Abstract
Prior work indicates that cerebral glycolysis is impaired following traumatic brain injury (TBI) and that pyruvate treatment acutely after TBI can improve cerebral metabolism and is neuroprotective. Since extracellular levels of glucose decrease during periods of increased cognitive demand and exogenous glucose improves cognitive performance, we hypothesized that pyruvate treatment prior to testing could ameliorate cognitive deficits in rats with TBI. Based on pre-surgical spatial alternation performance in a 4-arm plus-maze, adult male rats were randomized to receive either sham injury or unilateral (left) cortical contusion injury (CCI). On days 4, 9 and 14 after surgery animals received an intraperitoneal injection of either vehicle (Sham-Veh, n=6; CCI-Veh, n=7) or 1000 mg/kg of sodium pyruvate (CCI-SP, n=7). One hour after each injection rats were retested for spatial alternation performance. Animals in the CCI-SP group showed no significant working memory deficits in the spatial alternation task compared to Sham-Veh controls. The percent four/five alternation scores for CCI-Veh rats were significantly decreased from Sham-Veh scores on days 4 and 9 (p<0.01) and from CCI-SP scores on days 4, 9 and 14 (p<0.05). Measures of cortical contusion volume, regional cerebral metabolic rates of glucose and regional cytochrome oxidase activity at day 15 post-injury did not differ between CCI-SP and CCI-Veh groups. These results show that spatial alternation testing can reliably detect temporal deficits and recovery of working memory after TBI and that delayed pyruvate treatment can ameliorate TBI-induced cognitive impairments.
Keywords: Cerebral metabolism, Cortical contusion injury, Plus-maze, Pyruvate, Rat, Spatial alternation
Introduction
Performance of cognitive tasks increases the demand for metabolic fuel in the brain. For example, brain glucose utilization increases during task performance, extracellular glucose levels fluctuate dynamically and regionally during behavioral tests, and glucose concentrations in the hippocampus decrease rapidly and to an extent dependent on task complexity [5,23,25,31]. In normal young adult rats reductions of extracellular glucose during spatial alternation performance is prevented by systemic administration of exogenous glucose, which also improves cognitive performance [23,25,26]. Pre-trial glucose treatment in aged rats, who demonstrate cognitive impairments and more profound and prolonged decrease in hippocampal glucose compared to young adults, is also reported to elevate extracellular glucose levels and improve performance [24].
Traumatic brain injury (TBI) is a leading cause of death and disability worldwide, with an estimated 2% of the US population suffering from long-term neurological and cognitive impairments as a result of injury [19]. Treatment options, particularly in the chronic phase after TBI, are limited. Following an acute phase of neuronal excitation and anaerobic glycolysis, the chronic phase of TBI is characterized by neuronal depression and reductions in cerebral metabolism [3,4,13,17,20,33,42,46]. Interestingly, it was recently reported that systemic glucose injection prior to cognitive testing 11 to 15 days after injury improved spatial learning ability in rats with fluid percussion-induced TBI [18].
Since the acute pathophysiology of TBI can interfere with glucose metabolism or shunt glucose to alternative pathways [1,2] we, and others, have begun to examine the potential benefits of exogenous pyruvate treatments after experimental TBI. If administered acutely (<24 h) after TBI, systemic pyruvate treatment elevates the brain’s extracellular concentration of both pyruvate and glucose, attenuates cerebral inflammation and neuronal cell loss, improves cerebral cytochrome oxidase (CyO) activity and improves neurological recovery [10,28,48]. However, acute treatments with sodium pyruvate (SP) did not ameliorate working memory impairments in a spontaneous alternation task 1 week after TBI [28].
Based on the recent report showing that delayed glucose treatment improved cognitive outcome [18], the current study was conducted to determine if delayed SP treatments would improve spatial alternation performance in rats with experimental TBI. Potential treatment effects on contusion volume and regional cerebral metabolism were assessed after completion of the behavioral study.
Material and methods
All experimental procedures were reviewed and approved by the UCLA Chancellor’s Committee for Animal Research. Efforts were made to minimize pain and discomfort to the animals and the number of subject’s used in the study.
