Abstract
Privileged medicinal scaffolds based on the structures of tetra- and penta-substituted 2-aminopyrroles were prepared via one-pot multicomponent reactions of structurally diverse aldehydes and N-(aryl-, hetaryl-, alkyl-sulfonamido)-acetophenones with activated methylene compounds. This methodology was used in a four-step synthesis of alkaloids rigidins A, B, C and D in overall yields 61%, 58%, 60% and 53%, respectively. Of these, rigidins B, C and D were synthesized for the first time.
Polysubstituted pyrroles are an important class of heterocycles that display diverse pharmacological activities.1 Furthermore, they are useful building blocks in the synthesis of natural products and heterocyclic chemistry. Although a large number of new pyrrole syntheses,2 including multicomponent reactions (MCRs),3 have been reported in recent years, relatively few examples are known for the preparation of polysubstituted 2-aminopyrroles.4 Aminopyrroles are not readily available through general pyrrole ring-formation methods. At the same time the 2-aminopyrrole fragment is part of many different bioactive compounds and it is recognized as a privileged medicinal structure. Known bioactivities for this class of compounds include anti-inflammatory,5 anticancer,6 antiviral,7 antifungal,8 pesticidal,9 radioprotective,10 MEK inhibitory,11 MK2 inhibitory,12 FAK, KDR and Tie2 inhibitory,13 PDE inhibitory,14 anti-interleukin-6,15 TNF-α production inhibitory,16 and afferent pelvic nerve activity inhibitory.17 Moreover, 2-aminopyrroles are precursors for the synthesis of purine analogs – pyrrolopyrimidines, pyrrolotriazines and pyrrolopyridines.18–24 These pyrrole containing heterocycles are widely investigated for their multiple bioactivities, which, among many others, are known to include anti-inflammatory,18 anticancer,19 antiviral,20 antifungal,21 adenosine A1 receptor inhibitory,22 adenosine kinase23 and dihydrofolate reductase24 inhibitory. The pyrrolo[2,3-d]pyrimidine ring system is also a common motif in several natural products, such as nucleoside antibiotics tubercidin, toyocamyin, sangivamycin,25 and marine alkaloids rigidins A, B, C, D and E.26
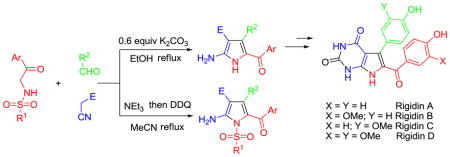
Previously, we described a novel method for the synthesis of multisubstituted pyrrolines using a multicomponent reaction of various N-(aryl-and alkylsulfonamido)-acetophenones with aldehydes and malononitrile (see Table 1 graphic).27 While the reaction is regioselective, it is not stereoselective and gives mixtures of cis and trans 2-pyrrolines, which are not easily separable. Utilizing this methodology as a starting point, we developed a new muticomponent one-pot method for the synthesis of tetra-and penta-diversely substituted 2-aminopyrroles. In addition, we utilized the new method for a short total synthesis of alkaloids rigidins A, B, C and D.
Table 1.
