Abstract
A substituted heterocyclic scaffold comprising a 1,4-benzodiazepine fused with a 1,2,3-triazole ring has been synthesized and diversified via a variety of refunctionalizations. The strategy features the rapid assembly of the scaffold by combining 3–4 reactants in an efficient multicomponent assembly process, followed by an intramolecular Huisgen cycloaddition.
Privileged scaffolds are uniquely suited to the preparation of molecular libraries for lead development in medicinal chemistry.1 Such frameworks are attractive for drug discovery because of the high hit rates and the pharmacological profiles of their derivatives relative to those of other ring systems. By varying substitutents on these privileged scaffolds, one can often identify potent and selective binders for multiple biological targets from a single library. Indeed, a retrospective analysis of all known drugs reveals that nearly 25% can be traced back to a mere 41 substructures that have since been catagorized as privileged.2
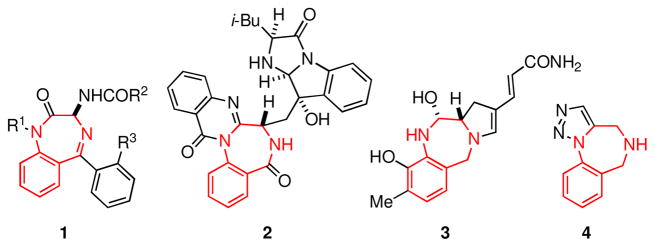
The 1,4-benzodiazepine ring system present in 1 is the archetypal privileged structure as defined by Evans more than 20 years ago.3 Compounds derived from the 1,4-benzodiazepine ring system, which has been suggested to serve as a structural mimic of peptide β-turns4 and α-helices,5 bind to a multitude of targets, including Gprotein coupled receptors, ligand-gated ion channels, and enzymes.1a Accordingly, it is hardly surprising that 1,4-benzodiazepines are found in a large number of pharmaceutical agents.2 Biologically-active natural products such as asperlicin (2)6 and anthramycin (3)7 also possess a 1,4-benzodiazepine substructure.
We recently developed a general approach for preparing a variety of substituted scaffolds of possible medical relevance by a strategy that involves sequencing multicomponent assembly processes (MCAPs) with subsequent cyclizations.8,9 During the course of these investigations, we developed an expedient route to a compound bearing the 1,2,3-triazolo-1,4-benzodiazepine scaffold 4.10 Although compounds derived from 4 are known to bind weakly to the benzodiazepine receptor11 and also to inhibit serine proteases,12 little else is known regarding the biological activity of compounds having this substructure. We now report some extensions of our initial discovery that have led to the facile synthesis of a number of novel members of this intriguing structural subclass of benzodiazepines, including more complex analogs possessing additional fused heterocyclic rings.
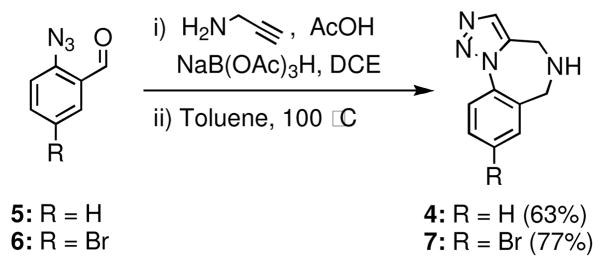
We first explored a simple variant of our original MCAP-cyclization approach in accord with the plan outlined in Scheme 1. The parent 1,2,3-triazolo-1,4-benzodiazepine scaffold 4 was prepared from known aldehyde 513 employing a reductive amination, followed by a thermally-induced, intramolecular Huisgen cycloaddition. This entry to 4 is both shorter and higher yielding than the reported method.14 The brominated scaffold 7 was accessed in a similar fashion from the known aldehyde 6.15
Scheme 1.
