Abstract
We review recent advances in brain imaging in humans, concentrating on advances in our understanding of the human brain in clinical chronic pain. Understanding regarding anatomical and functional reorganization of the brain in chronic pain is emphasized. We conclude by proposing a brain model for the transition of the human from acute to chronic pain.
Keywords: fMRI, VBM, brain activity, brain reorganization, acute pain, chronic pain
Descartes proposed that all observable human behavior could be divided into two categories, the simple and the complex. Simple behaviors were those in which a given sensation always, deterministically, produced the same behavioral response …. Complex behaviors, in contrast were those in which the linkage between sensation and action was unpredictable and subject to the vagaries of volition …. They were produced when sensory data were transmitted from the nervous system to the nonmaterial soul, the soul made a decision about what course of action to undertake, and this volitional command was then passed to the machinery of the body for execution.
From Paul W. Glimcher, 2003, Decisions, Uncertainty, and the Brain
Introduction
The state of our understanding of the interaction between pain and the brain is undergoing a veritable revolution with new surprising observations accumulating at a fast pace. A cursory search in PubMed for the term: “functional AND MRI AND pain AND brain” identifies 875 papers and 138 reviews. We will not go over this material. Here we highlight ideas regarding the transition of the human brain from acute to chronic pain, based primarily on human functional and anatomical brain imaging studies, where we examine current advances in understanding, possible underlying mechanisms, and discuss implications regarding both the properties of the brain, our understanding of pain, and possible novel therapeutic venues.
Aristotle categorized pain as an “affect” separating it from primary senses. On the other hand Descartes illustrated a skin to nerve to brain pathway for the transduction of a burning stimulus on the skin to a pain qualia. This dichotomy persists in the discussion of pain mechanisms to this day. It has been exemplified by opposing positions by classic pain scientists such as Hardy and Beecher, and Ed Perl, Ron Melzack and Pat Wall. Pat Wall in fact used Descartes drawings as a means of ridiculing the reductionist attitude that most pain scientist exhibited at his time and that continues unabated in current research. The IASP definition of pain (http://www.iasp-pain.org/) ducks the issue by marrying the two concepts together, and by also stating that pain is subjective and may not be related to an actual injury. This position, however, poses a new quandary: if pain is subjective and minimally related to a stimulus why, and how, do we study its related brain activity? Similarly if pain is both sensory and emotional how well have we faired in disentangling these components in the brain? And, how do these modalities differ between acute and chronic pain? We tackle these issues here, attempting to demystify current understanding of the brain in pain by emphasizing mechanistic implications of research in the field.
1. Brain representations for acute pain, how much further past phrenology?
Human functional brain imaging, since its inception in early 1990s, has concentrated heavily on examining brain properties for acute painful stimuli in healthy subjects. This is not surprising since mapping stimulus-response representations imposes fewer methodological challenges than studying chronic pain [97], and yet there still remain many unanswered critical questions regarding representation/encoding/processing of acute pain in the brain. We will not cover this topic in detail. However, important new concepts are highlighted providing the opportunity to then contrast properties of brain circuitry between acute pain and clinical pain.
A large number of studies have demonstrated a network of brain areas consistently activated with acute thermal, mechanical, and chemical painful stimuli [5; 16; 104; 103; 115]. These brain areas, or some sub-set, super-set, of them have been labeled as the “pain matrix” and used as a brain signature for an activity pattern associated with pain. The data reviewed here argues against this notion by indicating that there is no unitary set of brain regions that one can equate to presence of pain. This is especially true for distinct clinical chronic pain conditions that show unique brain activity patterns. Therefore, the spatial patterns seen for acute pain, although consistent across many labs and brain imaging paradigms, are only valid in healthy subjects and only for acute pain. Localizing a consistent set of brain regions activated with acute pain has been an important step forward, see for example review [21]. However, until we delineate functional roles of involved regions and the temporal dynamics of interactions (for example [6; 81]), our understanding of this circuitry remains at the level of modern phrenology (brain based phrenology) and even of epi-phenomenology. Unless the specificity, causality, capturing of features, and calculations of unique parameters of pain and its spatiotemporal dynamics are identified, the existing “pain maps” provide little more than evidence for responses that may all be unessential, e.g. attentional/motoric/autonomic/anxiety/fear shifts in the presence or in anticipation of impending pain [1].
Correlation to stimulus parameters, primarily its intensity, is one metric used to differentiate between brain regions activated with acute pain. However, this leads to a majority of brain regions that are identified to be active in acute pain showing such correlations [87; 26]. Yet, Coghill and colleagues used this approach and were able to identify that brain regions encoding painful stimulus intensities are primarily in the hemisphere contralateral to the stimulated body site [25]. The same group examined the discrimination between stimulus localization and stimulus intensity to further subdivide the brain areas related to acute pain [82], and advance the exciting notion that pain, similar to vision and audition, may also comprise of a ventral, in this case intensity coding, stream and a dorsal, spatial localization, stream. We as well as other groups have emphasized the need to identifying brain activity relative to the subjective perception of pain [28; 66; 42; 61; 70]. After all we all accept that the brain should reflect perception, and in fact the classical view has been that even peripheral primary afferent nociceptors show a close correspondence in their stimulus-response curves to the psychophysical power function that one observes for thermal or mechanical painful stimuli in humans. On the other hand group averaged afferent activity seems to fail to correlate with ratings of perceived pain [73]. Thus, it is not clear whether there even exist differences in brain activity for stimulus presentation versus subjective perception, and it is possible that brain activity based on stimulus or perception parameters are both looking at the same brain regions with minor changes in interpretational emphasis. Thus, we arrive to a fundamental question, namely, is subjective perception of pain distinguishable from brain regions better representing painful stimuli? If the distinction exists then the latter areas would be more properly labeled as nociceptive regions while the former would be pain perceptive areas. This distinction in fact would correspond to Descartes idea of the difference between the simple and complex categories, with the simple executed by sensory pathways which must be integrated with the subjectivity (Descartes’ soul) to give rise to perceived pain.
A. Distinct brain areas can be called nociceptive or perceptive
Implicit in all studies where brain activity is related to stimulus parameters is the notion that these parameters are uniformly reflected in perceived subjectivity of pain, and of course in a group average this is certainly correct (see for example Figure 1 in [10]). Yet, when one asks subjects to continuously rate their perception of pain, for a constant thermal stimulus applied to the same body site in all subjects, we observe large inter individual variability of responses [10], see Figure 1 top panel. Furthermore, based on differences in stimulus properties, there are marked effects of adaptation and sensitization on pain dynamics [52]. This observation per se indicates that brain imaging studies, where individual subjective differences in perceived pain are not taken into account, a large portion of the brain response variability is directly due to across subject differences in perceived pain. On the other hand, variability of perceived pain in contrast to the constancy of the stimulus provides the opportunity of differentiating between brain areas that better correlate with each, i.e. distinguishing between representation of physical stimulus parameters, nociceptive regions, from regions better reflecting subjectivity or perception of pain.
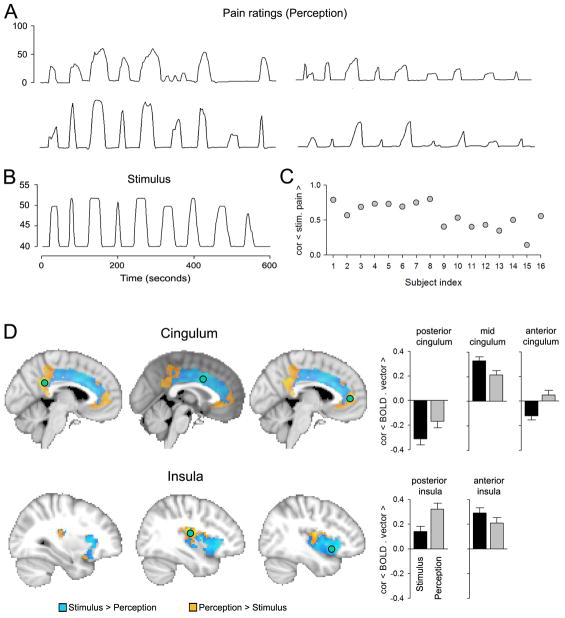
Figure 1.
Segregating the cingulate and insular cortices along the stimulus and subjective perception dimensions for acute thermal pain. A: Variability of subjective ratings of pain is illustrated for four subjects. B: Temporal and intensity properties for a constant thermal stimulus that was applied to the skin on the back. C: Variability of correlation between the stimulus and ratings for the 16 subjects in the study. D: Contrasting cingulate gyrus and insula activity between stimulus and perception identifies statistically significant regional differences in each area along these dimensions. Green circles are regions where activity was extracted and correlated either with the stimulus or individual subject perceptions, corresponding bar graphs are in the right, which indicate the sign and extent of difference between representation of stimulus and perception. Anterior cingulate and posterior insula are regions best related to pain perception. All data are derived from the study described in [10].
Parts of anterior cingulate (ACC) show high incidence of activation in pain tasks [5], as a result ACC is commonly dubbed as the main brain region signaling pain, or emotional pain. Yet single unit recordings from the region in awake humans does not show nociceptive responsive cells. Instead ACC neurons seem to respond to cognitively demanding situations, including those with emotional valence [30], implying that the region is more involved in anticipation of salient conditions. It is also important to note that a large number of non-painful tasks activate various portions of the cingulate cortex. Similar to the ACC, insular cortex shows a very high incidence of activation in pain tasks [5], and parts of the region are dubbed signaling either sensory or emotional/affective properties of pain. Electrical stimulation of posterior parts of the insula in humans does give rise to pain-like sensations [70]. Additionally some lesion studies imply that insults to the insula may decrease pain perception [14; 90]. A more recent study, on the other hand, shows that unilateral insula lesions increase, rather than decrease, thermal pain responsiveness [102]. Similarly to ACC, insula is also activated for a very large number of non-painful tasks [60].
Given the importance of the cingulate and insula in pain perception, we analyzed these regions for spatial segregation along the dimensions of nociceptive representation versus pain perception, using published data [10]. Figure 1 bottom panel shows the spatial segregation of the two brain regions along stimulus-perception representation. We observe that the anterior portion of the cingulate and the posterior insula better reflect pain perception, while large portions of both structures are mainly related to thermal stimulus properties.
