMDA was infused at a rate of 0.5 μl/min. Infusions were made using a gastight Hamilton syringe with an inner diameter of 0.13 mm and an outer diameter of 0.483 mm. For sham lesions, the syringe was lowered, but no infusion was conducted. In preparation for eyeblink conditioning, all rats with the exception of naïve controls in Experiment 2 were implanted with 4 eyelid electrodes (insulated stainless steel wire, 0.005 in) through the upper eyelid (orbicularis oculi muscle). Rats receiving hippocampal lesions or sham surgeries were given a minimum of 2 weeks recovery before eyeblink conditioning began due to the extent of the lesion. Rats used in Experiment 2 were given a minimum of 4 days recovery following placement of the eyeblink headstage before BrdU administration.
Trace Eyeblink Conditioning
For eyeblink conditioning, headstages were connected to a cable that allowed free movement within the conditioning chamber. Twenty-four hours prior to any behavioral manipulation, rats were acclimated to the conditioning apparatus for 1 h. During this acclimation period, spontaneous blink rates for each rat were recorded. The following day, rats began conditioning. Rats were placed in the eyeblink conditioning chambers and were trained with the trace eyeblink conditioning procedure for 200 trials a day for 4 consecutive days (800 trials total). The CS was an 83 dB, 250 msec white noise. The US was a 100 msec periorbital shock (0.65 mA). The CS and US were separated by either a 500 msec trace interval (Trace 500) or a 250 msec trace interval (Trace 250), in which no stimuli were delivered. For the Trace 500 Cue condition, the CS and US were presented as described, with the addition of a 100 msec key light illumination. The onset of the key light was 500 msec following the offset of the US. The intertrial interval (ITI) was 25 ± 5 sec. To detect the occurrence of an eyeblink, the maximum EMG response occurring during a 250 msec prestimulus baseline recording period was added to four times its standard deviation. Responses that exceeded that value and were longer than 3 ms were considered eyeblinks. Eyeblinks were considered conditioned responses (CRs) if they began 250 ms prior to US onset. Eyeblink performance was calculated as the percentage of trials on which a CR was produced in response to a CS.
Histology
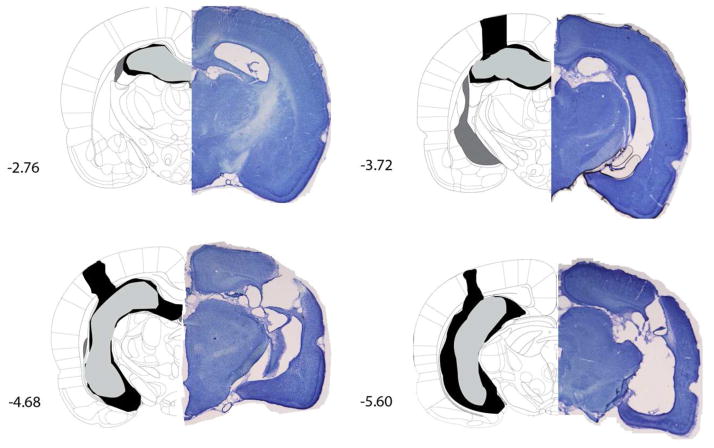
Following behavioral testing, rats were deeply anesthetized and perfused transcardially with 10% formalin. Brains were extracted and postfixed in a 10% formalin solution with 30% sucrose. Coronal sections (45 μm) throughout the rostrocaudal extent of the hippocampus were collected. Every 5th section was mounted onto gelatin coated slides and stained with cresyl violet. Only rats with bilateral damage to the dorsal and ventral hippocampus were included in data analysis (Figure 1). These criteria resulted in the following number of animals in each condition: Trace 250 Sham, n=8; Trace 250 Lesion, n=7; Trace 500 Sham, n=8; Trace 500 Lesion, n=7; Trace 500 Cue Sham, n=8; Trace 500 Cue Lesion, n=7.
Figure 1.
