Abstract
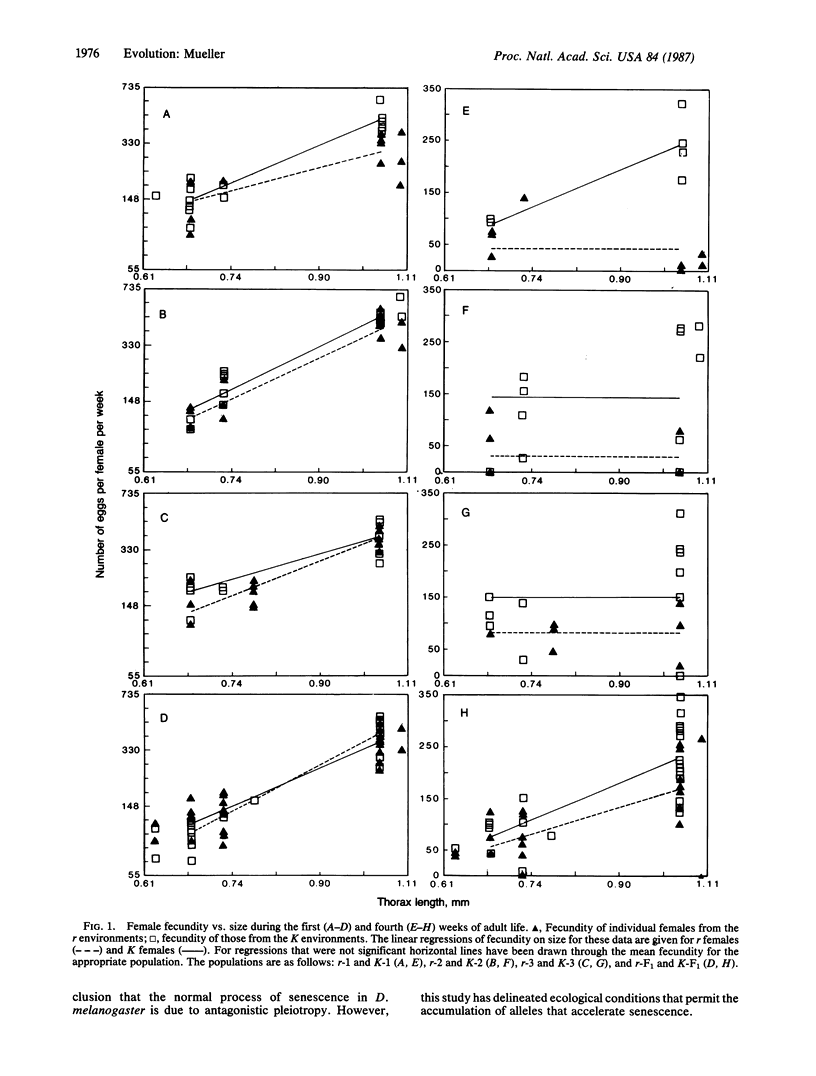
Ecological theories of life history evolution predict that natural selection should favor semelparous life histories in environments where juvenile survival is high relative to adult survival and rates of population growth are high. That is, organisms should complete their entire reproductive effort in a short period of time following maturation. Direct empirical verification of this idea has been lacking. Six independent populations of Drosophila melanogaster were maintained in two different environments, called r and K, for more than 120 generations. In the r environment population size was small, larval survival and rates of population growth were high, and reproduction was limited to a few days after eclosion. In the K environment population size was large and larval survival low, but adults were allowed to reproduce indefinitely. The fecundity of females of different sizes from each environment was measured daily for 4 weeks. No differences in fecundity were seen during the first week of adult life for females from the two environments. By the fourth week, however, the fecundity of large females from the r environment was 47-83% less than that of females from the K environment. The accelerated senescence exhibited by females from the r environment appears to be due to the accumulation of deleterious alleles whose effects are expressed late in life, which is consistent with the mutation accumulation hypothesis for the evolution of senescence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COLE L. C. The population consequences of life history phenomena. Q Rev Biol. 1954 Jun;29(2):103–137. doi: 10.1086/400074. [DOI] [PubMed] [Google Scholar]
- Edney E. B., Gill R. W. Evolution of senescence and specific longevity. Nature. 1968 Oct 19;220(5164):281–282. doi: 10.1038/220281a0. [DOI] [PubMed] [Google Scholar]
- Kosuda K. The aging effect on male mating activity in Drosophila melanogaster. Behav Genet. 1985 May;15(3):297–303. doi: 10.1007/BF01065984. [DOI] [PubMed] [Google Scholar]
- Mueller L. D., Ayala F. J. Trade-off between r-selection and K-selection in Drosophila populations. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1303–1305. doi: 10.1073/pnas.78.2.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. R., Charlesworth B. Genetics of life history in Drosophila melanogaster. I. Sib analysis of adult females. Genetics. 1981 Jan;97(1):173–186. doi: 10.1093/genetics/97.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M., Charlesworth B. A test of evolutionary theories of senescence. Nature. 1980 Sep 11;287(5778):141–142. doi: 10.1038/287141a0. [DOI] [PubMed] [Google Scholar]