B. Strength of relationship between brain activity and pain consciousness
The fact that we can localize brain areas that preferentially encode subjective perception of pain first implies that the transformation from stimulus space to subjectivity is a cortical process, the details of which remain to be understood. It also raises the important question of the extent to which this region reflects pain subjectivity, which also impinges on issues regarding consciousness and pain. To date humans lack physical theories of consciousness. As a consequence the best evidence we can advance of relationships between consciousness and biological processes can be correlative. The strength of evidence for the portion of insula better related to pain perception is shown in Figure 2. The figure illustrates that the brain activity, as determined by fMRI, in this insular region reflects the magnitude of subjective pain every time a participants indicates her/his perceived magnitude of pain, with a linear correlation between brain activity and magnitude of subjective perception. Therefore, there is a very tight relationship between this area of the brain and pain consciousness. Besides a second more lateral extension of the region in the prefrontal cortex, no other brain region showed such representation of subjective pain. On the other hand, as Figure 2 shows, the region also faithfully reflected brain activity for rating the magnitude of visual stimuli. Therefore, we can state that this area is unique in reflecting pain subjectivity but is not specific to any particular sensory modality. Therefore, we have proposed that the area is instead specialized in extracting subjective magnitudes in general [10], and argue the notion that the insular stream of information processing, in analogy to the dorsal and ventral visual streams that encode ‘where’ and ‘what’, may be extracting a signal in relation to subjective ‘how much’. Thus we propose that conscious perception of pain is the end product of the transformation of nociceptive information to a magnitude, achieved in a brain region that also extracts magnitudes for visual lengths, and in analogy also perhaps visual brightness, auditory loudness, olfactory pungentness, etc, relationships that remain to be systematically studied.
Figure 2.
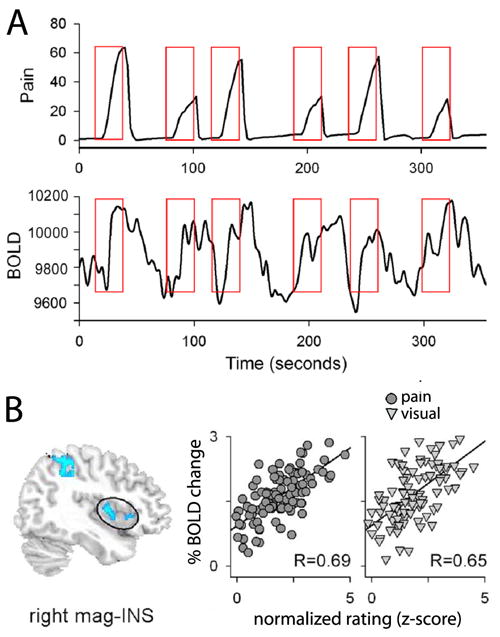
Insular region that reflects pain perception shows a one-to-one relationship with each epoch where pain is reported. Moreover, this area also just as faithfully encodes perceived magnitudes for bars displayed visually. Thus, the brain area accurately reflecting pain subjectivity seems to be encoding magnitudes in general and thus lacks specificity. A. Illustrates the method used for generating the scattergrams in B. For each epoch of perceived pain, the magnitude of peak pain rating is extracted and correlated to the peak fMRI BOLD activity identified within the thermal stimulus time window (illustrated in red boxes). B. Left figure shows the region of the insula that encodes perceived pain and perceived magnitude of lengths of visual bars (circled region is right magnitude related insula, mag-INS). First scattergram is the epoch-by-epoch pain perception to BOLD relationship, across all subjects. Second scattergram is for the visual magnitude-rating task. Correlation coefficients are indicated and closely match for both sensory dimensions. Data and figure adapted from the study described in [10].
C. Temporal transformation of pain representation: from anticipation to perception to relief
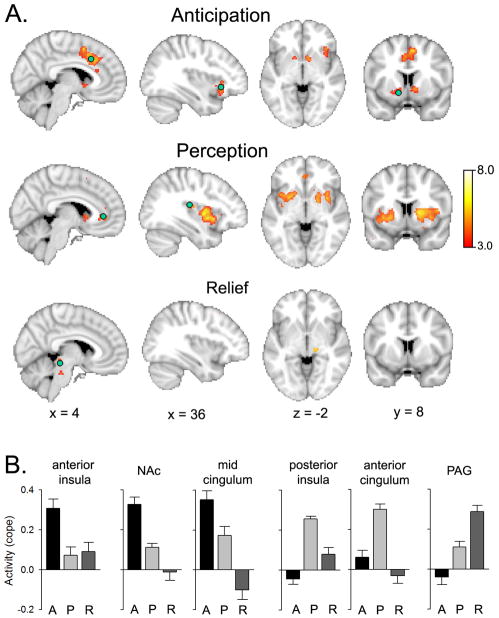
The fact that pain is processed far more slowly by the nervous system than any of the other sensory modalities makes it an ideal sensation with which fMRI brain activity can be segmented in the time domain to component circuitry that process incoming information and spatiotemporally extract specific features. Yet another fundamental property of pain is its salience, which simply corresponds to the common experience that the prospect of impending pain or the experience of pain both engage one’s attention, forcing a motivational state where the subject needs to make decisions as to the immediate future. Of course proper anticipation of impending pain is fundamental for survival and must thus be closely linked to reward, motivation and decision making [34]. Brain activations to signals anticipating impending pain has been studied by a number of groups [85; 84; 13; 86; 110; 20]. For the most part these studies concentrate on examining distortions of brain activity for the stimulus in the presence of anticipatory cues, as well as brain responses to the cue itself. In a recent study we demonstrated that nucleus Accumbens (NAc) signals anticipation of pain perception even when the stimuli are applied randomly and in the absence of any external cues [12]. At the start of an unpredictable painful thermal stimulus NAc activity captured the rate of change of the stimulus applied on the skin, an activity that preceded the stimulus itself as well as the related perception. In figure 3 we reanalyze those data by contrasting brain activity between three epochs, anticipation (early activity at start of stimulus), perception, and relief (late activity at stimulus end). The results indicate segregated networks that preferentially are involved in each epoch, with anticipation engaging NAc, mid-cingulate and anterior insula; perception activating anterior cingulate, mid and posterior insula, and large portion of dorsal striatum; and pain relief engaging brainstem activity especially the periaqueductal grey (PAG). The brain networks identified in figure 3 complement those illustrated in figure 2, in that regions involved in anticipation are also regions that better relate to stimulus, rather than perception, encoding. The PAG activity is similar to earlier brain imaging observations of the region signaling pain relief [32; 113; 33] and consistent with the region being involved in descending anti-nociceptive modulation, as repeatedly shown in animal studies. The main point of figure 3 is the illustration that distinct portions of brain regions known to be involved in acute pain show a specific spatiotemporal evolution as these areas extract distinct types of information in relation to the nociceptive stimulus and ensuing subjective perception of pain.
Figure 3.
Segregating brain activity for acute thermal pain perception between anticipation, perception and pain relief identifies distinct networks sequentially activated during perception of acute pain. Three vectors separated in time were contrasted for a thermal painful stimulus that was a randomized sequence of stimuli where intensity duration and inter-stimulus intervals were not predictable. The anticipation vector identified increased activity preferentially related to only the start of the stimulus; while perception was identified by subjective ratings of pain; and relief period was identified for increased activity in the time window when the stimulus was returning to baseline. A. Three separate and complimentary networks are identified. B. Differential activity for the three phases of pain perception is illustrated for indicated regions (green circles in A) (A = anticipation, P = perception, R = relief). Data are derived from the study described in [12].
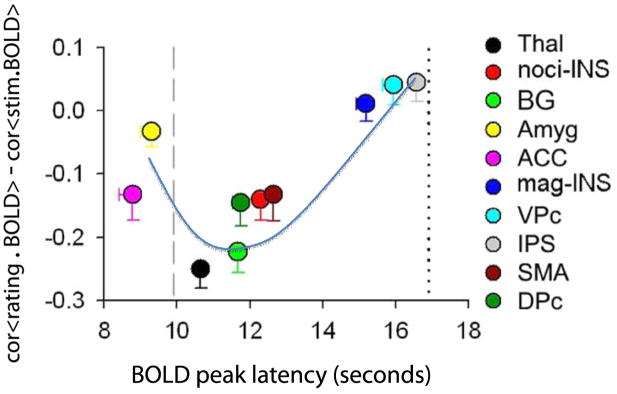
The networks identified in figure 3 were determined using a-priori vectors that would define the types of information that can be extracted within a given time window. An alternative approach to determining the spatiotemporal evolution of information flow during pain perception would be to interrogate the properties of brain areas identified to be active during the task. The latter approach was used in a recent study [10], and the main outcome is illustrated in figure 4. Fourteen brain regions were observed active during rating of acute thermal pain. The thermal stimulus peak (after convolving with fMRI hemodynamic function) on average preceded the peak perception by about 8 seconds. Within this time window one could then examine the sequence of activations across the 14 brain areas by determining each region’s peak activity. Moreover, as the shape of the brain activity differs between brain regions, its similarity can be tested to the stimulus and perception. These two parameters provide a 2-diemnsional space within which the fourteen brain regions can be localized. Figure 4 shows that the amygdala and mid ACC peak before the stimulus itself peaks. Thus, the mid ACC, now together with amygdala, is again showing an anticipatory response. We then observe a sequence of activations in brain regions that are consistent with being more nociceptive including the thalamus, dorsal striatum, supplementary motor area, and nociceptive insula, all of which peak just after the stimulus peaks and their activity patterns show better similarity to the stimulus. One the other hand perception related magnitude region of the insula peaks much later, just preceding perception and its activity better correlates to perception. Thus we observe a spatiotemporal sequence of activity that is consistent and complimentary to the sequence identified in figure 3. The information transformation from NAc, amygdala and mid ACC, to the thalamus and nociceptive insula, and finally to perceptive/magnitude encoding insula closely corresponds to Coghill’s notion of a ventral stream specialized in processing intensity of painful stimuli [82]. Thus, detailed information processing steps can be observed and parceled in the human with fMRI, especially for acute pain where the sequence of transitions from stimulus to perception is slow enough to enable such analyses.
Figure 4.
Temporal sequence of brain activity for thermal painful stimuli determined in relation to anticipated peak for the stimulus (vertical line at 10 seconds from stimulus start) and actual reported peak of pain perception (vertical line at 17 seconds). Vertical scale is similarity of brain activity shape to the stimulus and perception shapes (negative values are better correlations to stimulus shape while positive values are better correlations to perception, determined for specific BOLD activity extracted for all regions listed on the right). The anterior cingulate (ACC) and amygdala (Amyg) peak at times prior to the stimulus peak, implying that these regions are more related to anticipation of impending pain. The thalamic activity (Thal) peaks just after the stimulus and best reflects stimulus shape. Nociceptive insula (noci-INS) and magnitude insula (mag-INS) are segregated by time and shape similarity, reflecting their respective functional labels. The blue curve approximates the brain spatio-temporal evolution of nociceptive information being transformed into subjective consciousness of pain. BG = basal ganglia; VPc = ventral prefrontal cortex; IPS = inferior parietal sulcus; SMA =supplementary motor area; DPc = dorsal prefrontal cortex. Adapted from [10].