Twenty adult male (77 days of age, weighing 336–398 g on the day of surgery) Sprague-Dawley rats (Charles River, Hollister, CA) were pair-housed in plastic cages and maintained in a light (12:12 h light:dark cycle) and temperature controlled (21 ± 1°C) environment. After one week of acclimatization the rats were tested in a plus-maze to assess pre-surgical spatial alternation ability. After placing the rat in the center of the maze it was allowed to explore freely during a 15 min test and the number and sequence of entries into each arm was recorded. Alternations were counted when each arm was visited within a span of 5 arm entries and this number was divided by total possible alternations (total entries minus four) and multiplied by 100 to calculate the percent four/five alternation score [23,25,28,44]. Chance performance is 45–50%. Based on these alternation scores animals were randomized to 1 of 3 treatment groups. Surgical procedures have been previously reported in detail [10,28]. In brief, isoflurane-anesthetized rats were subjected to either a left hemisphere cortical contusion injury (CCI; using a 5 mm flat-tip impactor at 2.32 m/sec velocity, 2 mm tissue compression for 250 msec) or sham injury (no craniotomy).
On days 4, 9 and 14 post-surgery all rats were given an additional 15 min test in the plus-maze to assess working memory performance. One h prior to each post-surgical test sham injury controls (Sham-Veh: n=6) and one CCI group (CCI-Veh: n=7) were injected (2.5 ml/kg, i.p.) with 0.1 M phosphate buffered saline (PBS, pH 7.6). Another CCI group was injected (i.p.) with 1000 mg/kg of SP (400 mg/ml, dissolved in PBS immediately prior to injection) 1 h prior to each post-surgical test (CCI-SP: n=7). This SP dosage increases cerebral extracellular levels of pyruvate for at least 75 min post-injection [10]. Sham-SP effects were not examined since pyruvate had no effect on alternation performance in young adult rats [35].
Fifteen days after surgery all rats underwent [14C]2-Deoxy-D-Glucose (2DG) autoradiography procedures to evaluate regional cerebral metabolic rates of glucose (rCMRG) [32,41,42,46]. Briefly, under isoflurane anesthesia the right femoral artery and vein were catheterized and the rats were restrained on a cardboard plank during a 2 h period to allow recovery from anesthesia. 2DG (120 µCi/kg; American Radiolabeled Chemicals Inc., St. Louis, MO) was infused over 30 sec via the femoral vein and arterial blood samples were drawn at predetermined intervals over 45 min for plasma 14C and glucose assays. At 45 min rats were given a lethal dose of sodium pentobarbital (100 mg/kg, i.v.), decapitated and their brains were removed and flash frozen (−55°C, dry ice cooled 2-methylbutane).
Coronal brain sections were cut at 20 µm, saving 3 sequential sections for 2DG autoradiography and 2 sequential sections for CyO histochemistry every 500 µm. Tissue sections and 14C standards were apposed to Kodak Biomax film for 48 h and images were digitally captured with a flat-bed scanner (256 dpi, 8 bit gray scale). A minimum of 5 optical density readings from 8 cortical and 7 subcortical regions of interest (see Figure 2 for list of regions) were obtained bilaterally using ImageJ software (version 1.42q: National Institutes of Health, Bethesda, MD) and the rCMRG was calculated [41].
Figure 2.
Mean ± SEM regional cerebral metabolic rates of glucose (rCMRG) assessed on post-injury day 15. Cortical contusion injury (CCI) resulted in global rCMRG depression in the injured/left hemisphere, but rCMRG not differ between CCI rats treated with vehicle (Veh) or sodium pyruvate (SP) on days 4, 9 and 14.
*: p<0.05, **: p<0.01, ***: p<0.01 compared to Sham-Veh group.
CyO histochemistry was performed as previously reported [13,28] to assess cerebral oxidative capacity in tissue sections digitally captured at 600 dpi, obtaining optical density readings in the same regions evaluated using rCMRG. Tissue standards (10, 14, 20 and 25 µm-thick brain sections of naïve rat brain) were included in each batch of CyO reaction medium to establish correlations between section thickness and staining intensity (average Pearson r2: 99.3±0.002). Slopes of each standard curve were similar (2.002±0.02), so regional optical density readings were normalized (by subtracting the difference between the intercept of the co-stained standards and that of the average for all standards) to control for potential batch staining differences.