Rats were given excitotoxic lesions of the entire hippocampus. The drawings (left hemisphere) represent the largest lesion (black) and smallest lesion (gray) of rats included in analysis for Experiment 1. Photographs of representative lesions comprise the right hemisphere. Rats were included if the majority of the dorsal and ventral hippocampus was lesioned. Rats with excessive neocortical damage were excluded. Coordinates are anterior/posterior from bregma as determined by Paxinos & Watson (2007).
BrdU Injections and Inclusion Criteria
Rats were given a single intraperitoneal injection of BrdU in saline (200 mg/kg). Eyeblink conditioning began 7 days later. Rats were sacrificed 21 days after BrdU administration. Rats were dropped from analysis due to poor EMG signal in the eyeblink conditioning task (n=2) and absence of BrdU labeling (n=1). The remaining rats were distributed as follows: Naïve, n=9; Trace 250, n=7; Trace 500, n=8; Trace 500 Cue, n=7.
Immunohistochemistry
Rats were deeply anesthetized with sodium pentobarbital (200 mg/kg) and transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer. Brains were dissected from the skulls and post-fixed for at least 2 d. Coronal sections (40-μm) throughout the entire rostrocaudal extent of the dentate gyrus were cut with a Vibratome from one hemisphere into a bath of 0.1 M PBS (pH 7.5). For BrdU peroxidase staining, a 1:12 series of sections were mounted onto glass slides, dried and pretreated by heating in 0.1 M citric acid (pH 6.0). Slides were then rinsed in PBS, incubated in trypsin for 10 min, denatured in 2 M HCl:PBS for 30 min, rinsed and incubated with mouse antibodies to BrdU (diluted 1:250 with 0.5% Tween-20; Vector Laboratories, Burlingame, CA). The next day, slides were rinsed, incubated with biotinylated anti-mouse (1:200; Vector) for 60 min, rinsed, incubated with avidin-biotin complex, rinsed and reacted in 0.01% diaminobenzidine with 0.003% H2O2. Slides were counterstained with cresyl violet, dehydrated, cleared and coverslipped under Permount (Fisher Scientific, Fair Lawn, NJ).
Cell Counting
Quantitative analysis was conducted blind to behavioral condition. For BrdU peroxidase, estimates of total numbers of BrdU-labeled cells were determined using an unbiased stereology protocol previously reported to successfully quantify BrdU-labeling (West, et al., 1991; Gould et al., 1999). BrdU-labeled cells in the subgranular zone (SGZ) and granule cell layer (GCL) on every twelfth unilateral section throughout the entire rostrocaudal extent of the dentate gyrus were counted at 1,000X (100x objective with a 10x ocular) on an Olympus BX-50 light microscope, avoiding cells in the outermost focal plane. The number of BrdU-labeled cells per dentate gyrus was then multiplied by 24 to obtain an estimate of the total number of BrdU-labeled cells throughout the rostrocaudal axis.
Results: Experiment 1: Hippocampal lesions abolish trace eyeblink conditioning and this is partially reversed with the addition of a post-US cue
The percentage of conditioned responses across trial blocks for each training and lesion condition are presented in Figure 2. Each training condition is presented separately to ease inspection of the impact of hippocampal lesions. Repeated measures ANOVA across the 12 blocks of Trials with Training Condition and Lesion as between subjects factors revealed a significant effect of Trial, F(11,429)=42.17, p=.0001. The Trial x Lesion interaction was significant, F(11,429)=14.46, p=.0001. The Trial x Training Condition interaction was significant, F(22, 429)=1.93, p=.007. The Trial x Lesion x Training Condition interaction was also significant, F(22,429)= 2.34, p=.001. The main effect of Lesion was significant, F(1, 39)=48.22, p=.0001, as was the main effect of Training Condition, F(2,39)=11.09, p=.0001, and Lesion x Training condition interaction, F(2, 39)=9.63, p=.0001.
Figure 2.
