Abstract
Normal tissue cells survive and proliferate only while anchored to solid substrate. Conversely, transformed cells both survive and proliferate following detachment, having lost attachment context through unclear mechanisms. p66Shc is a focal adhesion-associated protein that reports cell attachment through a RhoA-dependent mechanosensory test. We find that human small cell lung cancer (SCLC) cells and mouse Lewis lung carcinoma (LLC), which display aggressive metastatic behavior, lack both p66Shc and retinoblastoma (pRB) and bypass anoikis. Re-expression of p66Shc in these cells restores anoikis and provides striking protection from metastasis by LLC cells in vivo. Notably, knockdown of p66Shc in normal epithelial cells leads to unrestrained Ras activation, preventing anoikis through downstream suppression of RhoA but blocking proliferation in a pRB-dependent manner, thus mimicking oncogenic Ras. Conversely, LLC and SCLC cells display constitutive Ras activation necessary to bypass anoikis, which is reversed by re-expression of p66Shc. p66Shc therefore coordinates Ras-dependent control of proliferation and anchorage sensation, which can be defeated in the evolution of highly metastatic tumors by combined loss of both p66Shc and pRB.
Keywords: anoikis, Shc, pRB, metastasis, small cell lung cancer
Introduction
Differentiated epithelial and endothelial cells have strong attachment requirements, presumably to prevent cellular vagrancy and consequent tissue disorganization. During transformation, cells lose such anchorage context and not only survive but also proliferate without attachment to solid matrix. Certain pathways downstream from integrins may influence detachment signals. Ectopic expression of integrin-linked kinase, TrkB, or oncogenic forms of Src and Ras have been shown to abrogate anoikis (Frisch and Francis, 1994; Frisch et al., 1996; Attwell et al., 2000; McFall et al., 2001; Douma et al., 2004). In addition, during certain stages of development and tissue repair, normal cells also bypass anoikis in order to migrate, suggesting native regulation of endogenous proteins that control attachment sensation.
The adapter protein p66Shc was recently found to localize to focal adhesions and permit attachment sensation by imposing RhoA-dependent tension across these integrin anchorage points, creating a mechanosensory test of anchorage (Ma et al., 2007). Upon detachment of cells from their underlying matrix, this p66-dependent tension test is met with load failure, initiating apoptosis. In contrast, the absence of p66Shc allows both survival and proliferation of unanchored 293 cells. In this study, we test the relevance of p66Shc to metastatic capacity and control of proliferation in both normal and transformed cells. Surprisingly, we find that p66Shc functions to restrain Ras and allow its physiologic regulation. Upon loss of p66Shc expression, endogenous Ras becomes hyperactivated and phenocopies oncogenic Ras, suppressing proliferation through retinoblastoma (pRB) in normal cells but allowing anchorage independence in vitro and aggressive metastasis of lung cancer cells in vivo.
Results
p66Shc acts upstream of RhoA to control anoikis in normal and lung cancer cells
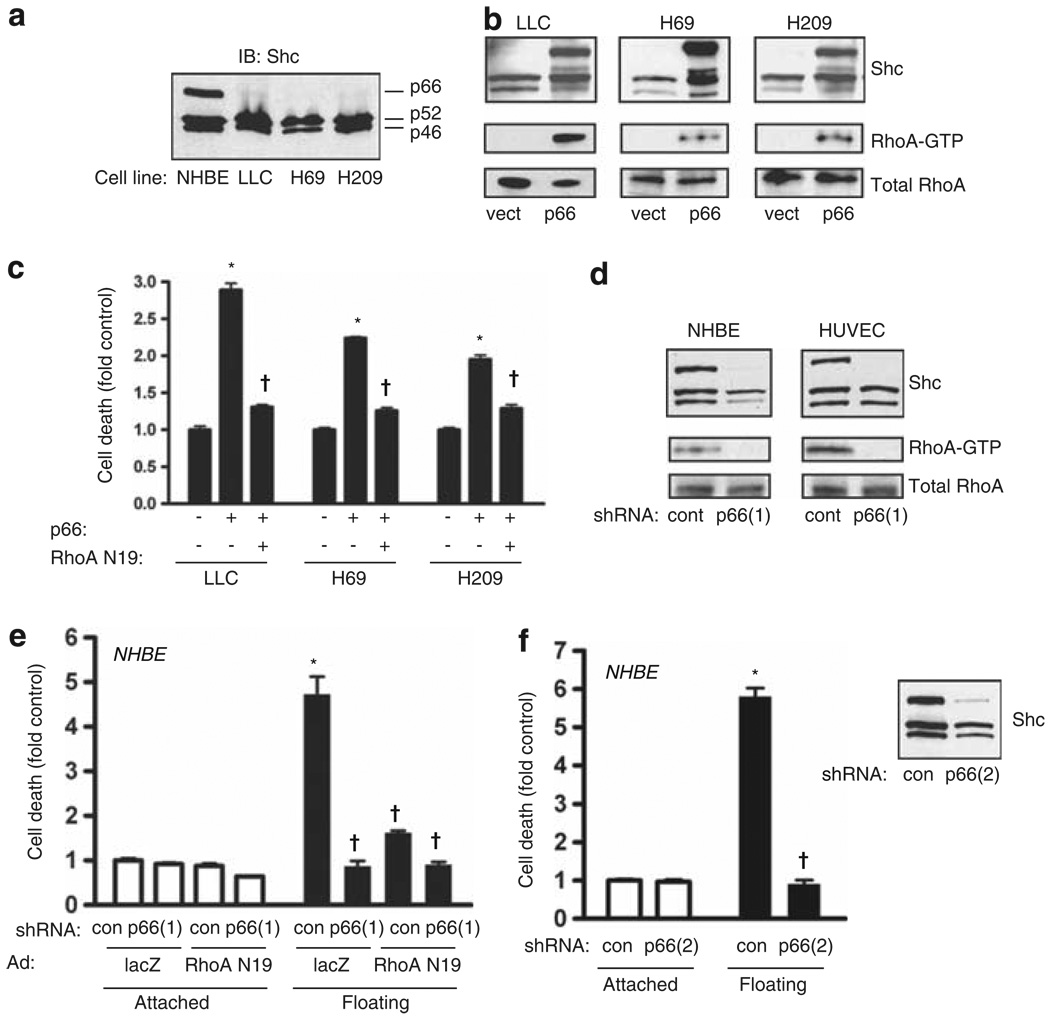