Synthesis of scaffolds 4 and 7
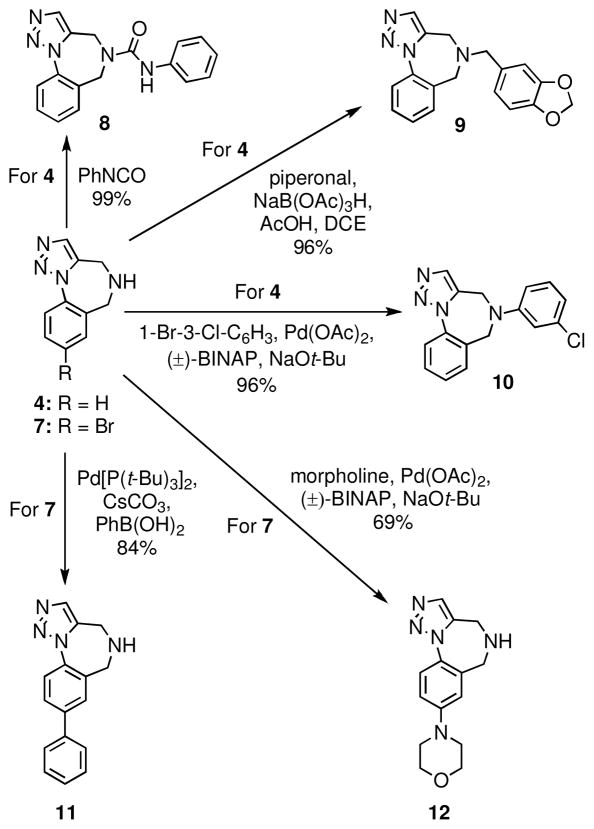
With an efficient route to amine 4 in hand, N-diversification was undertaken to afford various analogs. Representative examples of urea formation, reductive amination and cross-coupling using conditions developed by the Buchwald group are given in Scheme 2.16 The aryl bromide 7 is also a useful intermediate that can be used in diverse cross-coupling reactions. For example, biphenyl substrates are found in many biologically active compounds.1,2 It therefore occurred to us that hybrid biphenyl-benzodiazepine scaffolds might be of use in lead discovery. We found that 7 underwent a facile Suzuki cross-coupling to give the biaryl 11 in 84% yield. The bromide 7 could also be coupled with secondary amines under Buchwald conditions as exemplified by the use of morpholine to give the aniline derivative 12.16
Scheme 2.
Diversification of scaffolds 4 and 7
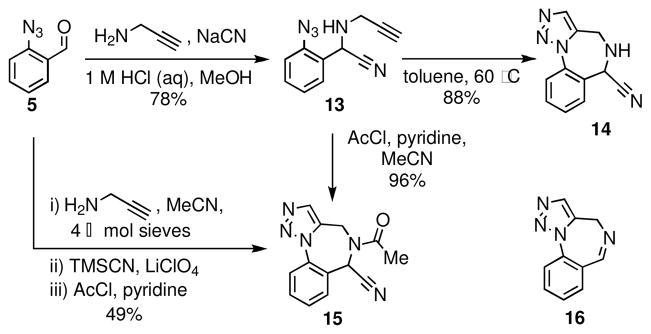
We envisioned that the triazolobenzodiazepine 14, which bears an α-amino nitrile function, would be a useful point of embarkation for the preparation of a number of tricyclic and tetracyclic analogs. The benzodiazepine scaffold 14 was thus prepared in 69% overall yield from aldehyde 5 via a facile two-step sequence that involved a Strecker reaction as the MCAP, followed by a dipolar cycloaddition (Scheme 3). Careful control of temperature in this conversion is essential because elimination of hydrogen cyanide to produce the known imine 1614 is observed at temperatures above 60 °C. N-Acetylation of the α-aminonitrile 13 gives an amide that undergoes dipolar cycloaddition at room temperature to afford benzodiazepine 15 in 96% yield; the marked difference in the reactivities of the amine 13 and its derived amide is notable. A ‘one-pot,’ four-component reaction was also developed that furnished benzodiazepine 15 in 49% yield.
Scheme 3.