Synthesis of Penta-substituted Pyrroles A
 | |||||
|---|---|---|---|---|---|
| Pyrrole | Ar | R1 | R2 | E | yield % |
| A1 | Ph | 4-O2N-Ph | 4-Cl-Ph | CN | 81 |
| A2 | Ph | 2,4,6-i-Pr-Ph | 3,4,5-MeO-Ph | CN | 91 |
| A3 | Ph | 4-MeO-Ph | 3,4,5-MeO-Ph | CN | 89 |
| A4 | 4-MeO-Ph | 4-MeO-Ph | 3-Br-4,5-MeO-Ph | CN | 78 |
| A5 | Ph | Me | 3-Br-4-MeO-Ph | CN | 37 |
| A6 | Ph | 4-MeO-Ph | 3,5-Br-Ph | CN | 56 |
| A7 | Ph | 4-MeO-Ph | 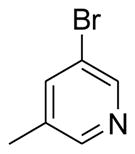 |
CN | 41 |
| A8 | Ph | 4-MeO-Ph | 2,6-Cl-Ph | CN | 78 |
| A9 | Ph | 4-F-Ph | 2,6-Cl-Ph | CN | 55 |
| A10 | Ph | Bu | 4-O2N-Ph | CN | 71 |
| A11 | Ph | 4-Me-Ph | Pr | CN | 55 |
| A12 | Ph | Me | 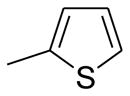 |
CN | 50 |
| A13 | Ph | 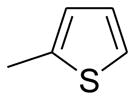 |
4-MeOOC-Ph | CN | 96 |
| A14 | Ph | Me | 2-Cl-6-F-Ph | CN | 88 |
| A15 | Ph | Me | 2,6-Cl-Ph | CN | 65 |
| A16a | Ph | 4-MeO-Ph | 4-Br-Ph | CONH2 | 38 |
| A17a | Ph | 4-MeO-Ph | 3,4,5-MeO-Ph | COOEt | 76 |
The intermediate pyrrolines were obtained in EtOH.
Penta-substituted 2-aminopyrroles A1–17 were prepared by a multicomponent reaction of N-(aryl-, hetaryl-and alkylsulfonamido)-acetophenones, aldehydes and cyanoacetic acid derivatives in acetonitrile, followed by oxidation with DDQ in one pot (Table 1). This three-component process works well for any tested combination of aliphatic, aromatic (including sterically hindered or heteroaromatic) aldehydes and malononitrile, cyanoacetamide or ethyl cyanoacetate. Because of the lower reactivity of the intermediate Knoevenagel products of cyanoacetamide or ethyl cyanoacetate, the reactions were sluggish in acetonitrile (A16 and A17). In these cases the pyrrolines were obtained in ethanol, the solvent was then evaporated and the crude material redissolved in acetonitrile for the subsequent oxidation with DDQ. When phenylsulfonyl-or 4-methoxybenzoylacetonitriles were used in this reaction, a mixture of pyrrolines was obtained, which did not undergo oxidation to the corresponding pyrroles.
This methodology was also used for the synthesis of tetra-substituted NH-pyrroles by base-promoted dehydrosulfinylation of the 2-pyrroline mixtures. It was previously reported that DBU was able to promote the elimination toluenesulfinic acid from N-tosyl-3-pyrrolines to give pyrroles.28 In our case, the treatment of mixtures of 2-pyrrolines with DBU in DMF leads to the formation tetra-substituted NH-pyrroles B1–5 (Table 2). We found that 4-MeO-PhSO2-and MeSO2-leaving groups are the best for this reaction, but it is necessary to change the solvent from acetonitrile to DMF. Furthermore, after the experimentation with different solvents, bases and reaction temperatures we found that simply refluxing a solution of the three reactants containing 0.6 equivalents K2CO3 in ethanol results in pyrroles B5–17 (Table 3).29
Table 2.
Synthesis of Tetra-substituted Pyrroles B
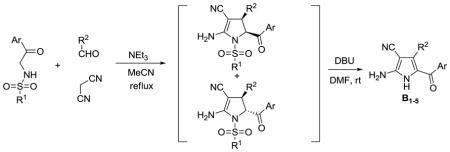 | ||||
|---|---|---|---|---|
| Pyrrole | Ar | R1 | R2 | yield % |
| B1 | Ph | Me | Pr | 43 |
| B2 | Ph | 4-MeO-Ph | 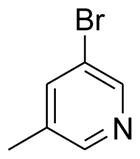 |
80 |
| B3 | Ph | 4-MeO-Ph | 2,6-Cl-Ph | 70 |
| B4 | Ph | 4-MeO-Ph | 3,5-Br-Ph | 70 |
| B5 | Ph | 4-O2N-Ph | 3,4,5-MeO-Ph | 19 |
| B5 | Ph | 2,4,6-i-Pr-Ph | 3,4,5-MeO-Ph | 24 |
| B5 | Ph | 4-Me-Ph | 3,4,5-MeO-Ph | 45 |
| B5 | Ph | 4-MeO-Ph | 3,4,5-MeO-Ph | 59 |
| B5 | Ph | Me | 3,4,5-MeO-Ph | 78 |
Table 3.