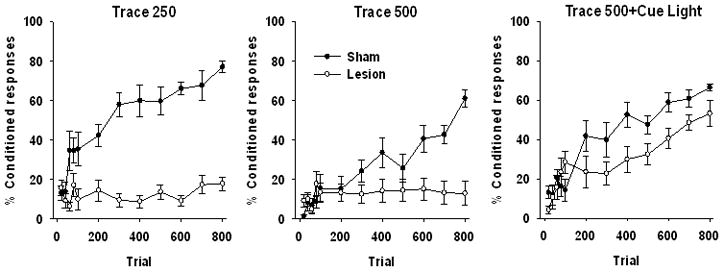
Behavioral results from animals trained with the Trace 250, Trace 500 and Trace 500 Cue procedures after receiving excitoxic hippocampus lesions or sham surgery. Data are presented as a mean percentage of conditioned responses ±SEM with the first 100 trials presented in 20 trial blocks with the remaining 700 trials in 100 trial blocks.
To clarify the impact of hippocampal lesions in each training condition, each condition was analyzed separately, with Lesion as the between subjects factor. Repeated measures ANOVA across the 12 blocks of conditioning in the Trace 250 condition, presented in the left panel of Figure 2, revealed a significant effect of Trial (11,143)=8.68, p=.0001, as well as a significant Trial x Lesion interaction, F(11, 143)=7.56, p=.0001. The main effect of Lesion was also significant, F(1,13)=104.28, p=.0001. As expected based on previous findings, hippocampal lesions abolished conditioned responding in the Trace 250 stimulus arrangement (Walker & Steinmetz, 2008; Weiss et al., 1999). Analysis of the Trace 500 groups using repeated measures ANOVA across the 12 blocks of conditioning revealed a significant effect of Trial (11,143)=12.47, p=.0001, as well as a significant Trial x Lesion interaction, F(11,143)=9.53, p=.0001. The main effect of lesion was significant, F(1,13)=4.29, p=.05. As expected, rats in the lesion condition failed to achieve over 20% conditioned responding at any point during training. The impact of hippocampal lesions on acquisition of trace conditioning with insertion of the post-US cue was analyzed using repeated measures ANOVA across the 12 blocks of conditioning. This ANOVA revealed a significant effect of Trial (11,143)=25.69, p=.0001, as well as a significant Trial x Lesion interaction, F(11,143)=2.78, p=.003. The main effect of Lesion was not significant, F(1,13)=3.78, p=.07. As depicted in the right panel of Figure 2, hippocampal lesions reduced asymptotic responding relative to sham operated controls. However, this group exhibited an increase in conditioned responding across trials similar to sham operated controls. Thus, insertion of the post-US cue attenuated the lesion induced deficit. This result is surprising, as the post-US cue was a third discontiguous stimulus.
Experiment 2: Rate of acquisition and not hippocampal-dependence predicts the impact of learning on survival of adult-generated cells
Repeated measures ANOVA on the 12 blocks of conditioning presented in Figure 4A revealed a significant effect of Trial F(11,209)=26.30, p=.0001. The Training Condition x Trial interaction was not significant F(22,209)<1, indicating that all three groups increased conditioned responding across trials. The main effect of Condition was significant, F(2,19)=4.35, p=.028, reflecting differences in the number of conditioned responses. Though all three groups reached the same level of asymptotic responding, rats in the Trace 500 condition expressed fewer conditioned responses across trial blocks compared to either Trace 250 or Trace 500 Cue.
Figure 4.