Synthesis of scaffold 14 and direct access to amide 15
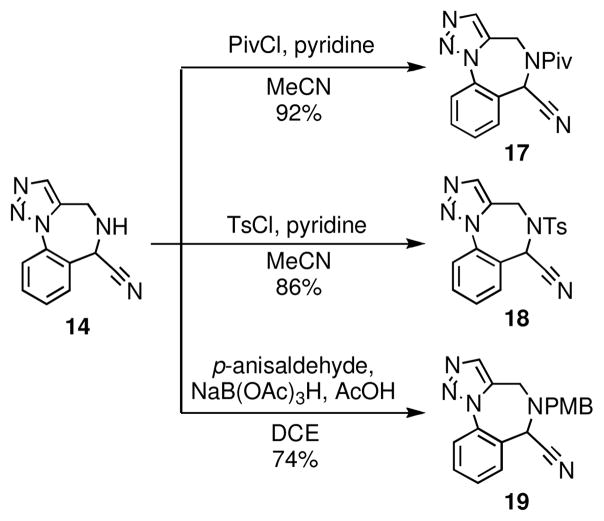
The N-diversification of 14 using a variety of different electrophiles was straightforward and generally proceeded in excellent yields (Scheme 4). Representative examples of N-diversification include N-acylation of 14 to give amide 17 and N-sulfonylation to provide sulfonamide 18. Reductive amination of 14 led to a variety of tertiary amines of the general type 19.
Scheme 4.
N-Diversification of scaffold 14
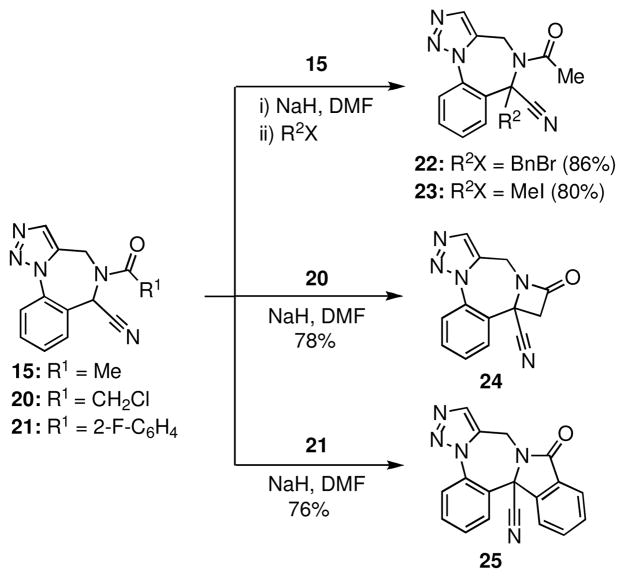
The nitrile function in 14 was incorporated at the outset to serve as a functional handle for introducing additional substituents and to facilitate the formation of other rings. We first exploited the acidic character of the proton adjacent to the nitrile to prepare benzodiazepine derivatives 22 and 23 through intermolecular alkylation of the anion generated by deprotonation of 15, with benzyl bromide and methyl iodide respectively (Scheme 5). The fused β-lactam 24 was prepared by sequential N-acylation of 14 with chloroacetyl chloride to give 20 and treatment of the intermediate amide with sodium hydride in DMF. The β-lactam motif is also a privileged substructure that is present in several pharmaceutical agents and numerous biologically active compounds.2,17 Under the same conditions, the 2-fluorobenzamide derivative 21 afforded isoindolinone 25 in 76% yield. To the best of our knowledge, this is the first example wherein a nitrile α-anion participates in an intramolecular ipso-substitution; this sequence also represents a new entry to isoindolinones, compounds that have also been identified as privileged scaffolds.18 For example, 1,4-benzodiazepine-fused isoindolinones are of interest as potassium channel antagonists in the treatment of cardiac arrhythmia19 and Meniere’s disease.20
Scheme 5.