Synthesis of Tetra-substituted Pyrroles B
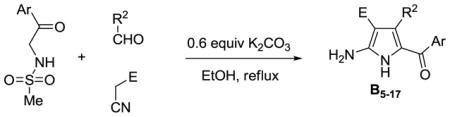 | ||||
|---|---|---|---|---|
| pyrrole | Ar | R2 | E | yield % |
| B5 | Ph | 3,4,5-MeO-Ph | CN | 80 |
| B6 | Ph | 2-Cl-6-F-Ph | CN | 76 |
| B7 | Ph | 4-MeO-Ph | CN | 55 |
| B8 | 4-MeO-Ph | 3,4,5-MeO-Ph | CN | 85 |
| B9 | Ph | 4-Br-Ph | CN | 73 |
| B10 | 4-MeO-Ph | 4-MeO-Ph | 4-MeO-Bz | 48 |
| B11 | Ph | 4-O2N-Ph | SO2Ph | 28 |
| B12 | Ph | 4-Br-Ph | SO2Ph | 40 |
| B13 | Ph | 3,4,5-MeO-Ph | SO2Ph | 52 |
| B14 | Ph | 3,4,5-MeO-Ph | COOEt | 57 |
| B15 | Ph | 2,6-Cl-Ph | CONH2 | 48 |
| B16 | Ph | 4-MeO-Ph | CONH2 | 93 |
| B17 | 4-MeO-Ph | 4-MeO-Ph | CONH2 | 89 |
The reaction scope encompasses the use of aliphatic, aromatic, and heterocyclic aldehydes as well as diverse activated methylene compounds including cyano, acyl, sulfono, alkoxycarbono and carbamido acetonitriles.
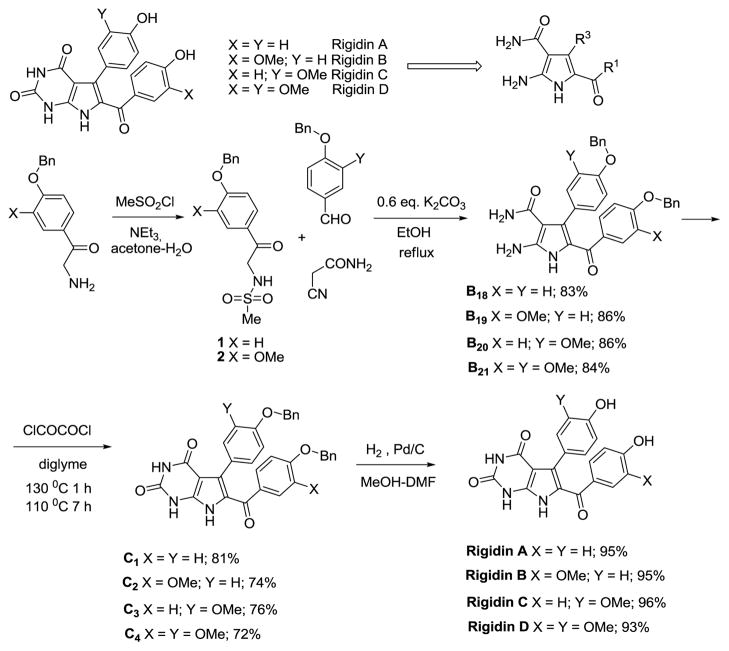
Tetra-substituted 2-aminopyrroles, containing a 3-carbamido-group, are a structural unit of marine alkaloids rigidins (Figure 1). These alkaloids have been isolated from tunicates obtained near Okinawa and New Guinea and have been shown to possess calmodulin antagonistic and cytotoxic activities.26 Several syntheses of rigidins A and E have been reported.30 Using our methodology for the synthesis of tetra-substituted pyrroles, we developed the shortest general approach to obtain these alkaloids. Moreover, rigidins B, C and D were synthesized for the first time (Figure 1). Commercially available aminoacetophenones were converted to N-(methanesulfonamido)-acetophenones 1 and 2 in almost quantitative yields and the latter were used in the three-component reaction to obtain 2-aminopyrroles B18-B21. Carbonylation was achieved with oxalyl chloride in diglyme to give pyrimidinediones C1-C4, these were subjected to hydrogenolysis to yield the desired rigidins with overall yields of 61% for A, 58% for B, 60% for C and 53% for D. Our synthesis compares favorably with the published approaches for rigidin A (7–9 steps and 26–40% overall yields).30 The spectral data are consistent with those published for the natural products.26 At the present time, we are preparing a library of rigidin analogues for biological testing.