The non-injured left and right cortical mantle in CyO stained tissue sections spaced at 1 mm intervals was viewed, traced and quantified (area and volume) using computer-assisted morphometry. The percent tissue loss in the left cortical mantle from +1.2 to −6.8 mm from bregma was calculated using the formula [100−((Left/Right) × 100)], as previously described [10,28,44].
All data are reported as the group average ± standard error of the mean (SEM). Plus-maze data were first analyzed by repeated measures analysis of variance (ANOVA), with further analysis of significant effects using one-way ANOVA at each time point followed by Tukey’s multiple comparison. Percent tissue loss, rCMRG and CyO data were analyzed using one-way ANOVA for each region of interest with Tukey’s post-hoc test. Two-tailed p-values < 0.05 were considered significant.
Results
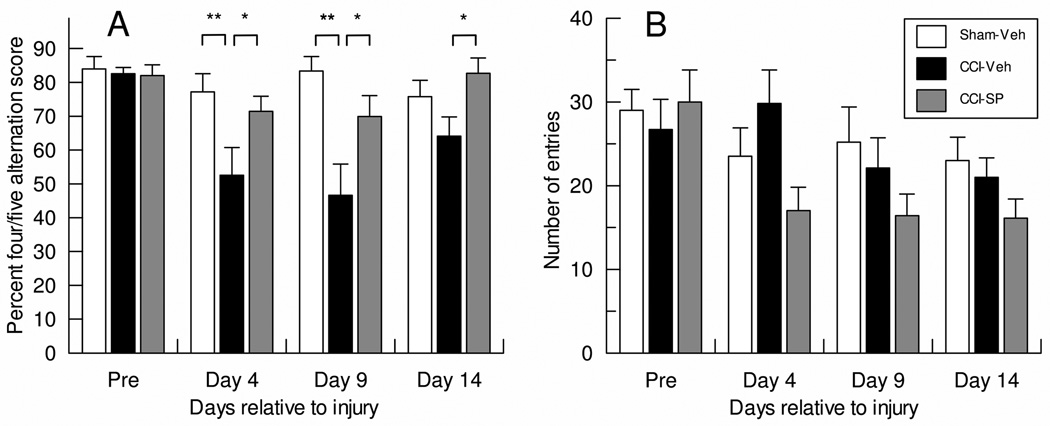
Baseline percent four/five alternation scores used for stratified random assignment were similar for all groups (Figure 1A). Sham-Veh spatial alternation performance remained stable over all test days (between 75.8±4.8 and 84.0±3.6). CCI-Veh scores (52.6±8.2) were significantly lower than those of the Sham-Veh (p<0.01) and the CCI-SP group (71.4±4.5, p<0.05) on day 4. Similar injury/drug effects persisted to day 9 post-injury. Alternation scores of CCI-Veh rats recovered to 64.1±5.7 by day 14, and although this performance level was not different from the Sham-Veh group it remained significantly worse than that of the CCI-SP group (82.7±4.5, p<0.05). The number of arm entries did not differ significantly between any groups on any of the test days (Figure 1B).
Figure 1.
Mean ± SEM percent four/five spatial alternation score (A) and number of arm entries (B) during plus-maze tests conducted pre- and post-injury in rats with sham or cortical contusion injury (CCI) and treatment with either vehicle (Veh) or sodium pyruvate (SP) 1 h prior to testing on days 4, 9 and 14. CCI significantly worsened the alternation scores on day 4 and 9 but SP treatment prevented this worsening. Number of arm entries did not differ between groups on any test day.
*: p<0.05, **: p<0.01.
The improved spatial alternation performance in the CCI-SP group did not appear to be related to any potential injury severity differences, as equivalent percent tissue loss was present in the CCI-Veh (30.4±3.9) and CCI-SP (26.7±5.6) groups on day 15. Both CCI groups had significant loss (p<0.001) of tissue volume in the left cortical mantle compared to Sham-Veh (−0.8±1.9) controls.