The SHC gene encodes three proteins that differ in the length of the amino terminus (p46, p52 and p66). We examined Shc proteins in three highly metastatic cancer cells, the mouse Lewis lung carcinoma (LLC) and two lines of human small cell lung cancer (SCLC; H69 and H209) harvested from metastatic sites. In contrast to primary normal human bronchial epithelial (NHBE) cells, which express all three forms of Shc, the LLC, H69 and H209 cell lines lacked detectable p66Shc (Figure 1a). Re-expression of p66Shc through lentiviral transduction caused constitutive activation of RhoA in all three cell lines (Figure 1b), consistent with the ability of p66Shc to act upstream of RhoA in attachment sensing (Ma et al., 2007). Indeed, re-expression of p66Shc restored anoikis in floating cultures of all three cancer cell lines, which was reversed by RhoA(N19) (Figure 1c). Conversely, primary epithelial and endothelial cells (NHBE and human umbilical vein endothelial cells) express p66Shc and display basal activation of RhoA. Knockdown of p66Shc by lentiviral delivery of short hairpin RNA (shRNA) completely suppressed basal RhoA activity (Figure 1d). NHBE cells, typical of normal epithelial cells, have intact anoikis mechanisms with significant cell death in floating culture for 16 h (Figure 1e). Knockdown of p66Shc or expression of RhoA(N19) significantly blocked detachment-induced cell death (Figure 1e). A second small hairpin against p66Shc (shRNA(2)) confirmed that p66Shc is necessary for anoikis (Figure 1f). Thus, in both normal and malignant epithelium, p66Shc acts upstream of RhoA to signal attachment status and initiate anoikis following detachment.
Figure 1.
p66Shc promotes anoikis through RhoA. (a) Immunoblot for Shc demonstrates expression of three Shc isoforms by normal human bronchial epithelium (NHBE) cells but loss of p66Shc expression by Lewis lung carcinoma (LLC) and SCLC (H69 and H209) cell lines. (b) LLC, H69 and H209 cells were transduced with p66Shc. The top panels demonstrate Shc isoform expression. Active RhoA was assessed by pulldown and was shown to consistently increase following expression of p66Shc. (c) LLC, H69 and H209 cells were grown in low attachment plates after lentiviral transduction with p66Shc or empty vector, and adenoviral transduction with dominant-negative RhoA(N19) or lacZ (control). After 16 h, cell death was assessed. Mean ± s.e.m. of four determinations is shown. *P < 0.001 compared with respective control, †P < 0.001 compared with p66Shc alone. (d) p66Shc was knocked down with shRNA in NHBE cells and human umbilical vein endothelial cells (HUVECs). The top panels demonstrate knockdown effect. The bottom panels show that p66Shc knockdown completely suppressed RhoA activity. (e) NHBE cells were transduced with the indicated shRNA by lentivirus and RhoA(N19) or lacZ by adenovirus, and allowed to adhere or forced to float for 16 h. Cell death was measured as above. Mean ± s.e.m. of four determinations is shown. *P < 0.001 compared with attached control, †P < 0.001 compared with floating control. (f) NHBE cells were transduced with a second shRNA against p66Shc (shRNA(2)) and cell death measured as above. Inset shows selective effect of shRNA(2) for p66Shc. Mean ± s.e.m. of four determinations is shown. *P < 0.001 compared with attached control, †P < 0.001 compared with floating control.
p66Shc prevents lung metastasis in vivo
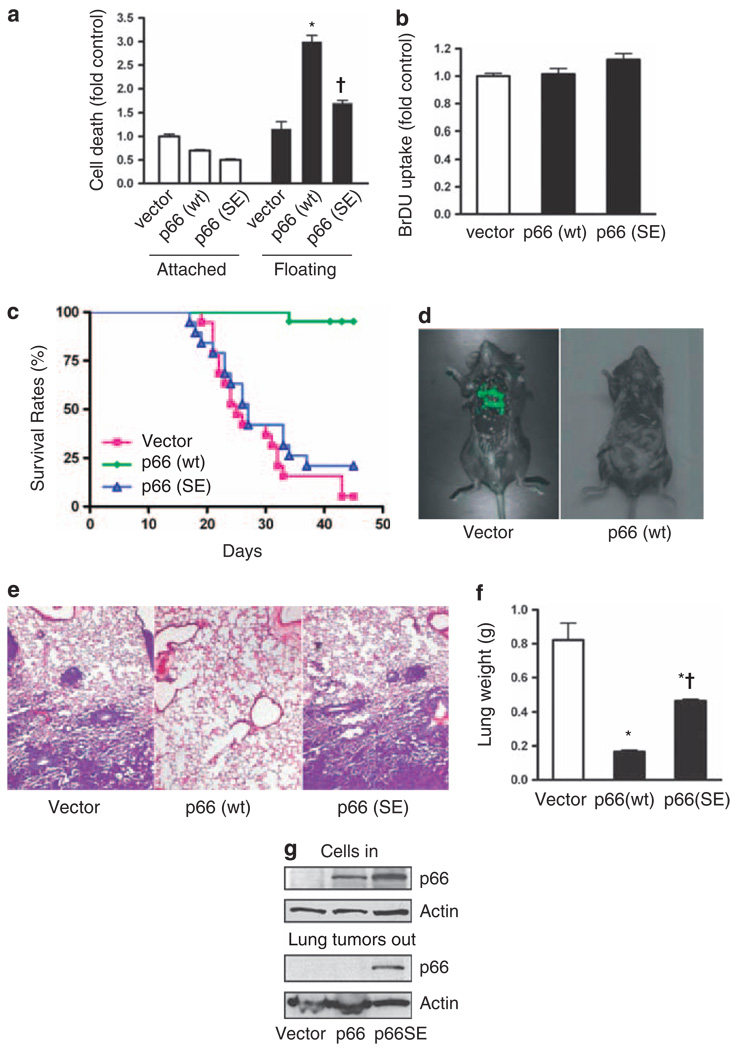
To test the significance of p66Shc-induced anoikis for metastatic capacity in vivo, we stably transfected LLC cells with either native p66Shc or p66(S36E), using retroviral transduction with the PINCO plasmid that expresses green fluorescent protein (GFP; Grignani et al., 1998). The S36E mutation lies within the N-terminal CH2 domain unique to the p66Shc isoform, and abrogates its attachment-sensing function (Ma et al., 2007). As expected, LLC cells expressing p66Shc had increased cell death following detachment but not while attached, whereas p66(S36E) had little effect (Figure 2a). Expression of p66Shc or p66(S36E) had no significant effect on the proliferation of adherent LLC cells (Figure 2b), suggesting a specific effect on anoikis in these cells.