D. Medial and lateral pain systems and emotional and sensory representations
Two elegant studies by Bushnell and colleagues first established that human brain activity may be sub-divided along emotional and sensory components [88; 54], where mid ACC was identified as being modulated by affect and primary somatosensory cortex by sensory properties of pain perception. Complementary evidence along these lines were studies by Zubieta and colleagues [114] who showed distinct brain activations correlated to the sensory or affective components of the McGill Pain Questionnaire (MPQ). These observations build on notions that date to the beginning of the twentieth century when Sherrington in 1906 [100] advanced the idea that pain has both sensory and emotional qualities and, Henry Head in 1911 [53] distinguished between protopathic and epicritic pathways. Ideas that were further propounded in the 1960s [71] regarding a medial and lateral spinothalamic pathways, with the former being involved in affect and the latter in sensory encoding. Yet this concept has not progressed much further and the notion of segregating brain activity for acute pain along these two dimensions remains relatively weak. For instance, it should be noted that many spino-cephalad pathways show considerable crosstalk [43; 50]. A major difficulty is inherent in the IASP definition, stating that pain is always unpleasant, based on the fact that intensity of pain and magnitude of unpleasantness of pain are almost always highly correlated with each other. Therefore, rendering distinctions between these components is hard. Despite the weak evidence regarding segregation of sensory and affective mechanisms of pain, authors, almost arbitrarily, assign the tag of sensory or affective to various brain regional activations, relying mostly on the classical anatomy that shows that lateral thalamic regions project to primary and secondary somatosensory regions, while medial thalamic projections target limbic and frontal cortex. The case for insular activity is worst as een the anatomical guide fails for this brain region.
It has recently been argued that an alternative approach to distinguishing between sensory representation and affect would be to examine the brain from the viewpoint of motivation [34] and, with this approach we were able to identify that NAc activity not only signals anticipation of impending pain, but seems to also calculate the reward value of pain relief at stimulus cessation by integrating information from the magnitude region of the insula, in healthy subjects [12]. The motivational approach provides the opportunity of testing the emotional valuation of pain by behavioral and decision-making measures independent of sensory encoding. Yet this line of work especially for pain remains in its infancy.
Overall summary
The section above illustrates the brain dynamics for acute pain. The brain regions involved and their properties provide a background relative to which we can contrast brain activity for clinical pain conditions.
2. Functional properties of the brain in chronic clinical pain
Clinically the most relevant conditions where human brain imaging can have a substantial impact are chronic conditions, as they remain most poorly understood and minimally treatable by existing therapies. The IASP definition of chronic pain is either based on duration of pain persisting past an inciting event (arbitrary number of 3–6 months) or pain extending past the healing process from the initial injury. We have commented on the inadequacy of this definition [4] and also proposed possible extensions based on the recent brain imaging evidence [2]. The challenge in studying clinical chronic pain conditions is to distil the relevant pain from other confounds and, relate brain activity directly to this clinical pain. As most chronic pain conditions remain minimally controlled by therapeutics, and as therapeutics, especially pharmacological approaches with central action, will also interfere non-specifically with brain activity, there remains little leeway of options for such brain imaging studies. A number of recent reviews summarize fundamental findings regarding brain activity and anatomical changes in chronic pain [4], [63], [103]. Advances in understanding CRPS in the context of cortical reorganization has also been reviewed [98].
A. Specificity of brain activity relative to spontaneous fluctuations of the pain of chronic back pain
Even though many chronic pain patients show signs of allodynia and/or hyperalgesia to various stimuli applied to the skin, by far the most prominent exhibition of chronic pain is uncontrolled ongoing pain in the absence of any external stimulation. We have uncovered that this spontaneous pain has characteristic fluctuations (in the scale of seconds to minutes) that are distinct for different types of chronic pain and that cannot be mimicked by healthy subjects pretending to have pain [36]. Therefore continuous ratings of these fluctuations can be used as a tool to study spontaneous pain related brain activity. In fact, given that continuous ratings can capture spontaneous pain, the subjective perceptual rating of pain becomes a universal tool with which one can tease out specific pain components, as the same exact approach can be used to rate a stimulus, such as described above for thermal pain, it can also be used to provoke clinically relevant pains, like localized allodynia and hyperalgesia, as well as spontaneous pain (after properly correcting for motor, cognitive and attentional confounds). We have used this technique to identify brain activity for spontaneous pain in chronic back pain patients [9]. The surprise was that the brain region best reflecting high magnitude of back pain was localized to the medial prefrontal cortex (mPFC), extending into anterior ACC; a region not anticipated by acute pain studies. Additionally, brain areas observed for acute pain, like portions of the insula and mid-ACC were only active transiently and only when the back pain magnitude was on the increase. These results are exciting because for the first time we are able to observe brain activity reflecting the subjective perception of the pain that chronic back pain come to the clinic to complain about (or an objective marker for the Cartesian soul of back pain). We interpret the transient activity as a nociceptive signal from the periphery, which then is converted into a sustained emotional suffering signal in mPFC.
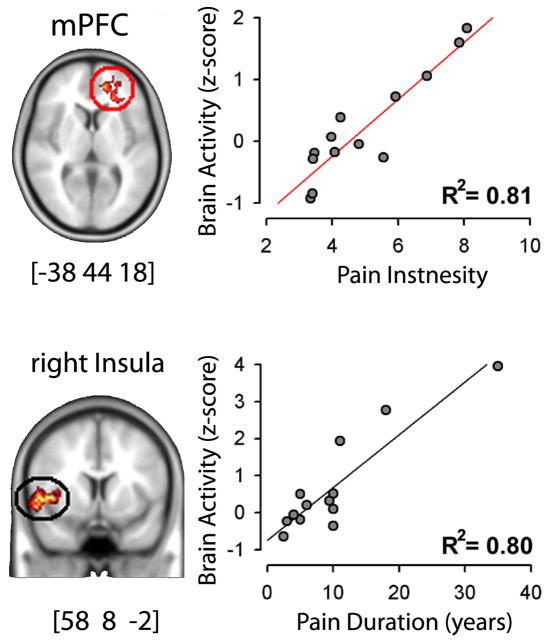
These observations are based on patients’ own ratings where the experimenter has no control and cannot discern if the patient is actually reporting anything factually related to the phenomenon of interest or is simply rating whatever comes to her/his mind. This in fact is a major weakness of many similar brain-imaging studies and requires an alternative approach to identify veracity of the results. We overcome this limitation by performing an across subject correlation analysis examining the relationship between intensity and duration of back pain to brain activity. Figure 5 shows that mPFC activity tightly correlates with amount of pain the patients reported just before starting brain scans, and also that the insular cortex activity correlates with the number of years each subject had been suffering from their back pain. Thus, the two fundamental clinical properties of the back pain are captured at a high enough correlation strength that we can state that looking at these brain regions in other such patients we should be able to predict their back pain magnitude and duration within 20% error. These findings were reproduced in a second group of chronic back pain patients and were also compared with brain activity for rating a thermal painful stimulus applied to the skin on the back. When insular and mPFC activity was examined in these patients and in healthy subjects we found that mPFC activity was related to spontaneous pain but not with thermal pain, while the insula activity was related to the thermal pain but not spontaneous pain. With this technique, we were able to demonstrate a double dissociation for representation of perceived magnitudes between acute thermal pain and spontaneous chronic back pain.
Figure 5.
Brain activity for rating spontaneous fluctuations of back pain in chronic back pain patients show a strong correlation with intensity and duration of the condition across all participating patients. The observed correlations are strong enough that we can assert that the task can be used to predict intensity and duration of chronic back pain in individual subjects within an error of 20%. Figure adapted from [9].
Thus, we can assert that, at least in this group of chronic pain patients, different brain areas encode the perceived magnitude for distinct types of pain. The prevalent expectation for brain activity in chronic pain is a sustained or enhanced activation of the brain areas already identified for acute pain. This view is partly implied by the chronic pain definition and by notions of specificity theory or labeled line theory of pain (where supraspinal organization and representation of pain is assumed to be through fixed and immutable routes). This is exactly what we do not see. Instead these results imply that functional anatomy or physiology or some combination of both have changed in the brain of chronic back pain patients. Below we elaborate on underlying possible mechanisms for this reorganization.
It is also important to remember that the close relationships between fundamental properties of back pain and activity in mPFC and insula are correlational, and both mPFC and insula respond to a long list of cognitive and emotional states. Thus, the phrenology of observing these areas activated and interpreting them as evidence for existence of pain is false. In fact even taking into account the underlying dynamical network is most likely a poor (linear) underestimation of the total brain state that constitutes such a condition, and we suspect that current concepts of the brain encoding any given perceptual/cognitive condition remains very limited.
B. Specificity of brain activity across multiple chronic pain conditions
As explained above the approach of continuous rating of fluctuations of pain may be applied for a variety of conditions. Besides back pain, we have now studied brain activity for spontaneous pain in post-herpetic neuralgia and its modulation by a lidocaine patch therapy [40], in chronic pelvic pain patients (manuscript in preparation), and in osteoarthritis (manuscript submitted); additionally in post-herpetic neuralgia we have examined brain activity for tactile allodynia, and in osteoarthritis for mechanical painful stimulation of the arthritic knee. The brain activations observed in these various pain groups are summarized in figure 6A. What is common between all the brain maps is the presence of pain. Thus, we can ask the simple question as to what brain areas are commonly active between these conditions, which would identify the center of pain perception, irrespective of the type of pain. Unfortunately the conjunction across all results in an empty map, suggesting that distinct clinical pain involve unique brain regions. The figure also illustrates that thermal or mechanical acute painful stimuli in healthy subjects or in chronic pain, in this case osteoarthritis, they essentially activate very similar regions. On the other hand brain activity for spontaneous pain seems to involve different brain regions for distinct chronic pain groups. To better illustrate this point we show group average magnitude of brain activity in two limbic brain areas and two sensory areas and for a variety of groups and stimuli, illustrating a complex gradient of brain activity across these brain areas that distinguish between stimulus types and chronic pain types.
Figure 6.