Amide derived diversification of scaffold 14
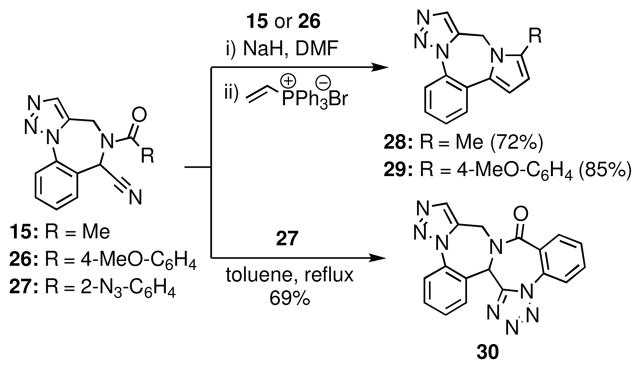
1,2,5-Trisubstituted pyrroles can be synthesized from N-acyl-α-aminoacetonitriles.21 Recognizing that pyrrole-fused 1,4-benzodiazepines have been reported as HIV-1 reverse transcriptase inhibitors,22 we were attracted to the possible application of this underutilized methodology to the synthesis of such compounds. In the event, treatment of the anions generated upon deprotonation of 15 or 26 with NaH with vinyltriphenylphosphonium bromide (Schweizer’s reagent) delivered the pyrroles 28 and 29 in 72% and 85% yields, respectively (Scheme 6). Notably, pyrrole formation was complete at ambient temperature within an hour, conditions significantly milder than the high temperatures and extended reaction times previously reported in the literature for related cyclizations.21
Scheme 6.
Preparation of pyrrole and tetrazole-fused 1,4-benzodiazepines
Tetrazole-fused 1,4-benzodiazepines are known benzodiazepine receptor binders23 and glycoprotein GPIIb/IIIb antagonists.24 Toward preparing such compounds, we found that 27, which was readily prepared from 14 by acylation with 2-azidobenzoyl chloride,25 underwent an intramolecular dipolar cycloaddition upon heating to give the hexacylic fused-tetrazole 30; a related approach to tetrazole-fused 1,4-benzodiazepines has been reported by Garanti.26
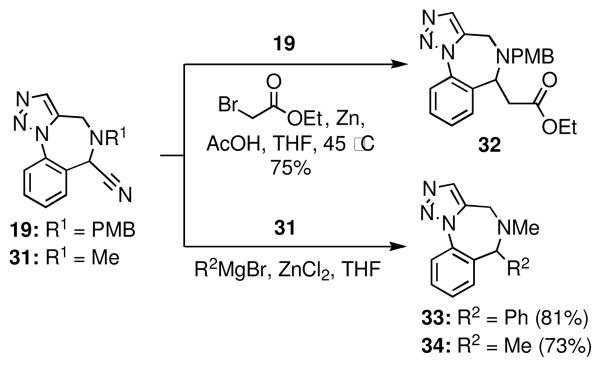
The capability of the nitrile function to serve as a leaving group was exploited to synthesize compounds via the Bruylants reaction (Scheme 7). Displacement of the nitrile from 19 under ‘Reformatsky-type’ conditions afforded ester 32 in 75% yield.27 Amines 33 and 34 were also prepared using a variant of the Bruylants reaction wherein organozinc reagents were used as nucleophiles. It was necessary to transmetallate the Grignard reagents to the corresponding organozinc species in order to obviate competing deprotonation alpha to the cyano function.
Scheme 7.
Diversification using the Bruylants reaction
In summary, we have demonstrated the synthesis of a number of diversely substituted 1,2,3-triazolo 1,4-benzodiazepines based upon scaffolds prepared using our approach to diversity oriented synthesis that features an MCAP involving imines followed by various cyclization reactions. A more efficient route to the parent scaffold 4 was developed, and representative derivatives of 4 were prepared. Brominated analogues of 4 are also readily available and may be used in a variety of cross-coupling reactions. Employing the Strecker reaction as the MCAP introduced an α-amino nitrile into the scaffold as a versatile functional handle. The nitrile functionality present in scaffold 14 can be exploited to enable the introduction of additional substituents by Bruylants reaction, α-alkylation or various arylation reactions. The nitrile group was also used in ring-forming reactions to give pyrroles and tetrazoles. Extensions of these discoveries to the efficient syntheses of novel targeted libraries are the subject of current investigations.