Figure 2.
p66Shc suppresses lung metastasis in vivo. (a) LLC cells stably expressing p66Shc or p66(S36E) were plated on normal or low attachment plates for 16 h and cell death assessed. Expression of wild-type p66Shc increased detachment-induced cell death (*P < 0.001 from vector control), whereas p66(S36E) had a reduced effect (†P < 0.001 from p66Shc floating). Mean ± s.e.m. of four determinations is shown. (b) 5-bromodeoxyuridine (BrDU) uptake of attached LLC cells expressing p66Shc (p66 wt) or p66(S36E) was not different from vector control. (c) LLC cells expressing p66Shc or p66(S36E)were injected into the tail vein of 6-week-old female C57BL/6 mice. Kaplan–Meier survival curve is shown; survival between groups was different, P < 0.0001. n = 19 (vector), n = 21 (p66 wt) and n = 19 (p66(SE)). (d) Representative mice receiving vector-LLC or p66-LLC cells. Multiple GFP-positive lung metastases are apparent in the former but not in the latter group. (e) Representative hematoxylin and eosin stain of lungs from mice injected with vector control, p66Shc or p66(S36E)-expressing LLC cells. Vector control and p66(S36E) lungs demonstrated dense interstitial replacement with tumor with perivascular tumor cuffing. (f) Wet lung weights were assessed, reflecting tumor burden. *P < 0.01 from vector control, †P < 0.01 from p66 (wt). (g) Immunoblot showing p66Shc expression of cells injected into animals (top panel). Lung tumors were dissected, pooled and immunoblotted for p66Shc from the single mouse in the p66Shc group that had metastatic tumors (middle lane), and representative tumors extracted from lungs of mice receiving LLC cells with empty vector (left lane) or p66(S36E) (right lane).
When injected into the tail vein of C57BL/6 mice, control LLC cells caused lethality in 18/19 mice by 45 days, with a median survival of 25 days (Figure 2c). In all cases, death was accompanied by a heavy lung metastatic burden, easily identified by GFP-positive masses and verified histologically and by lung weights (Figures 2d–f). In contrast, mice injected with LLC cells expressing p66Shc had minimal mortality, with only 1/21 mice dying from lung metastases. In this group, two mice were killed because of late outgrowths of tumors at the injection site; neither of these mice had detectable metastases in the lungs or abdominal compartment. The single mouse injected with p66Shc-expressing LLC cells that died from lung metastases was examined further by dissection of the metastatic nodules. Remarkably, the nodules had completely lost expression of p66Shc despite being GFP positive (Figure 2g). Because these LLC cells were not clonally selected, it is likely that the metastases in this animal represented a subclonal outgrowth of LLC cells, selected for their loss of p66Shc. In further support of the specific effect of p66Shc on metastatic capability, the mortality of mice injected with LLC cells expressing p66(S36E) resembled that of mice injected with control LLC cells, with 15/19 animals dying of lung metastases in 45 days for a median survival of 27 days. A representative sampling of metastatic nodules from this group showed no loss of presumably mutant p66 (Figure 1g). Thus, loss of metastatic capacity in vivo correlated accurately with anoikis in vitro and with expression of p66Shc with an intact CH2 domain.