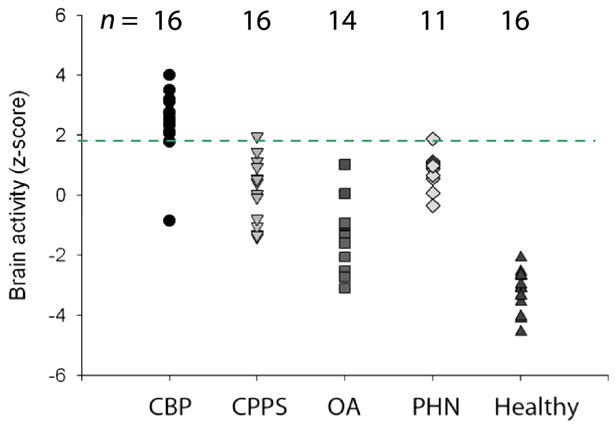
Figure 6A: Brain activity patterns for various clinical chronic pain conditions. Activity maps: are group-averaged responses for different pain conditions. Activity maps: Thermal pain, knee-pressure induced pain in healthy subjects, and in osteoarthritis patients (OA) show similar patterns of brain activity, implying that all three results correspond to acute pain activity. In contrast, brain activity for spontaneous pain in different clinical conditions (chronic back pain, CBP; osteoarthritis, OA; pelvic pain, CPPS; and post-herpetic neuralgia, PHN) show different activity patterns, engaging to different extents sensory and limbic brain areas. In PHN, tactile allodynia and spontaneous pain evoke relatively distinct brain regions too. Bar graphs: Magnitude of activity in 2 limbic regions (mPFC and Amygdala) and 2 sensory regions (thalamus and insula) are compared for four groups: healthy subjects for thermal pain, Healthy th; chronic back pain patients for spontaneous pain, CBP sp; post-herpetic neuralgia for spontaneous pain, PHN sp, and for tactile allodynia, PHN al). Thermal pain and PHN allodynia show larger activity in the sensory regions, while spontaneous pain in CBP and PHN evoke more limbic activity.
Figure 6B: Brain activity in medial prefrontal cortex (mPFC) shows high specificity for chronic back pain. Magnitude of mPFC regional activity (as identified in chronic back pain patients) across five groups of subjects. Each symbol is an individual subject. The threshold indicated by broken green line distinguishes chronic back pain (CBP) from pelvic pain (CPPS), osteoarthritis (OA), post-herpetic neuralgia (PHN) for spontaneous pain, and healthy subjects for acute pain (healthy) at an accuracy > 90%. The number of subjects studied in each group is indicated above.
Figure 6B addresses the issue of specificity of brain activity for various pain conditions from the viewpoint of a single brain region and in relation to individual subjects’ activity. As mPFC is activated for spontaneous fluctuations of chronic back pain, we interrogate its activity across a number of other acute and chronic pain conditions, and observe that it has a very high accuracy is distinguishing chronic back pain from other chronic pain conditions and from acute pain in healthy subjects.
C. Presence of chronic pain interferes with brain processing in general
Above we concentrated on the evidence for specific brain activity signatures for acute and chronic pain. Given the salience of pain one expects that presence of pain will distort brain processing other information. Interaction between acute pain and sensory and emotional distractions has been repeatedly documented [109; 65; 64; 108]. Here we briefly review the accumulating evidence regarding chronic pain. Our first main observation of chronic pain state disrupting brain processing was observed in postherpetic neuralgia patients. We have studied these patients before and after lidocaine patch treatment. An open labeled study where spontaneous pain was significantly decreased with the therapy. As the patients rated their ongoing pain and a visual bar we tested the idea that whole-brain activity may be modulated by the intensity of the chronic pain for both the pain and non-pain tasks [40]. Figure 7 shows that posterior parietal activity is generally decreased while prefrontal medial and lateral activity increased for both task with intensity of this pain. Parietal decreased activity suggests that brain attentional networks are attenuated by the chronic pain, while the frontal increased activity may be due to cognitive and emotional enhanced processing. Yet, the observed brain regions modulated are vast and complex and undoubtedly other processing abilities are distorted in these patients. In a second, more systematic approach to the question, we examined brain activity differences between healthy subjects and chronic back pain patients for rating the fluctuations of a visual bar. Performance was matched between the groups and increased brain activity also matched. On the other hand decreased activity was significantly different [11]. As the brain regions in the decreased activity corresponds to a specific brain network, default mode network, we tested the balance between positively and negatively correlated networks and could show the overall balance of these networks is shifted in the chronic back patients. These results suggest that the brain in resting state (brain activity in the absence of subjects doing anything specific) should also be distorted in chronic pain conditions, and this in fact has now been asserted for a number of clinical conditions [22; 68; 77; 23]. The study by Malinen et al. [68] is noteworthy as the authors extend the observations of distorted resting state networks to also report that the frequency of brain activity during rest is higher in the insula and anterior cingulate in chronic pain patients (Figure 8). We have been able to replicate the latter result in chronic back pain patients and additionally demonstrate that the increase in power at the high frequency range is related to the characteristics of the back pain (manuscript in preparation). It should be mentioned that studying chronic pain patients’ brain during rest promises to be yet another fruitful venue that should inform us of the global dynamical properties of the human brain in pain. How such changes come about? Are they specific for different clinical conditions? To what extent are they driven by anatomy, behavior, or peripheral reorganization? And, how are they modulated by therapeutics? These are all exciting and addressable questions.
Figure 7.
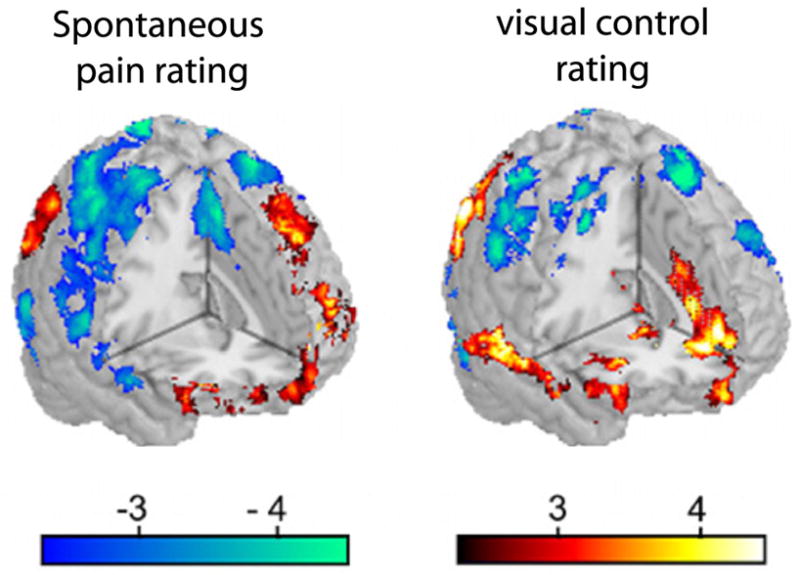
Intensity of ongoing chronic postherpetic neuropathy pain changes brain activity and thus cognitive processing in a complex pattern, for pain and non-pain tasks. The figure is adapted from a study [40] in which postherpetic neuropathy patients were studied before and after lidocaine application on the painful skin. Each patient was scanned at three time points relative to the drug therapy. In all cases the patients performed two different tasks: in the pain task they continuously rated the fluctuations of their spontaneous pain (left), and in the visual task they rated fluctuations of a bar varying in time (right). The relationship between brain activity and intensity of ongoing pain was determined using a covariate analysis, in which the related pain intensity for each fMRI scan was used to determine the effect of this parameter on brain responses. Across subjects and across all scans, average variation of brain activity is displayed for both tasks. The presence of the chronic pain effects activity in both tasks across large brain areas, rather similarly, increasing activity in some areas (red) and decreasing them in others (blue).
Figure 8.
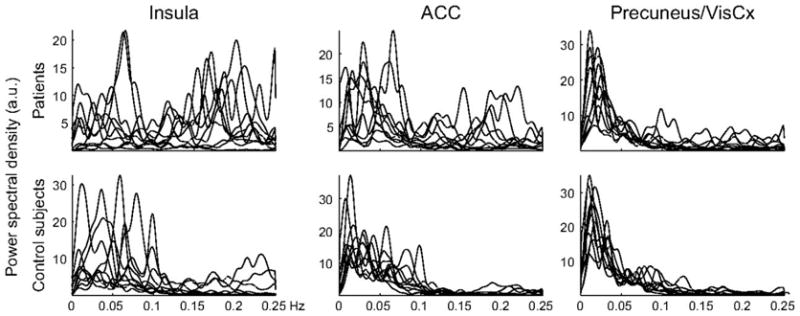
In chronic pain patients and in the absence of any specific task (resting state) activity in the insula and anterior cingulate, but not in the precuneus/visual cortex, fluctuate at higher frequencies than in control subjects. Power spectral density as a function of frequency is shown for each individual subject. Figure from [68].
D. Chronic pain distorts motivational value of analgesia
Above we concentrated on the evidence for specific brain activity signatures for acute and chronic pain. Here we identify interactions between motivational systems in acute pain and how they are modified by chronic pain. Acute pain plays a protective role in that its presence elicits the motivation to escape or avoid exposure to minimize further damage and its termination provides a sense of relief. Together these processes contribute to predicting the utility and costs of competing goals and in behavioral decisions in the presence of conflict between potential threat and reward. Chronic pain lacks these motivational advantages and instead imposes a persisting stress on these protective and adaptive systems. Only one paper has addressed chronic pain from the view of motivated behavior and the results are surprising and unexpected [12].
We [12; 9; 10] and an earlier study by Derbyshire and colleagues [31] have shown that the perceived magnitude of thermal pain and related representation of the stimulus and perception do not differ between chronic back pain patients and healthy subjects. On the other hand a number of studies do demonstrate increased brain activity for mechanical pain in distinct chronic pain conditions [44; 83; 42]. It is not clear where the discrepancy between these studies lies, is it due to differences in sensory modality tested, i.e. mechanical central sensitization but not thermal, or technical differences. Still the lack of evidence for thermal hyperalgesia in chronic back pain seems convincing. On the other hand when brain activity is contrasted for thermal pain between healthy controls and chronic back pain patients we observe NAc activity distinguishing between the groups [12]. Moreover, the difference in NAc activity between the groups could be attributed to its phasic response at the end of a thermal stimulus, with healthy subjects showing a signal that reflects prediction of reward, while the patients showing a signal that is instead more consistent with a lack of predicted reward and even perhaps a disappointment. These NAc responses reflect that chronic pain patients anticipate analgesia from acute pain and in this way the value associated with acute pain is distorted in these patients. In fact this reversal of signal was strong enough that it distinguished the groups at accuracy of more than 90%. The result is remarkable because it is independent of the subjective pain perception. Thus, this distorted assessment of analgesia is not captured in the conscious pain ratings, instead it is should be regarded as a subconscious evaluation that must in turn color subsequent actions/decisions.