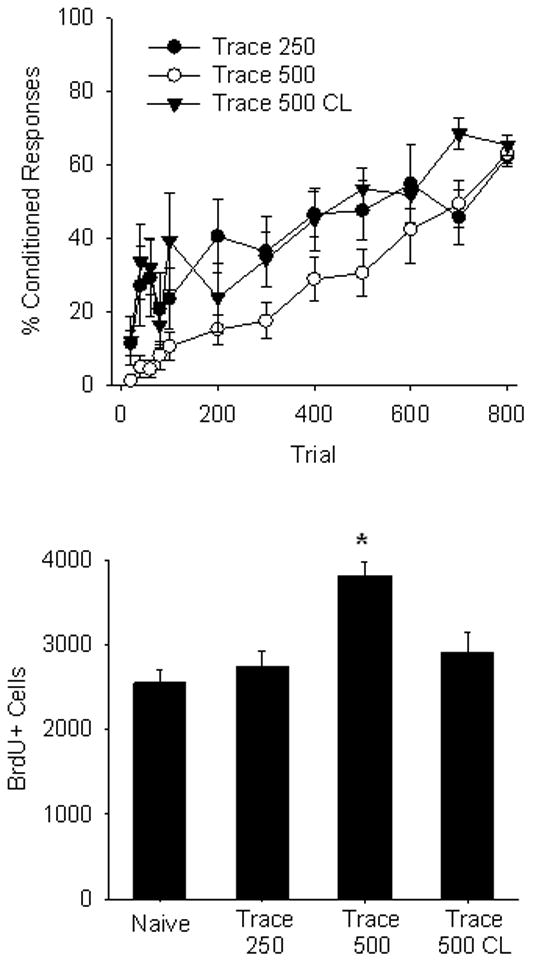
A) Behavioral results from animals trained with the Trace 250, Trace 500 and Trace 500 Cue procedures. Data are presented as a mean percentage of conditioned responses ±SEM with the first 100 trials presented in 20 trial blocks with the remaining 700 trials in 100 trial blocks. B) Bars represent the mean number of BrdU-labeled cells in the SGZ and GCL of the dentate gyrus ±SEM of naïve animals and groups Trace 250, Trace 500 and Trace 500 Cue conditions.
Only rats in the Trace 500 condition exhibited an increase in BrdU+ cells when sacrificed 21 d following BrdU administration (Figure 4B). This was confirmed with a one-way ANOVA, which confirmed a significant effect of Training Condition, F(3,27)=9.75, p=.0001. Multiple comparisons using Bonferoni’s posthoc test confirmed that the number of BrdU+ cells was significantly higher in the Trace 500 condition relative to Naïve, untrained controls (p=.0001). Trace 500 exhibited significantly more cells than Trace 250 (p=.001) and Trace CL (p=.01). Naïve, Trace 250 and Trace CL did not differ from one another (p>.05). Thus, hippocampal dependent training is not sufficient, as evidenced by the absence of a pro-survival effect in the Trace 250 condition. Further, facilitation of acquisition of trace eyeblink conditioning with the post-US cue abolished the increase in survival. This pattern of results suggests that hippocampal-dependence is not sufficient to rescue adult-generated hippocamapal cells from death, but rather, only rats exhibiting slow acquisition of hippocampal-dependent learning tasks exhibit increased survival.
Discussion
The experiments presented here demonstrate that hippocampal dependence of eyeblink conditioning is not sufficient to reduce death of adult generated hippocampal cells. Rather, within the range of hippocampal-dependent tasks tested, learning rate predicts the effect of learning on survival of adult-generated cells (Waddell & Shors, 2008). Training with a 250 msec trace interval resulted in faster acquisition compared to training with the 500 msec trace interval, though both arrangements require the hippocampus (Weiss et al., 1999; Beylin et al., 2001). Only training with the 500 msec trace interval increased survival. Surprisingly, addition of a third, discontiguous cue after delivery of the US facilitated the rate of acquisition and also rendered trace conditioning hippocampal-independent.
The strategy used to acquire trace conditioning is not clear. It has been proposed that the hippocampus is necessary to solve trace conditioning tasks because it is needed to detect the contingency between the discontiguous CS and US (Rodriguez & Levy, 2001). Other interpretations of hippocampal-dependence of trace conditioning propose that the hippocampus is necessary to discriminate between the trace interval and the intertrial interval (Bolles et al., 1978; Kaplan & Hearst, 1982; Mowrer & Lamoreaux, 1951). In support of this, signaling the end of a trace trial with a different stimulus than that used as the CS can reverse a trace conditioning deficit induced by a very long trace interval in a conditioned suppression preparation (Bolles, et al., 1978). The results presented here align with this second proposal, as addition of a third, discontiguous stimulus partially reversed the hippocampal-lesion induced deficit and facilitated acquisition of trace eyeblink conditioning in intact animals. Rats with permanent excitotoxic lesions of the hippocampus exhibited a significant increase in well-timed conditioned responses as presentation of training trials with the post-US cue progressed.