Absence of p66Shc arrests proliferation through activation of pRB
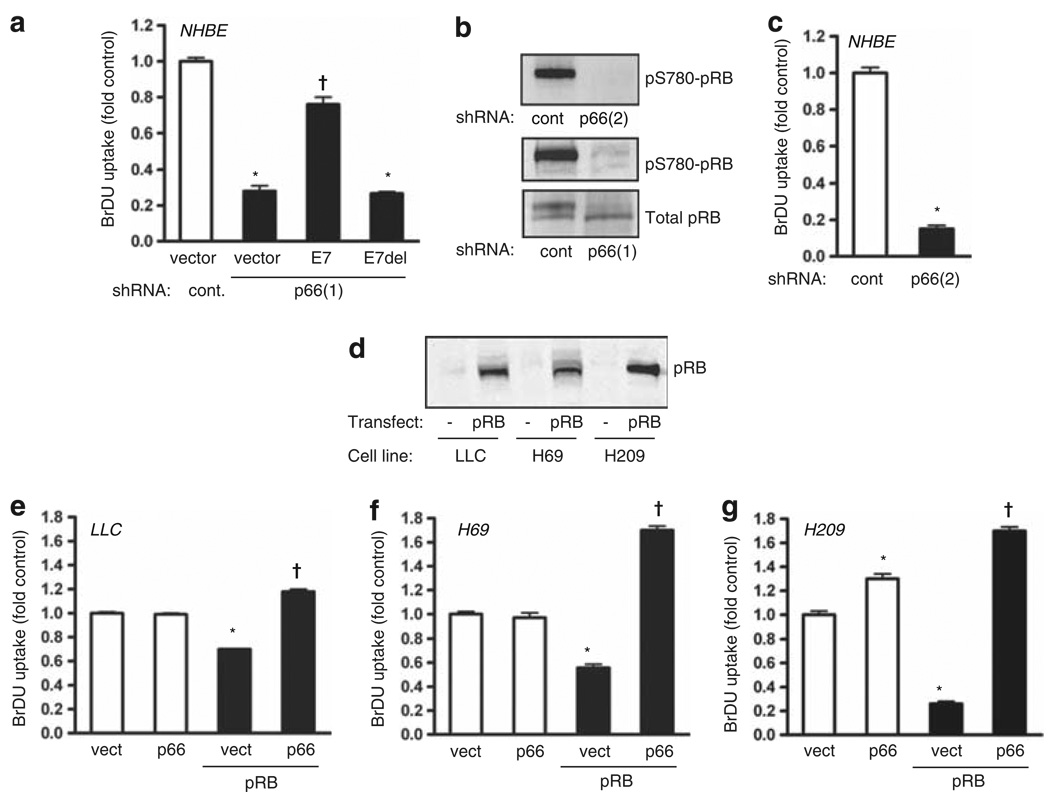
Because matrix attachment also permits proliferative signaling, we next asked whether loss of p66Shc also influences cell growth. Despite their continued attachment to culture dishes, knockdown of p66Shc effectively blocked proliferation of NHBE cells, suggesting that loss of attachment sensation may cause a default cell cycle arrest (Figure 3a). In contrast, LLC and SCLC cells proliferate despite the absence of p66Shc. Notably, ~90% of SCLC tumors are known to lack functional pRB (Wistuba et al., 2001), allowing for the possibility that pRB may normally enforce a proliferative arrest following the loss of p66Shc, an effect lost without functional pRB. In support of this hypothesis, expression of the pocket-binding viral protein E7 by NHBE effectively reversed the growth arrest induced by p66Shc knockdown, whereas the nonfunctional E7(Δ21–24) had no effect (Figure 3a). In parallel, p66Shc knockdown caused hypophosphorylation (activation) of endogenous pRB (Figure 3b). The effect of p66Shc knockdown on pRB phosphorylation and proliferation was confirmed with the second shRNA against p66Shc (Figures 3b and c). We further found that LLC, H69 and H209 cells lacked significant pRB expression (Figure 3d). Although expression of p66Shc alone did not reduce proliferation in any of these tumor cell lines, pRB expression reduced proliferation to a variable degree (Figures 3e–g). Strikingly, expression of p66Shc reversed the growth-suppressive effect of pRB in all three cell lines. These data suggest that in both normal and transformed epithelium, p66Shc acts upstream of pRB to suppress its function, allowing cell cycle progression. Thus, in the absence of p66Shc, pRB defaults to its hypophosphorylated state to halt proliferation. In SCLC and LLC cells, the absence of p66Shc is tolerated because of the additional loss of pRB, which permits cell growth.
Figure 3.
p66Shc suppresses proliferation through pRB. (a) NHBE cells were transduced with either HPV16 E7 or its nonbinding deletion mutant E7(Δ21–24), and proliferation assessed. Knockdown of p66Shc decreased 5-bromodeoxyuridine (BrDU) uptake (*P < 0.001 from vector control), and E7 but not E7(Δ21–24) restored proliferation (†P < 0.01 from p66Shc knockdown alone). Mean ± s.e.m. of six determinations is shown. (b) NHBE cells were transduced with control or p66Shc shRNA and immunoblotted for total and phospho-pRB, demonstrating hypophosphorylation of pRB following p66Shc knockdown. (c) Proliferation was assessed following transduction with indicated shRNAs. Mean ± s.e.m. of six determinations, *P < 0.001 from vector control. (d) Immunoblot for total pRB of the indicated cell lines before and after lentiviral delivery of pRB. (e–g) Lentivirus was used to transduce p66Shc and pRB as indicated. pRB decreased BrDU incorporation in LLC, H69 and H209 cells (*P < 0.001 from vector control) whereas p66Shc restored proliferation above baseline (†P < 0.01 from pRB alone). Mean ± s.e.m. of six determinations is shown.