In search of a brain source for the distorted NAC activity, we examined its connectivity to the rest of the brain in both groups. The identified networks were distinct. In healthy subjects NAc was mainly correlated to the insula, while in the patients it was better correlated with mPFC. Moreover, the strength of connectivity between NAc and mPFC was in direct proportion of the amount of back pain any given patient reported experiencing (figure 9). The strength of this across patient correlation of connectivity implies that the more someone is in back pain the more information is shared between the two brain regions, and this parameter can also be viewed as an objective measure than can identify the magnitude of back pain with a paradigm where the participant is not even aware of what is being measured. The patients were basically signaling that they prefer keeping this acute pain on their skin and are disappointed when it ceases, although their conscious rating of the painful stimulus and brain activity for the pain perception were identical to healthy subjects’ perception and brain activity. To capture the impact of this NAc signal on perception we examined ratings of spontaneous pain in another group of chronic back pain patients during the thermal stimulation and could demonstrate that this stimulus was actually analgesic to their spontaneous pain, to the surprise of the patients and only when patients specifically directed their attention to their own pain (figure 10). The mechanisms regarding the transition of NAc from sharing information with insula (healthy subjects) switching to sharing information with mPFC comes (chronic pain patients) remain to be studied. Also, how prevalent is this switch across different chronic pain groups, its relationship to conscious vs unconscious events, and a myriad related questions remain to be addressed. The study, in a sense, demonstrates that one can explore the impact of the emotion of pain outside of the sensory/affective, entangled, perception representation, and that the components of the Cartesian soul can be broken into their constituent subconscious parts in the brain, which in turn influence conscious behavior outside or independent of sensory representation.
Figure 9.
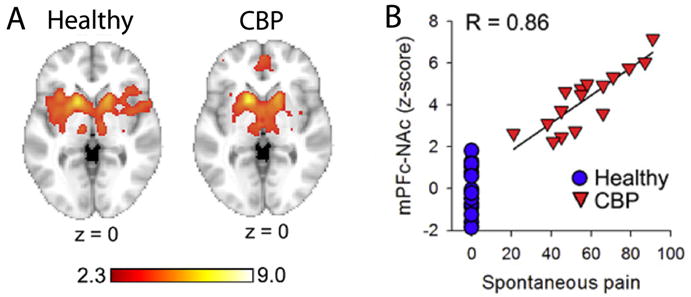
Distinct functional connectivity between nucleus accumbens and the rest of the brain are observed in chronic pain patients in contrast to healthy subjects. This shift in connectivity is tightly correlated to the magnitude of back pain reported by the patients. A. Healthy: Functional connectivity between nucleus accumbens and the rest of the brain in healthy subjects. We observe extensive bilateral insula involvement. A. CBP: Functional connectivity for nucleus accumbens in chronic back pain patients. Functional connectivity is shifted away from the insula to medial prefrontal cortex. B. Strength of connectivity between nucleus accumbens and medial prefrontal cortex in relation to the magnitude of back pain reported. Each red symbol is an individual chronic back pain patient; blue circles are healthy controls. The higher the magnitude of spontaneous pain of back pain the stronger is the correlation between mPFC and NAc, implying more information sharing between these two brain regions. Figure adapted from [12].
Figure 10.
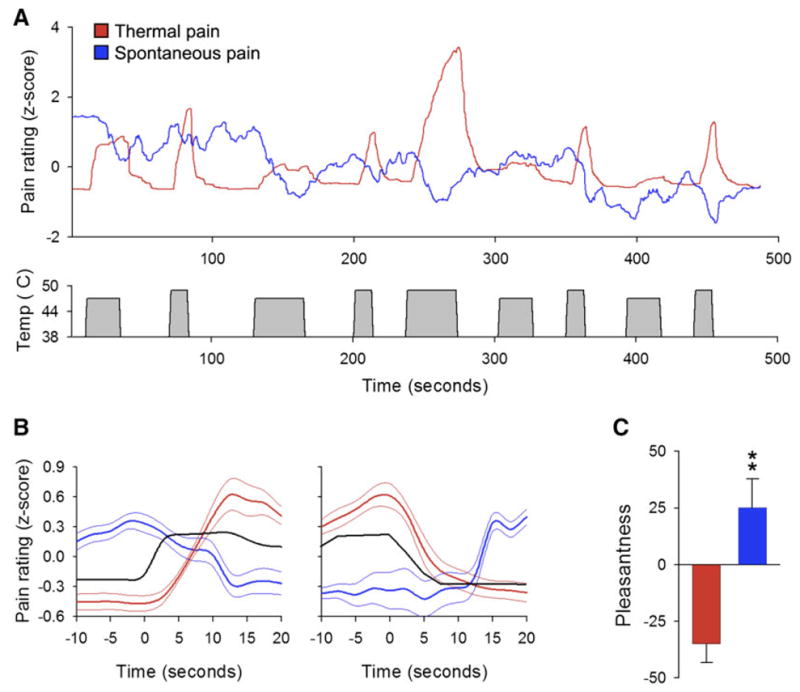
Chronic back pain patients report a decrease in the magnitude of their back pain during a thermal painful stimulus applied to the skin on their back. They seem to only realize this fact when they are specifically instructed to attend to their own back pain. A. Group average ratings of either the magnitude of thermal painful stimulus (red) or spontaneous fluctuations of back pain (blue), for a stimulus pattern shown below (grey) that is unpredictable in intensity and duration. Every time the stimulus is felt painful it seems to induce a decrease in spontaneous pain. B. Average stimulus pain perception (red) and spontaneous back pain perception (blue) relative to start and end of thermal stimulus (black). Rating of the stimulus intensity and back pain were done separately. C. When the back pain patients rate the stimulus, they judge the experience as unpleasant (red). But for the same stimulus when they rate their own back pain the experience is judged to be significantly more pleasant (blue). Figure adapted from [12].
3. Distortions of brain anatomy in chronic pain
About ten years ago we presented the first evidence for brain metabolite abnormalities, as measured by magnetic resonance spectroscopy (MRS), in chronic back pain patients [46; 45; 47]. We predicted in those studies that brain grey matter should show atrophy, and in 2004 we published the first results for brain grey matter distortions in chronic back pain [8], since then a steadily accumulating literature has been documenting evidence for brain morphological changes in multiple clinical pain conditions: back pain [92], fibromyalgia [58; 95; 67; 55], complex regional pain syndrome [41], knee osteoarthritis [89], irritable bowl syndrome [15; 29], headaches [93; 56; 91; 107], chronic vulvar pain [96], and in females suffering from menstrual pains [105], as well as in animal models of chronic pain [72; 99]. The evidence for metabolic changes with chronic pain has come more slowly as only a handful of studies have been done on the topic [48; 106; 37]. Metabolic and morphological approaches are complementary techniques and comparisons of outcomes with both should enhance understanding of underlying mechanisms. However, MRS remains technically underdeveloped and fraught with confounds, as a result its impact on brain neuroscience in general has lagged.
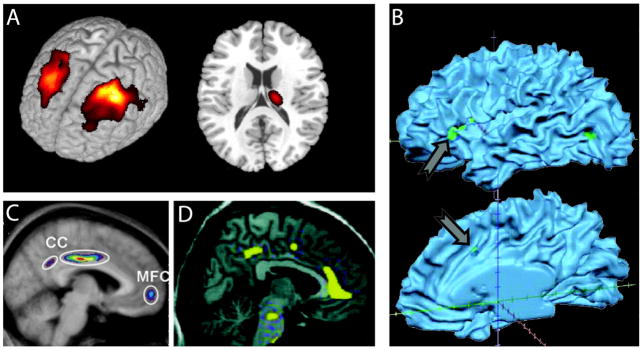
Figure 11 illustrates some of the pain conditions for which brain grey matter properties have been reported to be abnormal. Our initial morphometric study and a number of subsequent studies demonstrate that specific regional grey matter decreases correlate with duration of chronic pain, its intensity, and even the interaction between both factors [8; 59; 41], suggesting that the properties of the clinical conditions are influencing brain morphology. Brain morphometry studied in fibromyalgia patients by multiple researchers [59; 94; 112; 67; 19; 55] have shown a regional decrease in grey matter density [59; 112; 19], while others an increase [94], and yet one study shows no change at all [55]. Another study failed to demonstrate a relationship between pain intensity, duration and gray matter density and the authors interpret the lack of these relationships as evidence that observed anatomical abnormalities may be used as evidence for a brain predisposition for chronic pain [19]. Such variability of outcomes is unlikely to be a reflection of the patients studied. Instead it is more probably because of the fact that morphometric studies are complicated and subtle technical differences can bias outcomes. The most important confound being integrity of acquired anatomical MRI scans, where a few bad brain images may lead to opposite conclusions. Inadequate registration and/or segmentation can also give rise to false outcomes. Unfortunately there are no standard techniques for identifying such errors and very few groups perform quality control checks on acquired brain images. Using strict quality control procedures we have now studied this question across multiple chronic pain patient groups in contrast to healthy subjects and our results indicate that in chronic back pain, osteoarthritis, and complex regional pain syndrome we only observe decreased regional grey matter density and these decreases are specific to each type of chronic pain (manuscript in preparation). The most important message from these grey matter studies is the suggestion that distinct chronic pain conditions differentially impact on brain anatomy. A number of groups recently reported on the reversibility of brain morphometric changes with pain relief (figure 12), and most agree that at least some brain regional morphometric properties do reverse when pain is substantially relieved [49; 80; 89]. These early reports need to be examined more comprehensively and are yet important as they indicate that at least some of the morphological changes must be a direct consequence of the presence of the pain and most likely the underlying mechanism is based on synaptic plasticity that tracks the impact of the pain on the brain.
Figure 11.
Brain regional grey matter decreases in a number of chronic pain conditions. A. Bilateral dorsolateral prefrontal cortex and unilateral thalamic grey matter decreases in chronic back pain, from [8]. B. Insula and cingulate cortex grey matter decreases in irritable bowel syndrome, from [27]. Multiple brain regions show decrease grey matter density in C. fibromyalgia, from [58], and in D. tension headache, from [93]. The illustrated data are the earliest reports of brain morphological changes in various pain conditions. The list of additional pain conditions impacting brain anatomy is expanding very quickly.
Figure 12.

Evidence for brain grey matter density recovery with cessation of chronic pain. A: Left panel shows brain regions decreased in grey matter density in chronic osteoarthritis patients. Right panel shows brain regions where grey matter density increases following joint replacement and cessation of pain in osteoarthritis patients. Generally similar brain regions seem to changes with the presence and cessation of chronic pain. Adapted from [89]. B: Brain regional morphometry changes in post-traumatic headache. Grey matter density changes are shown for two brain regions in patients that develop chronic pain (3 months) and one year later when the pain subsided (1 year). In both areas grey matter signal recovers to original levels when pain symptoms subside. Adapted from [80].