Trace eyeblink conditioning and trace fear conditioning are particularly sensitive to hippocampal lesions (Bangasser et al., 2006; Beylin et al., 2001; Kim et al., 1995; Solomon et al., 1986; Weiss et al., 1999). Hippocampal lesions retard trace eyeblink conditioning even when the trace interval is extremely brief (i.e., 50 msec; Walker & Steinmetz, 2007). Recent evidence suggests that the hippocampus may be selectively necessary for aversive trace conditioning. Lesions of the hippocampus do not abolish acquisition of appetitive trace conditioning and this does not appear to be sensitive to parametric changes in CS and trace interval duration (Chan et al., 2004; Kyd et al., 2007; Oakeshott et al., 2006; Thibaudeau et al., 2007; Thibaudeau et al., 2009). This selectivity of motivational system may implicate the role of fear and therefore, the role of the post-US cue as a safety signal. Post-shock cues can reduce the expression of fear and even abolish helplessness associated with unpredictable shock (Mineka et al., 1984). Fear as indexed by freezing and ultrasonic vocalizations is high during early eyeblink conditioning trials and decreases as conditioning continues and the eyeblink CR emerges (Britton & Asthmeir, 2004; Lee & Kim, 2004). It is possible that aversive trace conditioning relies on the hippocampus because the US elicits fear that accrues to the context as well as the CS (Britton & Asthmeir, 2004). If the post-US cue is serving to reduce fear by signaling a period free of aversive stimulation, then the hippocampus is needed to distinguish between the reinforced and non-reinforced intervals comprising the experimental session. Further experiments are necessary to determine whether the post-US cue is acting to signal the intertrial interval.
Ablation of adult-generated hippocampal cells does not reliably disrupt hippocampal-dependent learning (e.g., Clelland et al., 2009; Jaholkowski et al., 2009; Shors et al., 2002). A recent report demonstrated that ablation of neurogenesis in the adult mouse only disrupted a spatial or visual discrimination task when the stimuli were very similar to each other (Clelland et al., 2009). When discrimination tasks were easy, in that the stimuli were very different, performance between irradiated and sham-irradiated mice did not differ (Clelland et al., 2009). This finding aligns well with the proposal that the dentate gyrus is involved in pattern separation and discrimination learning (e.g., Clelland et al., 2009; Kesner, et al., 2004; Lee & Kesner, 2004; Leutgeb, et al., 2007; Rolls, 1996). The rate of learning also appears to predict the impact of water maze learning on survival of new cells (Gould et al., 1999; van Praag et al., 1999; Greenough et al., 1999). Survival is increased by hippocampal-dependent water maze training only when animals require at least 2 days of training (Epp et al., 2007; Gould et al., 1999). Thus, learning rate appears to reliably predict the impact of learning on neurogenesis across preparations.
The mechanism through which learning reduces cell death of adult-generated cells is not known. Experience-dependent modulation of survival is critically dependent on the age of the cell (e.g., Döbrössy et al., 2003; Tronel et al., 2010). Because the pro-survival effect of learning directly relates to the rate of learning, we presume that new cells are modulated by local network activity when it is relatively protracted. Our laboratory consistently observes learning-dependent modulation of cell survival 1 week following BrdU administration (e.g., Dalla et al., 2007; Gould et al., 1999; Leuner et al., 2004; Leuner et al., 2006; Waddell & Shors, 2008), a developmental phase at which adult-generated cells in the rat extend axons into CA3 (Hastings & Gould, 1999). Similarly, water maze training increases survival 6–10 days following BrdU administration (Epp et al., 2007; Kee et al., 2007; Tronel et al., 2010). Environmental enrichment also increases survival within 2 weeks of cell development in the mouse and the BrdU-labeled cells selectively exhibit markers of activity when re-exposed to the same environment (Tashiro et al., 2007). A recent study found that spatial learning characterized by high cognitive demands facilitated maturation of adult-generated cells, and enhanced dendritic complexity of granule cells generated 1 week prior to training (Tronel et al., 2010). These findings suggest experience modulates survival and cell development, and further suggest that immature cells rescued by enrichment or learning encode at least some aspects of the experience.