p66Shc restrains K-Ras hyperactivation upstream from pRB
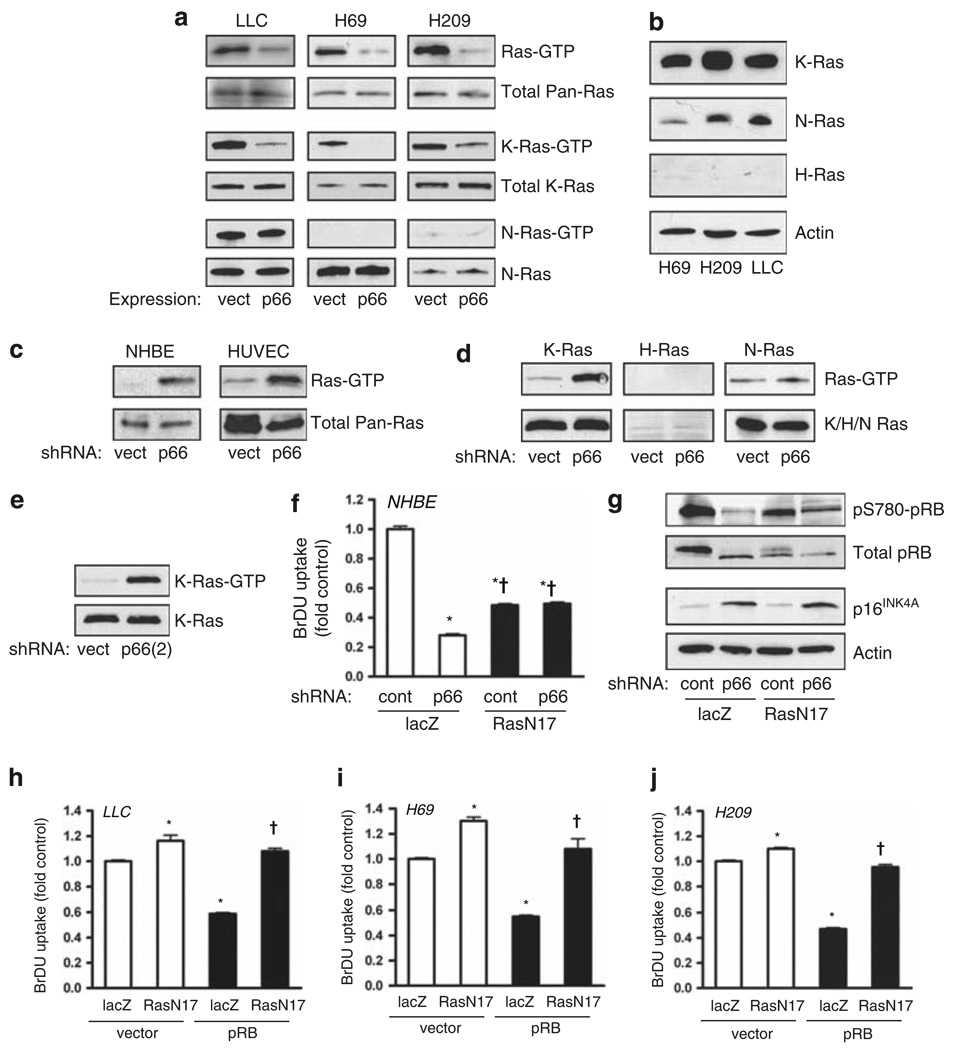
The effect of p66Shc on proliferation appears to be distinct from its effect on anoikis, which is RhoA dependent but pRB independent, as p66Shc restores anoikis in SCLC and LLC cells without pRB (Figure 1c). Because Ras potentially influences both pRB and RhoA, we next asked if Ras acts as a coordination point for both pathways. Notably, SCLC and LLC cells displayed high basal levels of Ras activation (Figure 4a). In all three cell lines, Ras activation was quantitatively decreased by expression of p66Shc, indicating suppression of Ras activity by p66Shc independent of pRB. Specifically, p66Shc suppressed K-Ras with no effect on N-Ras. In these cells, H-Ras was not expressed in significant levels (Figure 4b). Conversely, in primary NHBE and human umbilical vein endothelial cells, basal Ras activity was low but became hyperactivated by p66Shc knockdown (Figure 4c). In NHBE cells, this effect was also specific for K-Ras and not H-Ras or N-Ras (Figure 4d). Again, striking hyperactivation of K-Ras in NHBE cells was replicated using a second shRNA against p66Shc (Figure 4e). Thus, in both normal and malignant cells, p66Shc restrains hyperactivation of K-Ras.
Figure 4.
p66Shc restrains Ras hyperactivation in malignant and normal cells. (a) LLC, H69 and H209 tumor cell lines were transduced with lentivirus as indicated and active Ras was assessed by pulldown. Expression of p66Shc markedly suppressed Ras activation as assessed by immunoblot for pan-Ras (top panels) and K-Ras (middle panels) but not N-Ras (bottom panels). (b) Immunoblots of SCLC and LLC cells for different Ras isoforms, with actin loading control. (c) Knockdown of p66Shc in primary NHBE cells and human umbilical vein endothelial cells (HUVEC) caused activation of Ras. (d) p66Shc knockdown caused robust activation of K-Ras but not H-Ras or N-Ras in NHBE cells. (e) Similar conditions as in (d) except with shRNA(2) against p66Shc. (f) In NHBE cells, 5-bromodeoxyuridine (BrDU) uptake was assessed following knockdown of p66Shc and/or expression of Ras(N17). Mean ± s.e.m. of six determinations is shown, *P < 0.001 from lacZ control, †P < 0.001 from lacZ/p66Shc knockdown. (g) Immunoblot for phosphorylated and total pRB and p16INK4A of NHBE cells treated as indicated. (h–j) BrDU uptake was measured in LLC, H69 and H209 cells after expression of pRB and/or Ras (N17). pRB decreased BrDU uptake (*P < 0.001 from lacZ/vector control), whereas Ras(N17) restored proliferation (†P < 0.001 from lacZ/pRB, P > 0.05 from lacZ/vector control). Mean ± s.e.m. of six determinations is shown.
We next asked if the hyperactivation of K-Ras due to loss of p66Shc led to the acquisition of a constitutively active Ras-like state. In normal primary cells, endogenously controlled native Ras is transiently activated to suppress pRB function to allow cell cycle progression (Mittnacht et al., 1997; Peeper et al., 1997). In contrast, expression of constitutively active oncogenic Ras mutants such as Ras(V12) in normal cells causes cell cycle arrest, an effect requiring intact pRB function (Phelps et al., 1988; Serrano et al., 1997). Accordingly, suppression of basal Ras activity with Ras(N17) decreased NHBE cell proliferation at baseline (Figure 4f). However, in the presence of Ras(N17), knockdown of p66Shc no longer suppressed proliferation, and phosphorylation of pRB was partly restored (Figures 4f and g). p66Shc knockdown also induced the cyclin-dependent kinase 4 inhibitor p16INK4A, although Ras(N17) did not significantly block this induction (Figure 4g), suggesting both Ras-dependent and Ras-independent effects of p66Shc on pRB. These data indicate that loss of p66Shc may indeed leave native Ras in a dysregulated hyperactive state, leading to pRB-dependent proliferative arrest, comparable to the effects of oncogenic Ras mutations.