Mechanisms responsible for brain grey matter morphological changes remain unclear [69]. The available evidence suggests multiple mechanisms because morphological changes can be observed at early time points from initial injury as well as after long periods from injury, best illustrated in two animal studies [72; 99]. If regional grey matter changes are more than just a reflection of local extracellular changes in water, which may occur based on changes in concentration of channels of proteins within neurons and/or glia, then properties of axons connected to the regional neurons involved should change as well. In a very simple way if decreased grey matter density corresponds to neuronal death then underlying white matter density or connectivity should also be modified reflecting such changes. We probed this issue by examining the integrity of brain white matter in chronic pain patients and also by relating grey matter changes to underlying white matter properties in patients with complex regional pain syndrome (CRPS) relative to matched healthy controls [41]. White matter properties can now be studied by diffusion tensor imaging (DTI). This technology has dramatically matured in the last few years and provides powerful tools with which one can pose exciting new questions about axonal properties of the human brain, both regarding local integrity (based on measures of fractional anisotropy, FA) as well as the properties of white matter tracts (quantified by probabilistic tractography) [76]. Using a combination of voxel-based morphometry and DTI we could demonstrate regional decreased grey matter density and regional white matter abnormality demonstrated by local decrease in FA in the patients. Moreover, both FA value decreases and grey matter density decreases were accompanied by changes in white matter connectivity. However, changes in connectivity were target specific, with some projections showing increases and other decreases. These results again suggest that multiple complex mechanisms underlie brain morphometric changes, some of which may be regarded as markers for predisposition for the observed chronic pain condition. Additionally we documented the first evidence for global reorganization of the CRPS brain. We first showed a strong linear relationship between whole neocortex grey matter volume (after correcting for age effects) and whole neocortex white matter mean FA values across healthy subjects. To our knowledge this is the first evidence for such a global relationship and it implies that myelin properties or axonal density properties adjust in relation to brain size in normal subjects. This relationship was completely disrupted in CRPS patients, where we could find no significant relationship between whole brain FA and neocortical grey matter volume. Therefore, across the whole brain grey matter to white matter relationship is disrupted in these patients, implying that changes in one of these compartments is not being properly compensated for in the other compartment (figure 13), which in turn implies that above and beyond the local grey and white matter changes that we documented there are also across the brain large-scale morphological changes associated with chronic pain. Mechanisms and parameters controlling this larger scale reorganization also remain to be studied.
Figure 13.
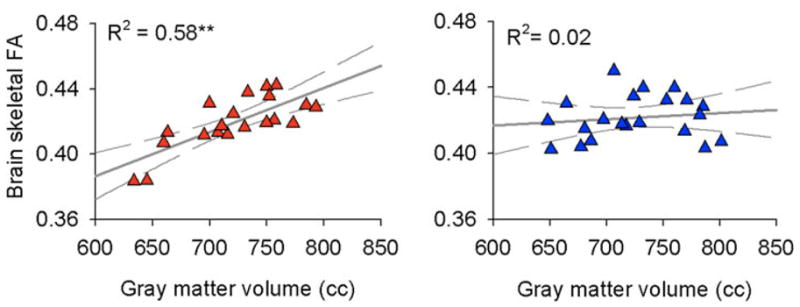
Whole-brain relationship between grey and white matter is disrupted in chronic complex regional pain syndrome subjects. Left panel shows that white matter fractional anisotropy (FA; measured for group average skeleton which insures that the tissue examined has 95% probability of being white matter) is correlated to total neocortical volume of the brain (after correcting for age effects). The right panel is similar data in complex regional pain syndrome patients, and shows that the relationship is destroyed. In both panels each symbol is the brain of a subject, red = healthy controls, blue = patients. Adapted from [41].
4. Learning, and forgetting, and a brain circuitry model for transition to chronic pain
We have previously argued that chronic pain can be viewed as a state of continuous learning coupled with reduced opportunity for forgetting [74; 3; 4]. Thousands of studies illustrate potency of painful events inducing learning where mechanisms of learning are investigated using Pavlovian paradigms, the majority of which use pain (electrical shock) as the unconditioned stimulus to which various events are associated to. Such studies repeatedly show that single painful stimuli are learned and remembered for weeks and months. Such studies also illustrate that once the associated learning occurs then the extinction of this association requires repeated exposure of the organism to the conditioned stimulus in the absence of the painful event. As chronic pain is fundamentally a state of continuous presence of pain, we can then conclude that it is also a state of continuous acquisition of associations with random events surrounding the organism especially at time points when the pain is high. Moreover, as the pain remains unremitting the organism does not have the opportunity of extinguishing these random associations, which requires frequent exposure to the same environment in the absence of pain. In fact we suggest that the contradiction between the subject’s conscious knowledge that the pain is not associated with the environment and the brain circuitry that continuously make such associations may be the core cognitive/emotional source of the suffering that chronic pain patients experience.
Within this theoretical construct of chronic pain we can now posit a general model of the brain circuitry regarding the transition from acute to chronic pain, based on the evidence presented above and coupling these observations to anatomical and physiological observations (figure 14). We first emphasize that nociceptive information accesses the cortex through multiple pathways and not just by the spinothalamic projection (majority of human brain imaging results continue to be interpreted in relation to the spinothalamic pathway). Even within the spinothalamic system medial thalamic cortical projections access large portions of the frontal cortex targeting mainly superficial layers [75]. This pathway is a reticulo-thalamocortical network that relays widespread nociceptive information to widespread cortical regions and most likely provides modulatory influences on large prefrontal cortical processes. Other nociceptive pathways such as the parabrachial-amygdala projections, spinal basal ganglia projections, spinal hypothalamic and spinal prefrontal projections [79; 57; 39; 18] and other monosynaptic and polysynaptic spinal-reticular-cortical projections provide ample opportunities for nociceptive information accessing various cortical circuitry and modulating such circuitry as a function of a continuous barrage of nociceptive inputs associated with chronic pain. Thus, continuous nociceptive input to supraspinal targets 1) reorganizes memories and their associations within the cortex; and 2) reorganizes motivational and memory consolidation properties of the limbic cortex.
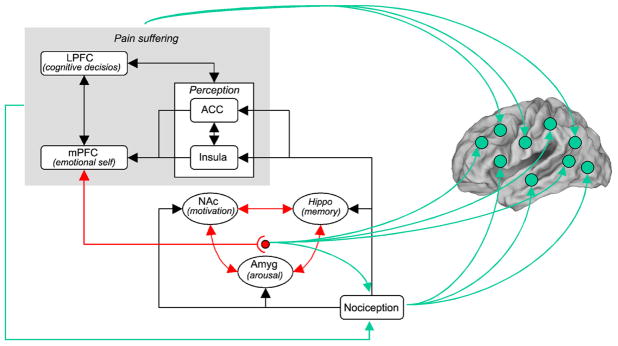
Figure 14.
A model regarding brain circuitry involved in the transition from acute to chronic pain. Nociceptive information, perhaps distorted by peripheral and spinal cord sensitization processes, impinges on limbic circuitry (Hippo, hippocampus; NAc, nucleus accumbens; and Amyg, amygdala). The interaction of limbic circuitry with prefrontal processes determines the level at which a certain pain condition transitions to a more emotional state. The limbic circuitry also provides learning/modulation signals to the rest of the cortex inducing functional and anatomical distortions that reflect the suffering and coping strategies of specific chronic pain conditions. Nociceptive signals also provide the brain with modulatory signals, and are in turn controlled by the state of suffering of the individual as well as limbic changes in arousal and motivation, through descending modulatory pathways.
The human functional brain imaging studies for chronic pain point to the recurring theme that chronic pain conditions engage preferentially medial prefrontal cortical areas as well as subcortical limbic regions, especially portions of the dorsal and ventral basal ganglia [17], amygdala [78], and hippocampus [62]. Even though different types of chronic pain and different perceptions in such patients engage distinct cortical and subcortical regions, by and large all of them show a shift away from brain regions engaged in sensory processing of pain towards regions encoding emotional and motivational subjective states. The continuous activation of these limbic/emotional structures must result in shifts in valuation (some of which are reviewed above) and these shifts would in turn modulate cortical learning and memory processes [35; 111]. Moreover, the shift in valuation then in turn influences nociceptive processing in the spinal cord, coupled with or further strengthened by shifts in prefrontal cortical emotional shifts. These descending modulatory systems provide yet another source of spinal cord central sensitization, as demonstrated in animals models [38], and are probably more important is human chronic pain conditions.
The details of the mechanisms across the brain network involved in chronic pain remain fuzzy, will undoubtedly continue to change with new observations, and certainly require more studies. As a result the model we present here (figure 14) is intentionally kept imprecise and is designed to indicate only a global construct.
5. Summary and conclusions
The functional imaging studies summarized here firstly show that the subjectivity of pain can be captured by objective markers of brain activity and that the general approach of searching for brain events closely related to the consciousness of pain provides a powerful concept with which acute and chronic pain can be studied and contrasted with each other. The approach even results in unraveling brain unconscious events that color motivation and valuation independent of conscious perception as a consequence of living with chronic pain. The comparison between different chronic pain conditions reveals that underlying brain activity is distinct with a general trend towards engaging limbic and paralimbic structures. The morphological studies show that the brain structure undergoes changes at multiple spatial and temporal scales, which are for the most part specific to the type of chronic pain studied. That some of these changes are reversible by cessation of chronic pain speaks to the specificity of the processes and also demonstrate that chronic pain may in fact by used as a unique tool with which the dynamics of brain plasticity can be studied at multiple spatial and temporal scales.
Based on the functional and morphological reorganization of the brain in chronic pain we advance a brain circuitry model for this transition where the primary drive is assumed to be the ability of pain to induce long-term memories by reinforcing learning mechanisms. We do not want to disregard the myriad of peripheral, spinal cord, and brainstem descending modulatory changes that have been described to accompany persistent or chronic pain as documented in animal models for such conditions. Instead we think that these peripheral and central sensitization events simply exaggerate the reorganization of limbic circuitry that in turn reflect their effects on prefrontal cortical evaluation of pain as well as provide the learning signals with which interactions between various cortical circuitry adapt to the suffering and coping that is specific to each clinical chronic pain condition.
Correspondences between physiological and morphological reorganization, as well as with metabolic changes should point to specific cognitive and sensory and motor ability changes that undoubtedly accompany distinct chronic pain conditions in specific patterns. We have taken some small steps in this direction [7; 101], but much more needs to be done as the array of cognitive impairments documented in chronic pain is large [51] but their unique contributions and relations to specific clinical cases remains to be systematically approached.
Anatomical and functional specificity of the brain in distinct chronic pain conditions perhaps seems as a new complication. However it suggests the possibility of unique therapies by targeting the underlying specific pathways for each type of chronic pain, see for example [74; 24; 103]. Moreover, it suggests that such therapies can be tested with very specific objective brain derived markers [35], even bypassing conscious reports.
Acknowledgments
This work is funded by NIH NINDS NS035115, NS057704, and by anonymous foundation for M.N. Baliki.