Figure 1.
Total Synthesis of Rigidins A, B, C and D.
We performed a preliminary biological evaluation of the synthesized tetra-and penta-substituted pyrroles for anticancer and antibacterial activities. The antiproliferative activity was assessed using the cancer cell line, HeLa, as a model for human cervical adenocarcinoma, through the measurements of mitochondrial dehydrogenase activities using MTT method.31 In addition, pyrroles A and B were tested against Staphylococcus epidermidis (ATCC 75984), where Minimum Inhibitory Concentrations (MICs) were determined by broth microdilution method.32 We found that selected synthesized pyrroles exhibit activities in these assays (Table 4), supporting the idea that diverse biological activities are likely to be found within the libraries of privileged medicinal structures such as 2-aminopyrroles. More detailed biological evaluation will be published elsewhere later.
Table 4.
Biological Activities of Pyrroles A and B.
| IC50,μM (HeLa) | MIC, μM (S. epi) | IC50, μM (HeLa) | MIC, μM (S. epi) | ||
|---|---|---|---|---|---|
| A2 | 12.5 | >200 | B2 | 37.5 | >200 |
| A3 | 17 | >200 | B3 | >100 | 30 |
| A6 | 20 | >200 | B4 | 3.1 | >200 |
| A7 | 6.2 | >200 | B5 | 75 | >200 |
| A8 | 50 | >200 | B6 | >100 | 25 |
| A13 | 75 | >200 | B8 | 37.5 | >200 |
| A14 | 25 | >200 | B10 | 20 | 50 |
| A15 | >100 | 25 |
In summary, a one-pot, multicomponent reaction of structurally diverse aldehydes, N-(aryl-, hetaryl and alkylsulfonamido)-acetophenones, with activated methylene compounds results in the formation of tetra-and penta-substituted 2-aminopyrroles. This methodology was used for a four-step total synthesis of natural products rigidins A, B, C and D. This synthesis is flexible and can be adapted to the preparation of a library of rigidin analogs.
Supplementary Material
Acknowledgments
This work is supported by the US National Institutes of Health (grants RR-16480 and CA-135579) under the BRIN/INBRE and AREA programs. Collaboration with Mass Spectroscopy Facility at University of New Mexico is acknowledged.
Footnotes
Dedicated to Prof. Yuri I. Smushkevich on the occasion of his 75th birthday.
Supporting Information Available: Experimental procedures and characterization of the compounds are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For recent reviews, see: Furstner A. Angew Chem, Int Ed. 2003;42:3582–3603. doi: 10.1002/anie.200300582.Fan H, Peng J, Hamann MT, Hu JF. Chem Rev. 2008;108:264–287. doi: 10.1021/cr078199m.Nakao Y, Fusetani N. J Nat Prod. 2007;70:689–710. doi: 10.1021/np060600x.Urban S, Hickkford SJH, Blunt JW, Munro MHG. Curr Org Chem. 2000;4:765–807.
- 2.For selected examples, see: Lu Y, Arndtsen B. Org Lett. 2009;11:1369–1372. doi: 10.1021/ol900185n.Blangetti M, Deagostino A, Prandi C, Tabasso S, Venturello P. Org Lett. 2009;11:3914–3917. doi: 10.1021/ol9015018.Aponick A, Li CY, Malinge J, Marques EF. Org Lett. 2009;11:4624–4627. doi: 10.1021/ol901901m.Egi M, Azechi K, Akai S. Org Lett. 2009;11:5002–5005. doi: 10.1021/ol901942t.Satito A, Konishi T, Hanzawa Y. Org Lett. 2010;12:372–374. doi: 10.1021/ol902716n.Wang HY, Mueller DS, Sachwani RM, Londino HN, Anderson LL. Org Lett. 2010;12:2290–2293. doi: 10.1021/ol100659q. and references cited therein.