We further tested the effect of Ras deregulation on proliferation in the lung cancer cell lines. First, we noted that Ras(N17) did not suppress proliferation of either the LLC or SCLC cell lines, which display basal Ras hyperactivation but lack pRB (Figures 4h–j). However, upon re-expression of pRB, proliferation decreased and was restored by Ras(N17) (Figures 4h–j). These data confirm that Ras, rendered hyperactive by lack of p66Shc, acts similarly to constitutively active oncogenic Ras in its ability to suppress proliferation through pRB, even in fully transformed cells. These data also highlight the importance of pRB loss in the development of SCLC, without which hyperactive Ras would be poorly tolerated.
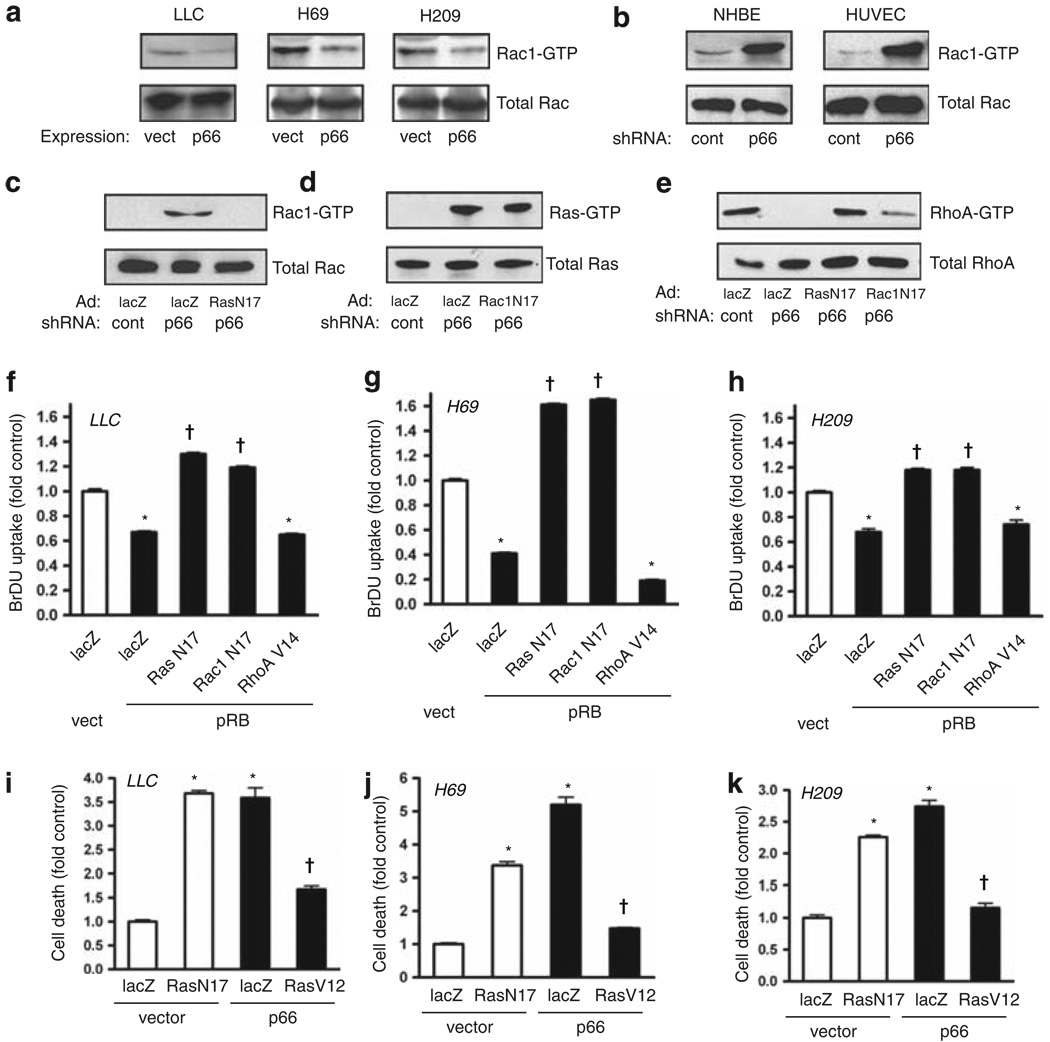
p66Shc restrains hyperactivation of Rac1 and deactivation of RhoA downstream from Ras
Transformation triggered by oncogenic Ras requires Rac1 (Qiu et al., 1995), which also mediates anchorage-dependent proliferative signals (Mettouchi et al., 2001). We therefore explored a potential role for Rac1 in the p66Shc-Ras pathway. Similar to its effect on Ras, we found that re-expression of p66Shc in LLC and SCLC cells reduced basal Rac1 activity; conversely, p66Shc knockdown in primary NHBE and human umbilical vein endothelial cells caused robust activation of Rac1 (Figures 5a and b). In NHBE cells, Rac1 hyperactivation was suppressed by Ras(N17), whereas Rac1(N17) had no effect on hyperactivation of Ras (Figures 5c and d), indicating activation of Rac1 downstream from Ras following loss of p66Shc. In contrast, the activity of RhoA was suppressed by p66Shc knockdown and restored by Ras(N17) and Rac1(N17) (Figure 5e). Thus, loss of p66Shc blocks RhoA downstream from constitutive activation of Ras and Rac1.
Figure 5.
Loss of p66Shc suppresses RhoA through Ras and Rac1 activation. (a) Re-expression of p66Shc through lentiviral delivery suppressed active Rac1, assessed by pulldown. (b) Knockdown of p66Shc by shRNA caused intense activation of Rac1 in NHBE cells and human umbilical vein endothelial cells (HUVECs). (c–e) Ras(N17) or Rac1(N17) were expressed and p66Shc knocked down as indicated in NHBE cells, and active Rac1, Ras and RhoA were assessed by pulldown. (f–h) Dominant negatives Ras(N17) and Rac1(N17) and constitutively active RhoA(V14) were delivered by adenovirus; pRB was expressed by lentiviral delivery in LLC, H69 and H209 cells. pRB suppressed BrDU uptake (*P < 0.001 from lacZ/vector control), whereas both Ras(N17) and Rac1(N17) restored proliferation above baseline (†P < 0.001 from lacZ/pRB). Active RhoA(V14) did not rescue proliferation from pRB. Mean ± s.e.m. of six determinations is shown. (i–k) LLC, H69 and H209 cells were grown in low attachment plates following transduction as indicated. DNA fragmentation was assessed 16 h later. Suppression of endogenous Ras with Ras(N17) caused death in floating cells for all three cell lines to levels comparable to those caused by p66Shc expression (*P < 0.001 from lacZ/vector control). In contrast, active Ras(V12) rescued all three cells from p66Shc-induced anoikis (†P < 0.001 from respective lacZ/p66 groups). Mean ± s.e.m. of four determinations is shown.