The contribution of new neurons to memory formation presumably relies in part upon the maturity of the cells at the time of learning. Adult-generated cell development parallels cell development in the neonate (Esposito et al., 2005; Ge et al., 2006; Overstreet Wadiche et al., 2005). Newly generated cells first exhibit tonic excitation in response to GABA followed by GABA-mediated synaptic inputs to glutamatergic-mediated synaptic inputs (Ben-Ari et al., 1997; Ge et al., 2006; Tozuka et al., 2005). The rate of this gradual switch from depolarizing GABA to hyperpolarizing GABA likely differs between rats and mice (Snyder et al., 2009). Cell maturation in the mouse is approximately 2 weeks slower than that demonstrated in rats, as indexed by neuronal markers and activity-dependent immediate early gene expression (Snyder et al., 2009). Development of retroviral techniques have enabled visualization of adult-generated cells in vivo to analyze cell maturation in the mouse (e.g., Ge et al., 2005; Ge et al., 2006; Song et al., 2005; Zhao et al., 2006), thus examining the more protracted cell maturation between rats and mice. It is notable that in the rat, newly generated neurons exhibit enhanced plasticity at an immature phase (Schmidt-Hieber et al., 2004). Newly generated cells expressing PSANCAM, a marker known to be expressed in new cells for the first 1–3 weeks after mitosis, exhibit a lower threshold for induction of long-term potentiation (Schmidt-Hieber et al., 2004; Snyder et al., 2009). Because this period of PSANCAM expression parallels the window during which many newly generated cells die (Dayer et al., 2003), it is possible that associative learning induces plasticity and recruits new cells into existing circuitry. Learning a particularly difficult spatial task enhanced survival and promoted development of complex dendritic arbors in immature cells, facilitating incorporation into existing circuitry (Tronel et al., 2010). Further experiments are necessary to understand how new neurons might contribute to memory formation. Increased survival induced by learning may produce a memory that is more stable and less susceptible to interference or decay.
Individual differences in learning rate may reflect differences in motivational state. Hippocampal theta is a rhythm proposed to index alterness or attentiveness (see Berry & Seager, 2001 for review). Rabbits exhibiting high theta ratio prior to eyeblink conditioning acquire the CR faster than rabbits exhibiting low theta activity (Berry & Thompson, 1978; Nokia et al., 2008). In slow learners, theta increases as training progresses and the CR emerges (Berry 1982; Berry & Seager, 2001; Nokia et al., 2009). Eyeblink conditioning tasks may increase survival only to the degree the task maintains attention across training sessions. Thus, adult-generated neurons may serve to promote learning when learning is particularly challenging. In summary, these data clearly dissociate the effects of learning on neurogenesis from those that are dependent on the hippocampus for learning.
Figure 3.
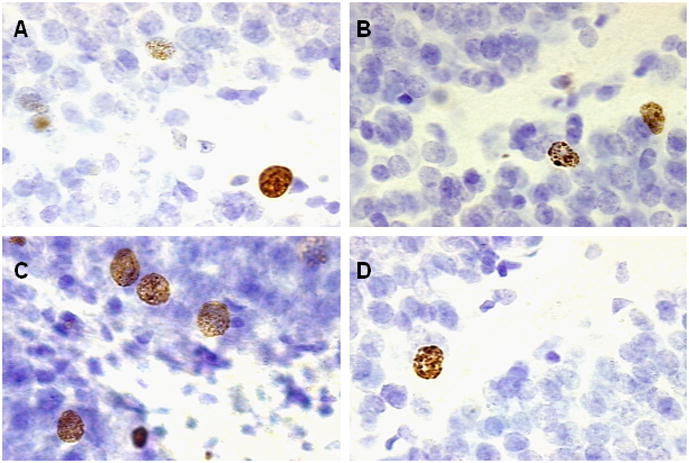
BrdU labeled cells in the dentate gyrus of the hippocampus from similar sections of a rat in the A) 21 day Naïve condition B) Trace 250 condition, C) Trace 500 condition and D) Trace 500 Cue condition. Images were magnified 1000x.