Supplementary Material
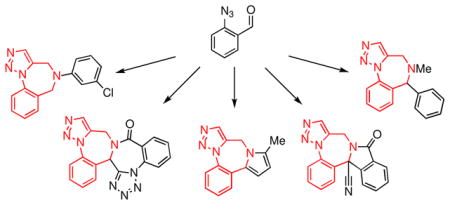
Figure 1.
Derivatives of the 1,4-benzodiazepine ring system
Acknowledgments
We thank the National Institutes of Health (GM 86192) and the Robert A. Welch Foundation (F-0652) for their generous support of this work.
Footnotes
Supporting Information Available: Experimental procedures, spectral data and copies of 1H and 13C NMR spectra for all new compounds and an improved method for preparing 4. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.For reviews, see: Horton DA, Bourne GT, Smythe ML. Chem Rev. 2003;103:893–930. doi: 10.1021/cr020033s.DeSimone RW, Currie KS, Mitchell SA, Darrow JW, Pippin DA. Comb Chem High T Scr. 2004;7:473–493. doi: 10.2174/1386207043328544.Costantino L, Barlocco D. Curr Med Chem. 2006;13:65–85.Duarte CD, Barreiro EJ, Fraga CAM. Mini-Rev Med Chem. 2007;7:1108–1119. doi: 10.2174/138955707782331722.Welsch ME, Snyder SA, Stockwell BR. Curr Opin Chem Biol. 2010;14:347–361. doi: 10.1016/j.cbpa.2010.02.018.
- 2.Bemis GW, Murcko MA. J Med Chem. 1996;39:2887–2893. doi: 10.1021/jm9602928. [DOI] [PubMed] [Google Scholar]
- 3.Evans BE, Rittle KE, Bock MG, Dipardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RSL, Lotti VJ, Cerino DJ, Chen TB, Kling PJ, Kunkel KA, Springer JP, Hirshfield J. J Med Chem. 1988;31:2235–2246. doi: 10.1021/jm00120a002. [DOI] [PubMed] [Google Scholar]
- 4.Ripka WC, De Lucca GV, Bach AC, II, Pottorf RS, Blaney JM. Tetrahedron. 1993;49:3593–3608. [Google Scholar]
- 5.Grasberger BL, Lu T, Schubert C, Parks DJ, Carver TE, Koblish HK, Cummings MD, LaFrance LV, Milkiewicz KL, Calvo RR, Maguire D, Lattanze J, Franks CF, Zhao S, Ramachandren K, Bylebyl GR, Zhang M, Mathney CL, Petrella EC, Pantoliano MW, Deckman IC, Spurlino JC, Maroney AC, Tomczuk BE, Molloy CJ, Bone RF. J Med Chem. 2005;48:909–912. doi: 10.1021/jm049137g. [DOI] [PubMed] [Google Scholar]
- 6.Chang RSL, Monaghan RL, Birnbaum J, Stapley EO, Goetz MA, Albersschonberg G, Patchett AA, Liesch JM, Hensens OD, Springer JP. Science. 1985;230:177–179. doi: 10.1126/science.2994227. [DOI] [PubMed] [Google Scholar]
- 7.(a) Leimgruber W, Stefanovic V, Schenker F, Karr A, Berger J. J Am Chem Soc. 1965;87:5791–5793. doi: 10.1021/ja00952a050. [DOI] [PubMed] [Google Scholar]; (b) Leimgruber W, Batcho AD, Schenker F. J Am Chem Soc. 1965;87:5793–5795. doi: 10.1021/ja00952a051. [DOI] [PubMed] [Google Scholar]
- 8.For the first example of this process, see: Martin SF, Benage B, Geraci LS, Hunter JE, Mortimore M. J Am Chem Soc. 1991;113:6161–6171.