Next, we placed Rac1 and RhoA in the pathways leading to control of proliferation and anoikis downstream from Ras. In LLC and SCLC cells, Rac1(N17) reversed the pRB-induced suppression of proliferation to a similar extent as Ras(N17) (Figures 5f–h). This finding suggests that hyperactive Ras suppresses growth in these malignant cells in the presence of pRB through Rac1. In contrast to Ras and Rac1, RhoA activity is suppressed in LLC and SCLC cells (Figure 1b). However, restoration of RhoA activity with RhoA(V14) had no effect on pRB-induced suppression of proliferation (Figures 5f–h). Therefore, hyperactive Ras signals bifurcate at Rac1 to suppress proliferation and anoikis through distinct pathways, with RhoA controlling anoikis and not proliferation in this context.
Lastly, to confirm that RhoA-dependent anoikis requires Ras restraint by p66Shc, we noted that suppression of Ras hyperactivation with Ras(N17) restored anoikis in floating cultures of LLC, H69 and H209 cells (Figures 5i–k). Furthermore, re-expression of p66Shc, which suppresses Ras (Figure 4a), also restored anoikis; however, concomitant expression of Ras(V12) overrode this effect, blocking p66Shc-induced anoikis in all three cell lines (Figures 5i–k). These data confirm that the effects of p66Shc on Ras restraint govern RhoA-dependent anoikis.
Discussion
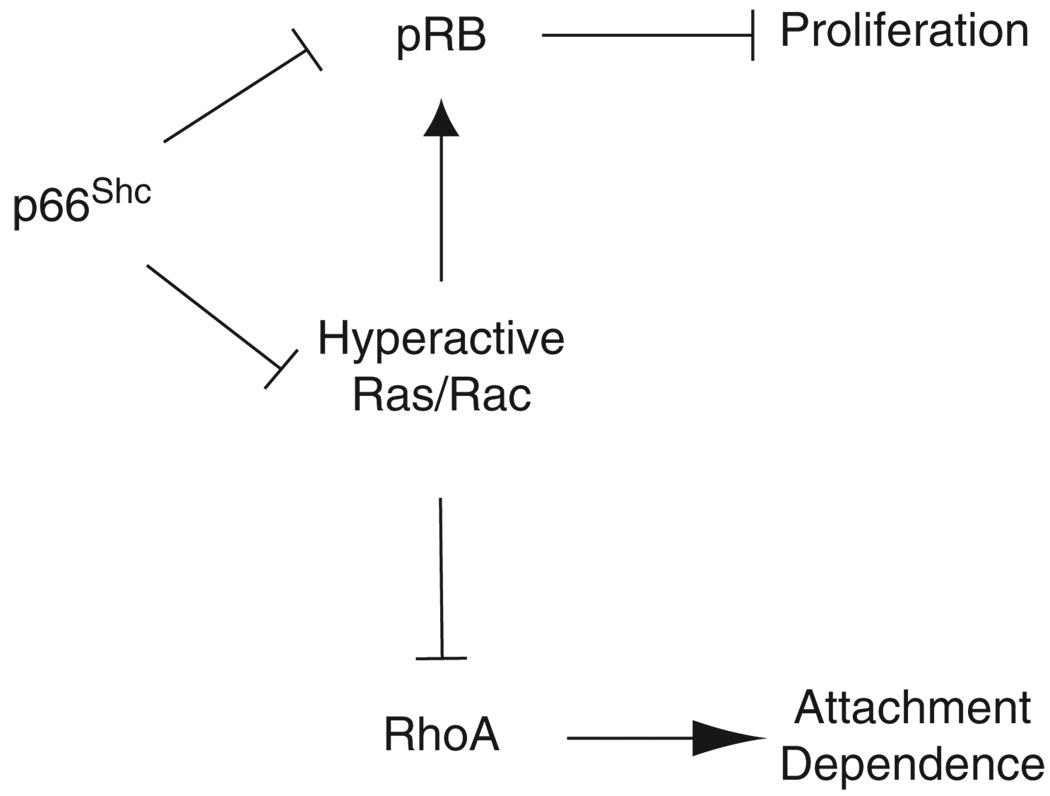
The model that emerges from this study suggests that p66Shc serves as an important coordination point influencing Ras and pRB pathways (Figure 6). Given its role in initiating anchorage-sensing pathways (Ma et al., 2007), we speculate that p66Shc normally lends attachment context to cell-fate decisions through these two pathways. Ras and pRB have complex functional interactions controlling proliferation and differentiation. Detailed genetic studies suggest that endogenous N-Ras and K-Ras interact with Rb1 loss in mice to suppress differentiation of pituitary tumors and enhance malignant transformation (Takahashi et al., 2004, 2006). However, in these Rb1 heterozygotes, N-Ras but not K-ras appears to have an opposite role in the progression of C-cell adenomas to metastatic carcinomas (Takahashi et al., 2006), suggesting tissue- and isoform-specific effects of Ras. Notably, these studies suggest that in the setting of tumorigenesis following loss of pRB, Ras influences differentiation rather than proliferation. Studies using oncogenic Ras to initiate tumorigenesis generally support the role of Ras in reversing differentiation. In this context, pRB acts as a tumor suppressor by irreversibly arresting proliferation in the face of constitutively active Ras (Lundberg et al., 2000); however, suppression of the pRB pathway allows oncogenic Ras to facilitate anchorage-independent growth in vitro and promote tumorigenesis in vivo despite low proliferation rates, indicating loss of differentiation as the primary effect of oncogenic Ras (Phelps et al., 1988; Ho et al., 2009).