Footnotes
The authors claim no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Apkarian AV. Cortical pathophysiology of chronic pain. Novartis Found Symp. 2004;261:239–245. [PubMed] [Google Scholar]
- 2.Apkarian AV. Pain perception in relation to emotional learning. Curr Opin Neurobiol. 2008;18(4):464–468. doi: 10.1016/j.conb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apkarian AV. Pain perception in relation to emotional learning. Curr Opin Neurobiol. 2008;18(4):464–468. doi: 10.1016/j.conb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87(2):81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Apkarian AV, Chialvo DR. The shadows of pain. Pain. 2006;123(3):221–222. doi: 10.1016/j.pain.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 7.Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Apkarian AV, Sosa Y, Sonty S, Levy RE, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26(47):12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baliki MN, Geha PY, Apkarian AV. Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol. 2009;101(2):875–887. doi: 10.1152/jn.91100.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28(6):1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 66(1):149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32(5):927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- 14.Berthier M, Starkstein S, Leiguarda R. Asymbolia for pain: a sensory-limbic disconnection syndrome. Ann Neurol. 1988;24(1):41–49. doi: 10.1002/ana.410240109. [DOI] [PubMed] [Google Scholar]
- 15.Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 138(5):1783–1789. doi: 10.1053/j.gastro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 16.Borsook D, Becerra L. Phenotyping central nervous system circuitry in chronic pain using functional MRI: considerations and potential implications in the clinic. Curr Pain Headache Rep. 2007;11(3):201–207. doi: 10.1007/s11916-007-0191-7. [DOI] [PubMed] [Google Scholar]
- 17.Borsook D, Upadhyay J, Chudler EH, Becerra L. A key role of the basal ganglia in pain and analgesia--insights gained through human functional imaging. Mol Pain. 6:27. doi: 10.1186/1744-8069-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47(6):787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Burgmer M, Gaubitz M, Konrad C, Wrenger M, Hilgart S, Heuft G, Pfleiderer B. Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosom Med. 2009;71(5):566–573. doi: 10.1097/PSY.0b013e3181a32da0. [DOI] [PubMed] [Google Scholar]
- 20.Burgmer M, Pogatzki-Zahn E, Gaubitz M, Wessoleck E, Heuft G, Pfleiderer B. Altered brain activity during pain processing in fibromyalgia. Neuroimage. 2009;44(2):502–508. doi: 10.1016/j.neuroimage.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Bushnell MC, Apkarian AV, McMahon SB, Koltzenburg M. Textbook of pain. Vol. 5. London: Elsevier; 2006. Represntation of pain in the brain; pp. 107–124. [Google Scholar]
- 22.Cauda F, D’Agata F, Sacco K, Duca S, Cocito D, Paolasso I, Isoardo G, Geminiani G. Altered resting state attentional networks in diabetic neuropathic pain. J Neurol Neurosurg Psychiatry. 81(7):806–811. doi: 10.1136/jnnp.2009.188631. [DOI] [PubMed] [Google Scholar]
- 23.Cauda F, Sacco K, Duca S, Cocito D, D’Agata F, Geminiani GC, Canavero S. Altered resting state in diabetic neuropathic pain. PLoS One. 2009;4(2):e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centeno MV, Mutso A, Millecamps M, Apkarian AV. Prefrontal cortex and spinal cord mediated anti-neuropathy and analgesia induced by sarcosine, a glycine-T1 transpoter inhibitor. Pain. 2009;145(1–2):176–183. doi: 10.1016/j.pain.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coghill RC, Gilron I, Iadarola MJ. Hemispheric lateralization of somatosensory processing. J Neurophysiol. 2001;85(6):2602–2612. doi: 10.1152/jn.2001.85.6.2602. [DOI] [PubMed] [Google Scholar]
- 26.Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82(4):1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 27.Davis KD, Pope G, Chen J, Kwan CL, Crawley AP, Diamant NE. Cortical thinning in IBS: implications for homeostatic, attention, and pain processing. Neurology. 2008;70(2):153–154. doi: 10.1212/01.wnl.0000295509.30630.10. [DOI] [PubMed] [Google Scholar]
- 28.Davis KD, Pope GE, Crawley AP, Mikulis DJ. Neural correlates of prickle sensation: a percept-related fMRI study. Nat Neurosci. 2002;5(11):1121–1122. doi: 10.1038/nn955. [DOI] [PubMed] [Google Scholar]
- 29.Davis KD, Tasker RR, Kiss ZH, Hutchison WD, Dostrovsky JO. Visceral pain evoked by thalamic microstimulation in humans. Neuroreport. 1995;6(2):369–374. doi: 10.1097/00001756-199501000-00035. [DOI] [PubMed] [Google Scholar]
- 30.Davis KD, Taylor KS, Hutchison WD, Dostrovsky JO, McAndrews MP, Richter EO, Lozano AM. Human anterior cingulate cortex neurons encode cognitive and emotional demands. J Neurosci. 2005;25(37):8402–8406. doi: 10.1523/JNEUROSCI.2315-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derbyshire SW, Jones AK, Creed F, Starz T, Meltzer CC, Townsend DW, Peterson AM, Firestone L. Cerebral responses to noxious thermal stimulation in chronic low back pain patients and normal controls. Neuroimage. 2002;16(1):158–168. doi: 10.1006/nimg.2002.1066. [DOI] [PubMed] [Google Scholar]
- 32.Dunckley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang L, Tracey I. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci. 2005;25(32):7333–7341. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fairhurst M, Wiech K, Dunckley P, Tracey I. Anticipatory brainstem activity predicts neural processing of pain in humans. Pain. 2007;128(1–2):101–110. doi: 10.1016/j.pain.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Fields HL. Book A motivation-decision model of pain: the role of opioids. City: IASP press; 2006. A motivation-decision model of pain: the role of opioids; pp. 449–459. [Google Scholar]
- 35.Flor H. Maladaptive plasticity, memory for pain and phantom limb pain: review and suggestions for new therapies. Expert Rev Neurother. 2008;8(5):809–818. doi: 10.1586/14737175.8.5.809. [DOI] [PubMed] [Google Scholar]
- 36.Foss JM, Apkarian AV, Chialvo DR. Dynamics of pain: fractal dimension of temporal variability of spontaneous pain differentiates between pain States. J Neurophysiol. 2006;95(2):730–736. doi: 10.1152/jn.00768.2005. [DOI] [PubMed] [Google Scholar]
- 37.Fukui S, Matsuno M, Inubushi T, Nosaka S. N-Acetylaspartate concentrations in the thalami of neuropathic pain patients and healthy comparison subjects measured with (1)H-MRS. Magn Reson Imaging. 2006;24(1):75–79. doi: 10.1016/j.mri.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 38.Gardell LR, Vanderah TW, Gardell SE, Wang R, Ossipov MH, Lai J, Porreca F. Enhanced evoked excitatory transmitter release in experimental neuropathy requires descending facilitation. J Neurosci. 2003;23(23):8370–8379. doi: 10.1523/JNEUROSCI.23-23-08370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87(2):251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- 40.Geha PY, Baliki MN, Chialvo DR, Harden RN, Paice JA, Apkarian AV. Brain activity for spontaneous pain of postherpetic neuralgia and its modulation by lidocaine patch therapy. Pain. 2007;128(1–2):88–100. doi: 10.1016/j.pain.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60(4):570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, Clauw DJ. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50(2):613–623. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- 43.Giesler GJ, Jr, Yezierski RP, Gerhart KD, Willis WD. Spinothalamic tract neurons that project to medial and/or lateral thalamic nuclei: evidence for a physiologically novel population of spinal cord neurons. J Neurophysiol. 1981;46(6):1285–1308. doi: 10.1152/jn.1981.46.6.1285. [DOI] [PubMed] [Google Scholar]
- 44.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46(5):1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 45.Grachev ID, Fredickson BE, Apkarian AV. Dissociating anxiety from pain: mapping the neuronal marker N-acetyl aspartate to perception distinguishes closely interrelated characteristics of chronic pain. Molecular Psychiatry. 2001;6(3):256–258. doi: 10.1038/sj.mp.4000834. [DOI] [PubMed] [Google Scholar]
- 46.Grachev ID, Fredrickson BE, Apkarian AV. Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. Pain. 2000;89(1):7–18. doi: 10.1016/S0304-3959(00)00340-7. [DOI] [PubMed] [Google Scholar]
- 47.Grachev ID, Fredrickson BE, Apkarian AV. Brain chemistry reflects dual states of pain and anxiety in chronic low back pain. J Neural Transm. 2002;109(10):1309–1334. doi: 10.1007/s00702-002-0722-7. [DOI] [PubMed] [Google Scholar]
- 48.Gussew A, Rzanny R, Gullmar D, Scholle HC, Reichenbach JR. 1H-MR spectroscopic detection of metabolic changes in pain processing brain regions in the presence of non-specific chronic low back pain. Neuroimage. doi: 10.1016/j.neuroimage.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 49.Gwilym SE, Fillipini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty; a longitudinal voxel-based-morphometric study. Arthritis Rheum. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- 50.Haber LH, Moore BD, Willis WD. Electrophysiological response properties of spinoreticular neurons in the monkey. J Comp Neurol. 1982;207(1):75–84. doi: 10.1002/cne.902070107. [DOI] [PubMed] [Google Scholar]
- 51.Hart RP, Wade JB, Martelli MF. Cognitive impairment in patients with chronic pain: the significance of stress. Curr Pain Headache Rep. 2003;7(2):116–126. doi: 10.1007/s11916-003-0021-5. [DOI] [PubMed] [Google Scholar]
- 52.Hashmi JA, Davis KD. Effect of static and dynamic heat pain stimulus profiles on the temporal dynamics and interdependence of pain qualities, intensity, and affect. J Neurophysiol. 2008;100(4):1706–1715. doi: 10.1152/jn.90500.2008. [DOI] [PubMed] [Google Scholar]
- 53.Head H, Holmes G. Sensory disturbances from cerebral lesions. Brain. 1911;34:102–254. [Google Scholar]
- 54.Hofbauer RK, Rainville P, Duncan GH, Bushnell MC. Cortical representation of the sensory dimension of pain. J Neurophysiol. 2001;86(1):402–411. doi: 10.1152/jn.2001.86.1.402. [DOI] [PubMed] [Google Scholar]
- 55.Hsu MC, Harris RE, Sundgren PC, Welsh RC, Fernandes CR, Clauw DJ, Williams DA. No consistent difference in gray matter volume between individuals with fibromyalgia and age-matched healthy subjects when controlling for affective disorder. Pain. 2009;143(3):262–267. doi: 10.1016/j.pain.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JH, Suh SI, Seol HY, Oh K, Seo WK, Yu SW, Park KW, Koh SB. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008;28(6):598–604. doi: 10.1111/j.1468-2982.2008.01550.x. [DOI] [PubMed] [Google Scholar]
- 57.Kostarczyk E, Zhang X, Giesler GJ., Jr Spinohypothalamic tract neurons in the cervical enlargement of rats: locations of antidromically identified ascending axons and their collateral branches in the contralateral brain. J Neurophysiol. 1997;77(1):435–451. doi: 10.1152/jn.1997.77.1.435. [DOI] [PubMed] [Google Scholar]
- 58.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27(15):4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27(15):4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 214(5–6):519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwan CL, Diamant NE, Pope G, Mikula K, Mikulis DJ, Davis KD. Abnormal forebrain activity in functional bowel disorder patients with chronic pain. Neurology. 2005;65(8):1268–1277. doi: 10.1212/01.wnl.0000180971.95473.cc. [DOI] [PubMed] [Google Scholar]