Global depression of rCMRG was observed in the left injured hemisphere at day 15 after CCI (Figure 2). In most ipsilateral cortical regions the rCMRG values of both CCI groups were significantly reduced compared to Sham-Veh. However, there were no differences between CCI-Veh and CCI-SP groups in any region. The rCMRG in thalamic regions and the CA3 ipsilateral to injury was also significantly depressed in CCI-Veh and CCI-SP groups compared to Sham-Veh controls. Again, there were no differences between CCI-Veh and CCI-SP groups. Cortical and subcortical rCMRG values did not differ between the three groups in any contralateral region (data not shown).
CyO activity at 15 days post-injury did not differ significantly between the three experimental groups in any ipsilateral or contralateral brain regions. For example, optical density readings were 85.1±3.9, 88.6±4.5 and 85.8±3.9 in the left peri-contusional cortex for Sham-Veh, CCI-Veh and CCI-SP groups, respectively. The only ipsilateral region showing a mild depression of CyO activity was the dorsolateral geniculate nucleus (78.9±4.9, 69.8±2.2 and 68.8±4.3 for Sham-Veh, CCI-Veh and CCI-SP groups). Thus, even those ipsilateral regions with the largest magnitude of rCMRG depression (see Figure 2) did not show significantly reduced oxidative capacity at day 15.
Discussion
Several novel findings are revealed in the current results. The spontaneous spatial alternation test has previously detected cognitive impairments in CCI rats at single time points [28,44]. Current results indicate this task can assess temporal working memory impairments and their spontaneous recovery after CCI. This plus-maze task involves minimal aversive motivation (exposure to room light and open space), and likely induces a smaller stress response than does the aversive motivation (swimming) inherent in Morris water maze tests [11] frequently used to evaluate spatial learning or working memory after experimental TBI. Thus, the plus-maze task may be of future use for evaluating TBI animals known to have altered neuroendocrine responses to stress [44].
The current results demonstrate that delayed SP treatments significantly ameliorate working memory impairments after CCI. This is consistent with the report that pre-testing glucose treatments improved spatial learning 11 to 15 days after fluid percussion-induced TBI [18]. Early glucose treatments (1–10 days) did not improve cognitive outcome after TBI [18], and acute SP treatments (at 1, 12 and 24 h) did not improve spatial working memory 7 days after CCI [28]. Current results for delayed SP and those for delayed glucose treatments [18] suggest that exogenous metabolic substrates might be beneficial to TBI patients, even chronically after injury. Further research in this area is certainly warranted.
The mechanisms by which delayed pyruvate treatment improves cognitive outcome remain to be clarified. Measures of cortical tissue loss indicated equivalent injury in both CCI groups, so improved outcome in the SP-treated group was not likely due to neuroprotective effects. Since TBI induces oxidative stress, nitric oxide, and mitochondrial dysfunction that impact on CyO to depress its activity for at least 7–10 days [8,13,15,28,40] and acute SP treatments improved cortical CyO activity 3 days post-CCI [28], we evaluated this marker of oxidative capacity in the current study. However, by 15 days CyO activity recovered to control values in CCI-Veh and CCI-SP groups. While SP treatment might improve cerebral oxidative capacity at earlier time points to impact on cognitive performance, further research is needed to verify this possibility. Spontaneous recovery of CyO activity may underlie delayed improvement (recovery) of spatial working memory in the CCI-Veh group. This interpretation is consistent with studies where increased CyO activity is affiliated with improved neurological or cognitive performance [7,12,43,45] and evidence that energy demands during task-induced brain activation are primarily mediated by oxidative metabolism [21].