Acknowledgments
This work was funded by the National Institutes of Health (National Institute of Mental Health-59970) and the National Science Foundation (IOB-0444364) and (IOS-09143860) to TJS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in adult rats. Journal of Comparative Neurology. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Waxler DE, Santallo J, Shors TJ. Trace conditioning and the hippocampus: The importance of contiguity. Journal of Neuroscience. 2006;26:8702–8706. doi: 10.1523/JNEUROSCI.1742-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated a “menage a` trios”. Trends in Neuroscience. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Berry SD. Septo-hippocampal activity and learning rate. In: Woody CD, editor. Conditioning: Representation of involved neural functions. New York: Plenum; 1982. pp. 417–431. [Google Scholar]
- Berry SD, Seager MA. Hippocampal theta oscillations and classical conditioning. Neurobiology of Learning & Memory. 2001;76:298–313. doi: 10.1006/nlme.2001.4025. [DOI] [PubMed] [Google Scholar]
- Berry SD, Thompson RF. Prediction of learning rate from the hippocampal electroencephalogram. Science. 1978;200:1298–1300. doi: 10.1126/science.663612. [DOI] [PubMed] [Google Scholar]
- Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: Temporal discontinuity or task difficulty? Neurobiology of Learning and Memory. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Collier AC, Bouton ME, Marlin N. Some tricks for ameliorating the trace conditioning deficit. Bulletin of the Psychonomic Society. 1978;11:403–406. [Google Scholar]
- Britton GB, Astheimer LB. Fear develops to the conditioned stimulus and to the context during classical eyeblink conditioning in rats. Integrative Physiological and Biological Science. 2004;39:295–306. doi: 10.1007/BF02734168. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. Journal of Comparative Neurology. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Bangasser DA, Edgecomb C, Shors TJ. Neurogenesis and Learning: Acquisition and asymptotic performance predict how many new cells survive in the hippocampus. Neurobiology of Learning & Memory. 2006;88:143–148. doi: 10.1016/j.nlm.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Papachristos EB, Whetstone AS, Shors TJ. Female rats learn trace memories better than male rats and consequently retain a greater proportion of new neurons in their hippocampi. Proceedings of the National Academy of Sciences. 2009;106:2927–2932. doi: 10.1073/pnas.0809650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. The Journal of Comparative Neurology. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Döbrössy JR, Drapeau E, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Molecular Psychiatry. 2003;8:974–982. doi: 10.1038/sj.mp.4001419. [DOI] [PubMed] [Google Scholar]
- Epp JR, Spritzer MD, Galea LAM. Hippocampus-dependent learning promotes survival of new neurons in the dentate gyrus at a specific time during cell maturation. Neuroscience. 2007;149:273–285. doi: 10.1016/j.neuroscience.2007.07.046. [DOI] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. Journal of Neuroscience. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh ELK, Sailor KA, Kitabatake Y, Ming G, Song M. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Cohen NJ, Juraska JM. New neurons in old brains: learning to survive? Nature Neuroscience. 1999;2:203–205. doi: 10.1038/6300. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nature Neuroscience. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. The Journal of Comparative Neurology. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Jaholkowski P, Kiryk A, Jedynak P, Ben Abdallah NM, Knapska E, Kowalczyk A, Piechal A, Blecharz-Klin K, Figiel I, Lioudyno V, Widy-Tyszkiewicz E, Wilczynski GM, Lipp HP, Kaczmarek L, Filipkowski RK. New hippocampal neurons are not obligatory for memory formation; cyclin D2 knockout mice with no adult brain neurogenesis show learning. Learning and Memory. 2009;16:439–451. doi: 10.1101/lm.1459709. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kaplan PS, Hearst E. Bridging temporal gaps between CS and US in autoshaping: insertion of other stimuli before, during, and after CS. Journal of Experimental Psychology: Animal Behavioral Processes. 1982;8:187–203. [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nature Neuroscience. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Lee I, Gilbert P. A behavioral assessment of hippocampal function based on a subregional analysis. Reviews in the Neurosciences. 2004;15:333–351. doi: 10.1515/revneuro.2004.15.5.333. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely acquired trace eyeblink conditioned responses. Behavioral Neuroscience. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Kyd RJ, Pearce JM, Haselgrove M, Amin E, Aggleton JP. The effects of hippocampal lesions on a novel temporal discrimination task for rats. Behavioural Brain Research. 2007;187:159–171. doi: 10.1016/j.bbr.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Encoding versus retrieval of spatial memory: Double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus. 2004;14:66–76. doi: 10.1002/hipo.10167. [DOI] [PubMed] [Google Scholar]