- 9.For a review of related strategies, see: Sunderhaus JD, Martin SF. Chem Eur J. 2009;15:1300–1308. doi: 10.1002/chem.200802140.
- 10.(a) Sunderhaus JD, Dockendorff C, Martin SF. Org Lett. 2007;9:4223–4226. doi: 10.1021/ol7018357. [DOI] [PubMed] [Google Scholar]; (b) Sunderhaus JD, Dockendorff C, Martin SF. Tetrahedron. 2009;65:6454–6469. doi: 10.1016/j.tet.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertelli L, Biagi G, Giorgi I, Livi O, Manera C, Scartoni V, Martini C, Giannaccini G, Trincavelli L, Barili PL. Farmaco. 1998;53:305–311. doi: 10.1016/s0014-827x(98)00025-1. [DOI] [PubMed] [Google Scholar]
- 12.Mohapatra DK, Maity PK, Shabab M, Khan MI. Bioorg Med Chem Lett. 2009;19:5241–5245. doi: 10.1016/j.bmcl.2009.06.107. [DOI] [PubMed] [Google Scholar]
- 13.Pelkey ET, Gribble GW. Tetrahedron Lett. 1997;38:5603–5606. [Google Scholar]
- 14.Alajarín M, Cabrera J, Pastor A, Villalgordo JM. Tetrahedron Lett. 2007;48:3495–3499. [Google Scholar]
- 15.Main CA, Petersson HM, Rahman SS, Hartley RC. Tetrahedron. 2008;64:901–914. [Google Scholar]
- 16.Wolfe JP, Buchwald SL. J Org Chem. 2000;65:1144–1157. doi: 10.1021/jo9916986. [DOI] [PubMed] [Google Scholar]
- 17.del Pozo C, Macías A, López-Oritz F, Maestro MA, Alonso E, González J. Eur J Org Chem. 2004:535–545. [Google Scholar]
- 18.Salcedo A, Neuville L, Zhu J. J Org Chem. 2008;73:3600–3603. doi: 10.1021/jo800266y. [DOI] [PubMed] [Google Scholar]
- 19.Baldwin JJ, Claremon DA, Elliott JM, Liverton N, Remy DC, Selnick HG. Merck & Co. Inc; 9514471. World Patent WO. 1995;(A1)
- 20.Lynch JJ, Jr, Salata JJ. Merck & Co. Inc; 9800405. World Patent WO. 1998;(A1)
- 21.(a) Cooney JV, McEwen WE. J Org Chem. 1981;46:2570–2573. [Google Scholar]; (b) Cooney JV, Beaver BD, McEwen WE. J Het Chem. 1985;22:635–642. [Google Scholar]
- 22.De Lucca GV, Otto MJ. Bioorg Med Chem Lett. 1992;2:1639–1644. [Google Scholar]
- 23.Boulouard M, Gillard AC, Guillaumat PO, Daoust M, Legrand E, Quermonne MA, Rault S. Pharm Pharmacol Commun. 1998;4:43–46. [Google Scholar]
- 24.Robarge KD, Dina MS, Somers TC, Lee A, Rawson TE, Olivero AG, Tischler MH, Webb RR, II, Weese KJ, Aliagas I, Blackburn BK. Bioorg Med Chem. 1998;6:2345–2381. doi: 10.1016/s0968-0896(98)80013-8. [DOI] [PubMed] [Google Scholar]
- 25.Cledera P, Avendaño C, Menéndez JC. Tetrahedron. 1998;54:12349–12360. [Google Scholar]
- 26.Broggini G, Garanti L, Molenti G, Zecchi G. Heterocycles. 1999;51:1295–1301. [Google Scholar]
- 27.Bernardi L, Bonini BF, Capitò E, Dessole G, Fochi M, Comes-Franchini M, Ricci A. Synlett. 2003:1778–1782. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.