- 3.(a) Balme G. Angew Chem, Int Ed. 2004;43:6238–6241. doi: 10.1002/anie.200461073. [DOI] [PubMed] [Google Scholar]; (b) Balme G, Bouyssi D, Monteiro N. Heterocycles. 2007;73:87–123. [Google Scholar]; (c) Shiraishi H, Nishitani T, Sakaguchi S, Ishii Y. J Org Chem. 1998;63:6234–6238. doi: 10.1021/jo980435t. [DOI] [PubMed] [Google Scholar]; (d) Cadierno V, Gimeno J, Nebra N. Chem Eur J. 2007;13:9973–9981. doi: 10.1002/chem.200701132. [DOI] [PubMed] [Google Scholar]; (e) St Cyr DJ, Arndtsen BA. J Am Chem Soc. 2007;129:12366–12367. doi: 10.1021/ja074330w. [DOI] [PubMed] [Google Scholar]; (f) Lu Y, Arndtsen BA. Angew Chem, Int Ed. 2008;47:5430–5433. doi: 10.1002/anie.200801385. [DOI] [PubMed] [Google Scholar]; (g) Yavari I, Kowsari E. Mol Divers. 2009;13:519–528. doi: 10.1007/s11030-009-9146-8. [DOI] [PubMed] [Google Scholar]; (h) Chen X, Hou L, Li X. Synlett. 2009:828–832. [Google Scholar]; (i) Anary-Abbasinejad M, Charkhati K, Anaraki-Ardakani H. Synlett. 2009:1115–1117. [Google Scholar]; (j) Merkui E, Boersch C, Frank W, Muller TJJ. Org Lett. 2009;11:2269–2272. doi: 10.1021/ol900581a. [DOI] [PubMed] [Google Scholar]; (k) Shanthi G, Perumal RT. Tetrahedron Lett. 2009;50:3959–3962. [Google Scholar]; (l) Liu W, Jaang H, Huang L. Org Lett. 2010;12:312–315. doi: 10.1021/ol9026478. [DOI] [PubMed] [Google Scholar]; (m) Maiti S, Biswas S, Jana U. J Org Chem. 2010;75:1674–1683. doi: 10.1021/jo902661y. [DOI] [PubMed] [Google Scholar]; (n) St Cyr DJ, Morin MST, Belanger-Gariepy F, Arndtsen BA, Krenske EH, Houk KN. J Org Chem. 2010;75:4261–4273. doi: 10.1021/jo1008383. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 4.(a) Verhe R, De Kimpe N, De Buyck L, Tilley M, Schamp N. Tetrahedron. 1980;36:131–142. [Google Scholar]; (b) Kusumoto T, Hiyama T, Ogata K. Tetrahedron Lett. 1986;27:4197–4200. [Google Scholar]; (c) Chatani N, Hanafusa T. J Org Chem. 1991;56:2166–2170. [Google Scholar]; (d) Marco JL, Martinez-Grau A, Martin N, Seoane C. Tetrahedron Lett. 1995;30:5393–5396. [Google Scholar]; (e) Nair V, Vinod AU, Rajesh C. J Org Chem. 2001;66:4427–4429. doi: 10.1021/jo001714v. [DOI] [PubMed] [Google Scholar]; (f) Chien TC, Meade EA, Hinkley JM, Townsend LB. Org Lett. 2004;6:2857–2859. doi: 10.1021/ol049207d. [DOI] [PubMed] [Google Scholar]; (g) Shaabani A, Teimouri MB, Arab-Ameri S. Tetrahedron Lett. 2004;45:8409–8413. [Google Scholar]; (h) Demir A, Emrullahoglu M. Tetrahedron. 2006;62:1452–1458. [Google Scholar]; (i) Al-Mousawi SM, Moustafa MS, Meier H, Kolsshorn H, Elnagdi MH. Molecules. 2009;14:798–806. doi: 10.3390/molecules14020798. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Barnea E, Majumder S, Staples RJ, Odom AL. Organometallics. 2009;28:3876–3881. [Google Scholar]; (k) Fontaine P, Masson G, Zhu J. Org Lett. 2009;11:1555–1558. doi: 10.1021/ol9001619. [DOI] [PubMed] [Google Scholar]