In contrast to the CyO results, this study found reduced rCMRG in several brain regions 15 days after CCI, with no effect of SP treatments. The “mismatch” between rCMRG and CyO activity was unexpected, and may reflect faster or more complete recovery of oxidative capacity in surviving neurons and/or glia or resolution of factors inhibiting CyO activity with ongoing impairment of glucose transport, uptake and/or glycolytic metabolism. This rCMRG depression at day 15 is consistent with prior reports in CCI models [9,33], but appears attenuated compared to earlier time points after CCI [20,32,42]. Partial recovery of rCMRG may have influenced behavioral recovery after CCI as functional outcome after brain injury parallels improvements in rCMRG in rats [9,27,31,32], and in some [14,29,30], but not all [3], human TBI studies. Since frontal cortex and hippocampus mediate spatial learning [7], recovery of rCMRG (and CyO) in these brain regions may have supported working memory in both the CCI-Veh and CCI-SP groups by day 14.
Although the current results show no effect of SP treatments on rCMRG assessed 24 h after the final injection, we cannot rule out the possibility that behavioral improvements after SP administration are related to changes in glucose levels or glycolysis. SP treatment can increase circulating levels of both pyruvate and glucose and increase levels of these energy substrates in the cerebral extracellular space [10]. Pyruvate is known to stimulate pyruvate dehydrogenase activity [39], facilitate glycolysis and oxidative phosphorylation (see [28] and references therein), and increase cellular energy function and adenosine triphosphate production [16,47]. Provision of exogenous glucose or pyruvate prior to testing in mazes, either systemically or via local brain infusion, has been shown to alter activity-induced fluctuations in hippocampal glucose and/or to improve spatial alternation performance or memory processing [22–24,34–37]. This body of work suggests that if metabolic fuel is sufficiently increased to satisfy the increased energy demand, then cognitive performance can be improved. However, glucose (or pyruvate) also serves as a precursor to numerous neurotransmitters, and microdialysis studies indicate glucose effects on cholinergic neuron activity and acetylcholine levels in hippocampus may underlie beneficial effects on spontaneous alternation performance [36,37]. Similar effects or peripherally-mediated effects for glucose-enhanced memory [26] could also underlie the current findings of working memory improvements induced by delayed SP treatment after CCI.
In summary, the current results show that the spontaneous alternation task detects temporal working memory deficits in rats with TBI and that pre-trial SP treatment ameliorates these deficits. Given the current results for SP, those reported for delayed glucose treatment after TBI [18], and reports that cortical blood flow in head trauma patients is attenuated during performance of spatial working memory tasks [6,38], additional studies of delayed metabolic substrate treatment after TBI are needed.
Acknowledgements
Supported by the UCLA Brain Injury Research Center and Award Number P01NS058489 from the National Institute of Neurological Disorders and Stroke (NINDS). The content is the sole responsibility of the authors and does not necessarily represent official views of the NINDS or the National Institutes of Health.
Abbreviations
- ANOVA
analysis of variance
- 2DG
[14C]2-deoxy-D-glucose
- CCI
cortical contusion injury
- CyO
cytochrome oxidase
- PBS
phosphate buffered saline
- rCMRG
regional cerebral metabolic rates of glucose
- SEM
standard error of the mean
- SP
sodium pyruvate
- TBI
traumatic brain injury
- Veh
vehicle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: There are no conflicts of interest to report.
Contributor Information
Nobuhiro Moro, Email: nmoro@mednet.ucla.edu.
Sima S. Ghavim, Email: sghavim@mednet.ucla.edu.
David A. Hovda, Email: dhovda@mednet.ucla.edu.