- Lee, Kim Differential effects of cerebellar, amygdalar, and hippocampal lesions on classical eyeblink conditioning in rats. Journal of Neuroscience. 2004;24:3242–3250. doi: 10.1523/JNEUROSCI.5382-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. Journal of Neuroscience. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. Journal of Neuroscience. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Waddell J, Gould E, Shors TJ. Temporal discontiguity is neither necessary nor sufficient for learning-induced effects on neurogenesis. Journal of Neuroscience. 2006;26:13437–13442. doi: 10.1523/JNEUROSCI.2781-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. The Journal of Comparative Neurology. 1999;406:449–460. [PubMed] [Google Scholar]
- Mineka S, Cook M, Miller S. Fear conditioned with escapable and inescapable shock: Effects of a feedback stimulus. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:307–323. [Google Scholar]
- Mowrer OH, Lamoreaux RR. Conditioning and conditionality (discrimination) Psychological Review. 1951;58:196 –212. doi: 10.1037/h0061399. [DOI] [PubMed] [Google Scholar]
- Overstreet Wadiche L, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. Journal of Neurophysiology. 2005;94:4528–4532. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. New York: Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- Rodriguez P, Levy WB. A model of hippocampal activity in trace conditioning: where’s the trace? Behavioral Neuroscience. 2001;115:1224 –1238. doi: 10.1037//0735-7044.115.6.1224. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proceedings of the National Academy of Sciences. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. Adult-born hippocampal neurons are more numerous, faster maturing and more involved in behavior in rats than in mice. Journal of Neuroscience. 2009;26:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843– 852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz J. Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behavioral Neuroscience. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: A critical period during an immature stage. Journal of Neuroscience. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaudeau G, Potvin O, Allen K, Dore FY, Goulet S. Dorsal, ventral and complete excitotoxic lesions of the hippocampus in rats failed to impair appetitive trace conditioning. Behavioural Brain Research. 2007;185:9–20. doi: 10.1016/j.bbr.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Thibaudeau G, Dore FY, Goulet S. Additional evidence for intact appetitive trace conditioning in hippocampal-lesioned rats. Behavioral Neuroscience. 2009;123:707–712. doi: 10.1037/a0015239. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Tronel S, Fabre A, Charrier V, Oliet SHR, Gage FH, Abrous DN. Spatial learning sculpts dendritic arbor of adult-born hippocampal neurons. Proceedings of the National Academy of Sciences. 2010;107:7963–7968. doi: 10.1073/pnas.0914613107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning and long-term potentiation in mice. Proceedings of the National Academy of Sciences. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Shors TJ. Neurogenesis, learning and associative strength. European Journal of Neuroscience. 2008;27:3020–3028. doi: 10.1111/j.1460-9568.2008.06222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AG, Steinmetz JE. Hippocampal lesions in rats differentially affect long- and short-trace eyeblink conditioning. Physiology & Behavior. 2008;93:570–578. doi: 10.1016/j.physbeh.2007.10.018. [DOI] [PubMed] [Google Scholar]
- Weiss C, Bouwmeester H, Power JM, Disterhoft JF. Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behavioural Brain Research. 1999;99:123–132. doi: 10.1016/s0166-4328(98)00096-5. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. The Anatomical Record. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Ming G, Gage F. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. The Journal of Neuroscience. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]