- 62.Lang S, Kroll A, Lipinski SJ, Wessa M, Ridder S, Christmann C, Schad LR, Flor H. Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. Eur J Neurosci. 2009;29(4):823–832. doi: 10.1111/j.1460-9568.2009.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee MC, Tracey I. Unravelling the mystery of pain, suffering, and relief with brain imaging. Curr Pain Headache Rep. 14(2):124–131. doi: 10.1007/s11916-010-0103-0. [DOI] [PubMed] [Google Scholar]
- 64.Loggia ML, Mogil JS, Bushnell MC. Empathy hurts: compassion for another increases both sensory and affective components of pain perception. Pain. 2008;136(1–2):168–176. doi: 10.1016/j.pain.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 65.Loggia ML, Mogil JS, Bushnell MC. Experimentally induced mood changes preferentially affect pain unpleasantness. J Pain. 2008;9(9):784–791. doi: 10.1016/j.jpain.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 66.Lorenz J, Cross DJ, Minoshima S, Morrow TJ, Paulson PE, Casey KL. A unique representation of heat allodynia in the human brain. Neuron. 2002;35(2):383–393. doi: 10.1016/s0896-6273(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 67.Luerding R, Weigand T, Bogdahn U, Schmidt-Wilcke T. Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: structural correlates of pain-cognition interaction. Brain. 2008;131(Pt 12):3222–3231. doi: 10.1093/brain/awn229. [DOI] [PubMed] [Google Scholar]
- 68.Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, Kalso E, Hari R. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc Natl Acad Sci U S A. 107(14):6493–6497. doi: 10.1073/pnas.1001504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.May A. Chronic pain may change the structure of the brain. Pain. 2008;137(1):7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 70.Mazzola L, Isnard J, Peyron R, Guenot M, Mauguiere F. Somatotopic organization of pain responses to direct electrical stimulation of the human insular cortex. Pain. 2009;146(1–2):99–104. doi: 10.1016/j.pain.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 71.Melzack R, Casey K, Kenshalo DR. The skin senses. Springfield: Charles C. Thomas; 1968. Sensory, motivational, and central contol determinants of pain; pp. 423–443. [Google Scholar]
- 72.Metz AE, Yau HJ, Centeno MV, Apkarian AV, Martina M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc Natl Acad Sci U S A. 2009;106(7):2423–2428. doi: 10.1073/pnas.0809897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meyer RA, Campbell JN. Myelinated nociceptive afferents account for the hyperalgesia that follows a burn to the hand. Science. 1981;213(4515):1527–1529. doi: 10.1126/science.7280675. [DOI] [PubMed] [Google Scholar]
- 74.Millecamps M, Centeno MV, Berra HH, Rudick CN, Lavarello S, Tkatch T, Apkarian AV. D-cycloserine reduces neuropathic pain behavior through limbic NMDA-mediated circuitry. Pain. 2006 doi: 10.1016/j.pain.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monconduit L, Villanueva L. The lateral ventromedial thalamic nucleus spreads nociceptive signals from the whole body surface to layer I of the frontal cortex. Eur J Neurosci. 2005;21(12):3395–3402. doi: 10.1111/j.1460-9568.2005.04160.x. [DOI] [PubMed] [Google Scholar]
- 76.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 77.Napadow V, Lacount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 62(8):2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10(3):221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- 79.Newman HM, Stevens RT, Apkarian AV. Direct spinal projections to limbic and striatal areas: anterograde transport studies from the upper cervical spinal cord and the cervical enlargement in squirrel monkey and rat. J Comp Neurol. 1996;365(4):640–658. doi: 10.1002/(SICI)1096-9861(19960219)365:4<640::AID-CNE10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 80.Obermann M, Nebel K, Schumann C, Holle D, Gizewski ER, Maschke M, Goadsby PJ, Diener HC, Katsarava Z. Gray matter changes related to chronic posttraumatic headache. Neurology. 2009;73(12):978–983. doi: 10.1212/WNL.0b013e3181b8791a. [DOI] [PubMed] [Google Scholar]
- 81.Ohara S, Crone NE, Weiss N, Lenz FA. Analysis of synchrony demonstrates ‘pain networks’ defined by rapidly switching, task-specific, functional connectivity between pain-related cortical structures. Pain. 2006;123(3):244–253. doi: 10.1016/j.pain.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 82.Oshiro Y, Quevedo AS, McHaffie JG, Kraft RA, Coghill RC. Brain mechanisms supporting discrimination of sensory features of pain: a new model. J Neurosci. 2009;29(47):14924–14931. doi: 10.1523/JNEUROSCI.5538-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105(3):403–413. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 84.Ploghaus A, Tracey I, Clare S, Gati JS, Rawlins JN, Matthews PM. Learning about pain: the neural substrate of the prediction error for aversive events. Proc Natl Acad Sci USA. 2000;97(16):9281–9286. doi: 10.1073/pnas.160266497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science. 1999;284(5422):1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- 86.Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, Nichelli P. Does anticipation of pain affect cortical nociceptive systems? J Neurosci. 2002;22(8):3206–3214. doi: 10.1523/JNEUROSCI.22-08-03206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Porro CA, Cettolo V, Francescato MP, Baraldi P. Temporal and intensity coding of pain in human cortex. J Neurophysiol. 1998;80(6):3312–3320. doi: 10.1152/jn.1998.80.6.3312. [DOI] [PubMed] [Google Scholar]
- 88.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 89.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29(44):13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schmahmann JD, Leifer D. Parietal pseudothalamic pain syndrome. Clinical features and anatomic correlates. Arch Neurol. 1992;49(10):1032–1037. doi: 10.1001/archneur.1992.00530340048017. [DOI] [PubMed] [Google Scholar]
- 91.Schmidt-Wilcke T, Ganssbauer S, Neuner T, Bogdahn U, May A. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia. 2008;28(1):1–4. doi: 10.1111/j.1468-2982.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 92.Schmidt-Wilcke T, Leinisch E, Ganssbauer S, Draganski B, Bogdahn U, Altmeppen J, May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006 doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 93.Schmidt-Wilcke T, Leinisch E, Straube A, Kampfe N, Draganski B, Diener HC, Bogdahn U, May A. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65(9):1483–1486. doi: 10.1212/01.wnl.0000183067.94400.80. [DOI] [PubMed] [Google Scholar]
- 94.Schmidt-Wilcke T, Luerding R, Weigand T, Jurgens T, Schuierer G, Leinisch E, Bogdahn U. Striatal grey matter increase in patients suffering from fibromyalgia--a voxel-based morphometry study. Pain. 2007;132 (Suppl 1):S109–116. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 95.Schmidt-Wilcke T, Luerding R, Weigand T, Jurgens T, Schuierer G, Leinisch E, Bogdahn U. Striatal grey matter increase in patients suffering from fibromyalgia--a voxel-based morphometry study. Pain. 2007;132 (Suppl 1):S109–S116. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 96.Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008;140(3):411–419. doi: 10.1016/j.pain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 97.Schweinhardt P, Lee M, Tracey I. Imaging pain in patients: is it meaningful? Curr Opin Neurol. 2006;19(4):392–400. doi: 10.1097/01.wco.0000236620.89710.63. [DOI] [PubMed] [Google Scholar]
- 98.Schwenkreis P, Maier C, Tegenthoff M. Functional imaging of central nervous system involvement in complex regional pain syndrome. AJNR Am J Neuroradiol. 2009;30(7):1279–1284. doi: 10.3174/ajnr.A1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seminowicz DA, Laferriere AL, Millecamps M, Yu JS, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. Neuroimage. 2009;47(3):1007–1014. doi: 10.1016/j.neuroimage.2009.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sherrington CS. The integrative action of the nervous system. New Haven, CT: Yale university press; 1906. [Google Scholar]
- 101.Small DM, Apkarian AV. Increased taste intensity perception exhibited by patients with chronic back pain. Pain. 2006;120(1–2):124–130. doi: 10.1016/j.pain.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 102.Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, Coghill RC. Roles of the insular cortex in the modulation of pain: insights from brain lesions. J Neurosci. 2009;29(9):2684–2694. doi: 10.1523/JNEUROSCI.5173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: is chronic pain a disease? J Pain. 2009;10(11):1113–1120. doi: 10.1016/j.jpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 104.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55(3):377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 105.Tu CH, Niddam DM, Chao HT, Chen LF, Chen YS, Wu YT, Yeh TC, Lirng JF, Hsieh JC. Brain morphological changes associated with cyclic menstrual pain. Pain. 150(3):462–468. doi: 10.1016/j.pain.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 106.Valdes M, Collado A, Bargallo N, Vazquez M, Rami L, Gomez E, Salamero M. Increased glutamate/glutamine compounds in the brains of patients with fibromyalgia: a magnetic resonance spectroscopy study. Arthritis Rheum. 62(6):1829–1836. doi: 10.1002/art.27430. [DOI] [PubMed] [Google Scholar]
- 107.Valfre W, Rainero I, Bergui M, Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48(1):109–117. doi: 10.1111/j.1526-4610.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 108.Villemure C, Bushnell MC. Mood influences supraspinal pain processing separately from attention. J Neurosci. 2009;29(3):705–715. doi: 10.1523/JNEUROSCI.3822-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Villemure C, Wassimi S, Bennett GJ, Shir Y, Bushnell MC. Unpleasant odors increase pain processing in a patient with neuropathic pain: psychophysical and fMRI investigation. Pain. 2006;120(1–2):213–220. doi: 10.1016/j.pain.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 110.Wiech K, Kalisch R, Weiskopf N, Pleger B, Stephan KE, Dolan RJ. Anterolateral prefrontal cortex mediates the analgesic effect of expected and perceived control over pain. J Neurosci. 2006;26(44):11501–11509. doi: 10.1523/JNEUROSCI.2568-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends Cogn Sci. 2008;12(8):306–313. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 112.Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, Bushnell MC, Chizh BA. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25(12):3576–3582. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- 113.Zambreanu L, Wise RG, Brooks JC, Iannetti GD, Tracey I. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain. 2005;114(3):397–407. doi: 10.1016/j.pain.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 114.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293(5528):311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 115.Zubieta JK, Stohler CS. Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci. 2009;1156:198–210. doi: 10.1111/j.1749-6632.2009.04424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]