Reference List
- 1.Bartnik BL, Lee SM, Hovda DA, Sutton RL. The fate of glucose during the period of decreased metabolism after fluid percussion injury: a 13C NMR study. J. Neurotrauma. 2007;24:1079–1092. doi: 10.1089/neu.2006.0210. [DOI] [PubMed] [Google Scholar]
- 2.Bartnik BL, Sutton RL, Fukushima M, Harris NG, Hovda DA, Lee SM. Upregulation of pentose phosphate pathway and preservation of tricarboxylic acid cycle flux after experimental brain injury. J. Neurotrauma. 2005;22:1052–1065. doi: 10.1089/neu.2005.22.1052. [DOI] [PubMed] [Google Scholar]
- 3.Bergsneider M, Hovda DA, McArthur DL, Etchepare M, Huang SC, Sehati N, Satz P, Phelps ME, Becker DP. Metabolic recovery following human traumatic brain injury based on FDG-PET: time course and relationship to neurological disability. J. Head Trauma Rehabil. 2001;16:135–148. doi: 10.1097/00001199-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Bergsneider M, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, Phelps ME, McArthur DL, Caron MJ, Kraus JF, Becker DP. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J. Neurosurg. 1997;86:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- 5.Bontempi B, Jaffard R, Destrade C. Differential temporal evolution of post-training changes in regional brain glucose metabolism induced by repeated spatial discrimination training in mice: visualization of the memory consolidation process? Eur. J. Neurosci. 1996;8:2348–2360. doi: 10.1111/j.1460-9568.1996.tb01198.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen SH, Kareken DA, Fastenau PS, Trexler LE, Hutchins GD. A study of persistent post-concussion symptoms in mild head trauma using positron emission tomography. J. Neurol. Neurosurg. Psychiatry. 2003;74:326–332. doi: 10.1136/jnnp.74.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conejo NM, Gonzalez-Pardo H, Vallejo B, Arias JL. Changes in brain oxidative metabolism induced by water maze training. Neuroscience. 2007;145:403–412. doi: 10.1016/j.neuroscience.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 8.Dai W, Cheng HL, Huang RQ, Zhuang Z, Shi JX. Quantitative detection of the expression of mitochondrial cytochrome c oxidase subunits mRNA in the cerebral cortex after experimental traumatic brain injury. Brain Res. 2009;1251:287–295. doi: 10.1016/j.brainres.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Dunn-Meynell AA, Levin BE. Lateralized effect of unilateral somatosensory cortex contusion on behavior and cortical reorganization. Brain Res. 1995;675:143–156. doi: 10.1016/0006-8993(95)00050-z. [DOI] [PubMed] [Google Scholar]
- 10.Fukushima M, Lee SM, Moro N, Hovda DA, Sutton RL. Metabolic and histologic effects of sodium pyruvate treatment in the rat after cortical contusion injury. J. Neurotrauma. 2009;26:1095–1110. doi: 10.1089/neu.2008.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav. Brain Res. 2009;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hovda DA, Sutton RL, Feeney DM. Recovery of tactile placing after visual cortex ablation in cat: a behavioral and metabolic study of diaschisis. Exp. Neurol. 1987;97:391–402. doi: 10.1016/0014-4886(87)90099-9. [DOI] [PubMed] [Google Scholar]
- 13.Hovda DA, Yoshino A, Kawamata T, Katayama Y, Becker DP. Diffuse prolonged depression of cerebral oxidative metabolism following concussive brain injury in the rat: a cytochrome oxidase histochemistry study. Brain Res. 1991;567:1–10. doi: 10.1016/0006-8993(91)91429-5. [DOI] [PubMed] [Google Scholar]
- 14.Humayun MS, Presty SK, Lafrance ND, Holcomb HH, Loats H, Long DM, Wagner HN, Gordon B. Local cerebral glucose abnormalities in mild closed head injured patients with cognitive impairments. Nucl. Med. Commun. 1989;10:335–344. doi: 10.1097/00006231-198905000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Huttemann M, Lee I, Kreipke CW, Petrov T. Suppression of the inducible form of nitric oxide synthase prior to traumatic brain injury improves cytochrome c oxidase activity and normalizes cellular energy levels. Neuroscience. 2008;151:148–154. doi: 10.1016/j.neuroscience.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Izumi Y, Zorumski CF. Neuroprotective effects of pyruvate following NMDA-mediated excitotoxic insults in hippocampal slices. Neurosci. Lett. 2010;478:131–135. doi: 10.1016/j.neulet.2010.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kokiko ON, Hamm RJ. A review of pharmacological treatments used in experimental models of traumatic brain injury. Brain Inj. 2007;21:259–274. doi: 10.1080/02699050701209964. [DOI] [PubMed] [Google Scholar]