- 5.(a) Mohamed MS, Rashad AE, Adbel-monem M, Fatahalla SS. Z Naturforsch. 2007;62c:27–31. doi: 10.1515/znc-2007-1-205. [DOI] [PubMed] [Google Scholar]; (b) Berger M, Schaecke H, May E, Skuballa W, Kuenzer H. 2008098798 A1. PCT Int Appl WO. 2008
- 6.Cocco MT, Congiu C, Onnis V. Bioorg Med Chem. 2003;11:495–503. doi: 10.1016/s0968-0896(02)00465-0. [DOI] [PubMed] [Google Scholar]
- 7.Akiyoshi A, Takashi S, Naohisa O, Yasuo S, Motoniro S, Junji N, Masayosh K. JP2009179589 (A) PCT Int Appl. 2009
- 8.Onnis V, De Log A, Cocco MT, Fadda R, Meleddu R. Eur J Med Chem. 2009;44:1288–1295. doi: 10.1016/j.ejmech.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Chou D, Knauf W, Maier M, Malaska MJ, McIntyre D, Lochhaas F, Huber SK. 20070281976 A1. PCT Int Appl US. 2007
- 10.Ghorab MM, Heimy HI, Khalil AI, Abou El Ella DA, Noaman E. Phosphorus, Sulfur, and Silicon. 2008;183:90–104. [Google Scholar]
- 11.Wallace MB, Adams ME, Kanouni T, Mol CD, Dougan DR, Feher VA, O’Connell SM, Shi L, Halkowycz P, Dong Q. Bioorg Med Chem Lett. 2010;20:4156–4158. doi: 10.1016/j.bmcl.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 12.Heng R, Koch G, Schlapbach A, Seiler MP. 2008034600 A1. PCT Int Appl WO. 2008
- 13.Ronan B, Tabart M, Souaille C, Viviani F, Bacque E. FR2881742 A1. PCT Int Appl. 2006
- 14.Wang D, Kosh JW, Sowell JW, Sr, Wang T. 2005046676 A1. PCT Int Appl WO. 2005
- 15.Kenji K, Noriko K. 2010024227 A1. PCT Int Appl WO. 2010
- 16.Bullington JL, Fan X, Jackson PF, Zhang Y-M. 2004029040 A1. PCT Int Appl WO. 2004
- 17.Tanaka M, Sasaki Y, Kimura Y, Fukui T, Ukai Y. BJU International. 2003;92:1031–1036. doi: 10.1111/j.1464-410x.2003.04512.x. [DOI] [PubMed] [Google Scholar]
- 18.Mohamed MS, Kamel R, Fatahala SS. Eur J Med Chem. 2010;45:2994–3004. doi: 10.1016/j.ejmech.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 19.(a) Diana P, Barraja P, Lauria A, Montalbano A, Almerico M, Dattolo G, Cirrincione Eur J Med Chem. 2002;37:267–272. doi: 10.1016/s0223-5234(02)01339-9. [DOI] [PubMed] [Google Scholar]; (b) Willemann C, Grunert R, Bednarski PJ, Troschutz R, Cocco MT, Congiu C, Onnis V. Bioorg Med Chem. 2009;17:4406–4419. doi: 10.1016/j.bmc.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 20.(a) Bennet SM, Nguyen-Ba N, Ogilvie KK. J Med Chem. 1990;33:2162–2173. doi: 10.1021/jm00170a019. [DOI] [PubMed] [Google Scholar]; (b) Krawczyk SH, Nassiri MR, Kucera LS, Kern ER, Ptak RG, Wotring LL, Drach JC, Townsend LB. J Med Chem. 1995;38:4106–4114. doi: 10.1021/jm00020a026. [DOI] [PubMed] [Google Scholar]; (c) Migawa MT, Drach JC, Townsend LB. J Med Chem. 2005;48:3840–3851. doi: 10.1021/jm0402014. [DOI] [PubMed] [Google Scholar]