- 18.Kokiko-Cochran ON, Michaels MP, Hamm RJ. Delayed glucose treatment improves cognitive function following fluid-percussion injury. Neurosci. Lett. 2008;436:27–30. doi: 10.1016/j.neulet.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 19.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Lee SM, Wong DA, Samii A, Hovda DA. Evidence for energy failure following irreversible traumatic brain injury. Ann. N.Y. Acad. Sci. 1999;893:337–340. doi: 10.1111/j.1749-6632.1999.tb07849.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin AL, Fox PT, Hardies J, Duong TQ, Gao JH. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc. Natl. Acad. Sci. U.S.A. 2010;107:8446–8451. doi: 10.1073/pnas.0909711107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNay EC, Canal CE, Sherwin RS, Gold PE. Modulation of memory with septal injections of morphine and glucose: effects on extracellular glucose levels in the hippocampus. Physiol. Behav. 2006;87:298–303. doi: 10.1016/j.physbeh.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 23.McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNay EC, Gold PE. Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. J. Gerontol. Biol. Sci. Med. Sci. 2001;56:B66–B71. doi: 10.1093/gerona/56.2.b66. [DOI] [PubMed] [Google Scholar]
- 25.McNay EC, McCarty RC, Gold PE. Fluctuations in brain glucose concentration during behavioral testing: dissociations between brain areas and between brain and blood. Neurobiol. Learn. Mem. 2001;75:325–337. doi: 10.1006/nlme.2000.3976. [DOI] [PubMed] [Google Scholar]
- 26.Messier C. Glucose improvement of memory: a review. Eur. J. Pharmacol. 2004;490:33–57. doi: 10.1016/j.ejphar.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 27.Moore AH, Osteen CL, Chatziioannou AF, Hovda DA, Cherry SR. Quantitative assessment of longitudinal metabolic changes in vivo after traumatic brain injury in the adult rat using FDG-microPET. J. Cereb. Blood Flow Metab. 2000;20:1492–1501. doi: 10.1097/00004647-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Moro N, Sutton RL. Beneficial effects of sodium or ethyl pyruvate after traumatic brain injury in the rat. Exp. Neurol. 2010;225:391–401. doi: 10.1016/j.expneurol.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakashima T, Nakayama N, Miwa K, Okumura A, Soeda A, Iwama T. Focal brain glucose hypometabolism in patients with neuropsychologic deficits after diffuse axonal injury. AJNR Am. J. Neuroradiol. 2007;28:236–242. [PMC free article] [PubMed] [Google Scholar]
- 30.Nakayama N, Okumura A, Shinoda J, Nakashima T, Iwama T. Relationship between regional cerebral metabolism and consciousness disturbance in traumatic diffuse brain injury without large focal lesions: an FDG-PET study with statistical parametric mapping analysis. J. Neurol. Neurosurg. Psychiatry. 2006;77:856–862. doi: 10.1136/jnnp.2005.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prins ML, Hovda DA. Mapping cerebral glucose metabolism during spatial learning: interactions of development and traumatic brain injury. J. Neurotrauma. 2001;18:31–46. doi: 10.1089/089771501750055758. [DOI] [PubMed] [Google Scholar]
- 32.Prins ML, Hovda DA. The effects of age and ketogenic diet on local cerebral metabolic rates of glucose after controlled cortical impact injury in rats. J. Neurotrauma. 2009;26:1083–1093. doi: 10.1089/neu.2008.0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Queen SA, Feeney DM. Temporally changing patterns of hippocampal cerebral glucose utilization following sensorimotor cortical contusion in rats. Brain Res. 1996;724:246–250. doi: 10.1016/0006-8993(96)00308-3. [DOI] [PubMed] [Google Scholar]