- 21.El-Gaby MSA, Gaber AM, Atalla AA, Abd Al-Wahab KA. Il Farmaco. 2002;57:613–617. doi: 10.1016/s0014-827x(01)01178-8. [DOI] [PubMed] [Google Scholar]
- 22.Castelhano AL, McKibben B, Witter DJ. 6878716 B1. PCT Int Appl US. 2005
- 23.Bookser BC, Ugarkar BG, Matelich MC, Lemus RH, Allan M, Tsuchiya M, Nakane M, Nagahisa A, Wiesner JB, Erion MD. J Med Chem. 2005;48:7808–7820. doi: 10.1021/jm050394a. [DOI] [PubMed] [Google Scholar]
- 24.Gangjee A, Jain HD, Queener SF, Kisliuk RL. J Med Chem. 2008;51:4589–4600. doi: 10.1021/jm800244v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta PK, Daunert S, Nassiri MR, Wotring LL, Drach JC, Townsend LB. J Med Chem. 1989;32:402–408. doi: 10.1021/jm00122a019. [DOI] [PubMed] [Google Scholar]
- 26.(a) Kabayashi J, Cheng J, Kikuchi Y, Ishibashi M, Yamamura S, Ohizumi Y, Ohta T, Nozoe S. Tetrahedron Lett. 1990;31:4617–4620. [Google Scholar]; (b) Tsuda M, Nozawa K, Shimbo K, Kobayashi J. J Nat Prod. 2003;66:292–294. doi: 10.1021/np020393a. [DOI] [PubMed] [Google Scholar]; (c) Davis RA, Christensen LV, Richardson AD, Moreira da Rocha R, Ireland CM. Mar Drugs. 2003;1:27–33. [Google Scholar]
- 27.Magedov IV, Luchetti G, Evdokimov NM, Manpadi M, Steelant WFA, Van slambrouck S, Tongwa P, Antipin MYu, Kornienko A. Bioorg Med Chem Lett. 2008;18:1392–1396. doi: 10.1016/j.bmcl.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mastalerz H, Gavai AV, Fink B, Struzynski C, Tarrant J, Vite GD, Wong TW, Zhang G, Vyas DM. Can J Chem. 2006;84:528–533. [Google Scholar]
- 29.As our work was in progress ( Frolova LV, Magedov IV, Kornienko A. Abstracts of Papers, 239th National Meeting of the American Chemical Society; San Francisco, CA. Washington, DC: American Chemical Society; 2010. p. ORGN-1069.) a report of a similar synthesis of tetra-substituted pyrroles appeared, see: Wang K, Domling A. Chem Biol Drug Des. 2010;75:277–283. doi: 10.1111/j.1747-0285.2009.00942.x.
- 30.(a) Edstrom ED, Wei Y. J Org Chem. 1993;58:403–407. [Google Scholar]; (b) Sakamoto T, Kondo Y, Sato S, Yamanaka H. J Chem Soc, Perkin Trans I. 1996:459–464. [Google Scholar]; (c) Gupton JT, Banner EJ, Scharf AB, Norwood BK, Kanters RPF, Dominey RN, Hempel JE, Kharlamova A, Bluhn-Chertudi I, Hickenboth CR, Little BA, Sartin MD, Coppock MB, Krumpe KE, Burnham BS, Holt H, Du KX, Keertikar KM, Diebes A, Ghassemi S, Sikorski JA. Tetrahedron. 2006;62:8243–8255. [Google Scholar]
- 31.Mosmann T. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 32.LaPlante KL, Rybak MJ. Antimicrobal Agents and Chemotherapy. 2004;48:4665–4672. doi: 10.1128/AAC.48.12.4665-4672.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.