- 34.Ragozzino ME, Gold PE. Glucose injections into the medial septum reverse the effects of intraseptal morphine infusions on hippocampal acetylcholine output and memory. Neuroscience. 1995;68:981–988. doi: 10.1016/0306-4522(95)00204-v. [DOI] [PubMed] [Google Scholar]
- 35.Ragozzino ME, Hellems K, Lennartz RC, Gold PE. Pyruvate infusions into the septal area attenuate spontaneous alternation impairments induced by intraseptal morphine injections. Behav. Neurosci. 1995;109:1074–1080. doi: 10.1037//0735-7044.109.6.1074. [DOI] [PubMed] [Google Scholar]
- 36.Ragozzino ME, Pal SN, Unick K, Stefani MR, Gold PE. Modulation of hippocampal acetylcholine release and spontaneous alternation scores by intrahippocampal glucose injections. J. Neurosci. 1998;18:1595–1601. doi: 10.1523/JNEUROSCI.18-04-01595.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ragozzino ME, Unick KE, Gold PE. Hippocampal acetylcholine release during memory testing in rats: augmentation by glucose. Proc. Natl. Acad. Sci. U.S.A. 1996;93:4693–4698. doi: 10.1073/pnas.93.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez-Carrion R, Gomez PV, Junque C, Fernandez-Espejo D, Falcon C, Bargallo N, Roig-Rovira T, Ensenat-Cantallops A, Bernabeu M. Frontal hypoactivation on functional magnetic resonance imaging in working memory after severe diffuse traumatic brain injury. J. Neurotrauma. 2008;25:479–494. doi: 10.1089/neu.2007.0417. [DOI] [PubMed] [Google Scholar]
- 39.Sharma P, Benford B, Li ZZ, Ling GS. Role of pyruvate dehydrogenase complex in traumatic brain injury and measurement of pyruvate dehydrogenase enzyme by dipstick test. J. Emerg. Trauma Shock. 2009;2:67–72. doi: 10.4103/0974-2700.50739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soane L, Kahraman S, Kristian T, Fiskum G. Mechanisms of impaired mitochondrial energy metabolism in acute and chronic neurodegenerative disorders. J. Neurosci. Res. 2007;85:3407–3415. doi: 10.1002/jnr.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 42.Sutton RL, Hovda DA, Adelson PD, Benzel EC, Becker DP. Metabolic changes following cortical contusion: relationships to edema and morphological changes. Acta Neurochir. Suppl. (Wien.) 1994;60:446–448. doi: 10.1007/978-3-7091-9334-1_122. [DOI] [PubMed] [Google Scholar]
- 43.Sutton RL, Hovda DA, Chen MJ, Feeney DM. Alleviation of brain injury-induced cerebral metabolic depression by amphetamine: a cytochrome oxidase histochemistry study. Neural Plasticity. 2000;7:109–125. doi: 10.1155/NP.2000.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor AN, Rahman SU, Sanders NC, Tio DL, Prolo P, Sutton RL. Injury severity differentially affects short- and long-term neuroendocrine outcomes of traumatic brain injury. J. Neurotrauma. 2008;25:311–323. doi: 10.1089/neu.2007.0486. [DOI] [PubMed] [Google Scholar]
- 45.Wrubel KM, Riha PD, Maldonado MA, McCollum D, Gonzalez-Lima F. The brain metabolic enhancer methylene blue improves discrimination learning in rats. Pharmacol. Biochem. Behav. 2007;86:712–717. doi: 10.1016/j.pbb.2007.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshino A, Hovda DA, Kawamata T, Katayama Y, Becker DP. Dynamic changes in local cerebral glucose utilization following cerebral concussion in rats: evidence of a hyper- and subsequent hypometabolic state. Brain Res. 1991;561:106–119. doi: 10.1016/0006-8993(91)90755-k. [DOI] [PubMed] [Google Scholar]
- 47.Zeng J, Yang GY, Ying W, Kelly M, Hirai K, James TL, Swanson RA, Litt L. Pyruvate improves recovery after PARP-1-associated energy failure induced by oxidative stress in neonatal rat cerebrocortical slices. J. Cereb. Blood Flow Metab. 2007;27:304–315. doi: 10.1038/sj.jcbfm.9600335. [DOI] [PubMed] [Google Scholar]
- 48.Zlotnik A, Gurevich B, Cherniavsky E, Tkachov S, Matuzani-Ruban A, Leon A, Shapira Y, Teichberg VI. The contribution of the blood glutamate scavenging activity of pyruvate to its neuroprotective properties in a rat model of closed head injury. Neurochem. Res. 2008;33:1044–1050. doi: 10.1007/s11064-007-9548-x. [DOI] [PubMed] [Google Scholar]