Figure 6.
Schematic indicating relationship of p66Shc to proliferative and anoikis pathways through Ras and pRB, consistent with findings in both normal primary bronchial epithelium and malignant lung cancer lines studied. p66Shc restrains Ras and Rac1 from hyperactivation. Signals bifurcate beyond Rac1 to control proliferation through pRB and attachment sensing through RhoA. Loss of anchorage context following loss of p66Shc would allow survival while detached but suppress proliferation through pRB. The diagram includes possible Ras-independent effect of p66Shc on pRB suppression.
Interestingly, our data suggest that in the absence of p66Shc, native K-Ras assumes a hyperactive, deregulated state similar to oncogenic Ras. Knockdown of p66Shc activates pRB in normal cells, and re-expression of pRB by SCLC and LLC cells enforces this antiproliferative function of hyperactive Ras. Furthermore, in both normal and malignant cells, Ras activated by the absence of p66Shc acts primarily to disable anoikis rather than promote proliferation rates, again similar to the effects of oncogenic Ras on reversing differentiation. The loss of metastatic capacity upon re-expression of p66Shc by LLC cells effectively demonstrates the biological significance of this effect. Given its parallel effects on restoring anoikis in SCLC cells, these results suggest that p66Shc may act as an important metastasis suppressor.
An additional ramification of Ras deregulation may lie in the observation that oncogenic Ras mutations are rarely found in SCLC (Wistuba et al., 2001). In the H69 and H209 cell lines, we demonstrate that K-Ras is constitutively hyperactivated in the absence of p66Shc, leading to their characteristic anchorage-independent phenotype. Such basal hyperactivation would suggest that there would be little selective pressure favoring the outgrowth of cells harboring activating mutations of Ras. This interpretation is consistent with the lack of effect of oncogenic v-Ras on classic SCLC lines (Mabry et al., 1988).
The mechanism by which p66Shc restrains Ras and thus promotes RhoA activation remains unclear. p66Shc is known to antagonize the proliferative effects of EGF, which work in part through p52Shc and therefore suggests an antagonistic relationship between the two isoforms (Migliaccio et al., 1997; Okada et al., 1997). The basis for the divergent effects of the two Shc isoforms on EGF/EGFR signaling is thought to be related to competition for the Sos adapter Grb2, although one anticipated effect is activation of Rac1 at the expense of Ras (Khanday et al., 2006), an effect that we did not find. Furthermore, although p66Shc is felt to be a negative regulator of ERK, the direct effect of p66Shc on Ras itself has not been studied. A more widely recognized effect of p66Shc is its ability to initiate oxidative stress and cell death, through translocation to the mitochondrial inner membrane and redox cycling with cytochrome c (Giorgio et al., 2005). However, we have previously shown that p66Shc mutations that abrogate cytochrome c binding retain full capacity to initiate anoikis (Ma et al., 2007), consistent with its primary effect in conferring attachment context through regulation of Ras.
We conclude that p66Shc normally restrains Ras from becoming hyperactivated with subsequent loss of RhoA-dependent anoikis. As in the case of oncogenic Ras, pRB is activated as a downstream failsafe consequence following loss of p66Shc expression, halting proliferation. In the evolution of highly metastatic tumors such as SCLC, the loss of RB1 may allow subsequent repression of p66Shc with acquisition of metastatic capacity.
Materials and methods
Cloning and transduction
Human p66Shc (Ma et al., 2007), pRB (Addgene plasmid 10720; Sellers et al., 1998), HPV 16 E7 (Addgene plasmid 10720; Sellers et al., 1998) and the nonbinding E7 deletion mutant E7(Δ21–24) (Addgene plasmid 13687; Gonzalez et al., 2001) were ligated into the lentiviral shuttle pCCL.PPT.hPGK.IRES. eGFP/pre (gift of Philipp Scherer), and were used to produce lentivirus in Phoenix-293 cells with the packaging plasmids pMD2.BSBG, pMDLg/pRRE and pRSV-REV. Oligos encoding shRNA specific for the p66 isoform of Shc were ligated into pSUPER.retro.puro, and the fragment containing the H1 promoter and hairpin sequences was subcloned into the lentiviral shuttle pCCL.PPT.hPGK. GFP.Wpre and used to produce lentivirus as above. shRNA(1) targets coding nucleotides 42–60 of p66Shc and shRNA(2) targets nucleotides 252–270. p66Shc or p66(S36E) (Ma et al., 2007) were subcloned into the retroviral vector PINCO (gift of Pier Guiseppe Pelicci), which contains a separate EGFP cassette, and retrovirus was produced from the Phoenix-293 feeder line. Adenovirus containing Myc-Rac1(N17) was a gift of Kaikobad Irani, and adenoviruses containing RhoA(N19) and RhoA(V14) were gifts of Christopher Chen. Adenoviruses containing Ras(V12) and Ras(N17) were obtained from Vector Biolabs (Philadelphia, PA, USA).
Immunoblots
The following antibodies were used: Shc and Rac1 (BD Biosciences, San Jose, CA, USA); pRB and pS780-pRB (Cell Signaling, Danvers, MA, USA); RhoA, p16INK4A, pan-Ras and N-Ras (Santa Cruz, Santa Cruz, CA, USA), actin (Millipore, Billerica, MA, USA); and H-Ras and K-Ras (Abgent, San Diego, CA, USA).
GTPase pulldown assays
Active RhoA was assessed by pulldown using rhotekin-RBD-GST (Ma et al., 2007). Active Ras was assessed with pulldown using Raf-RBD-GST (Wu et al., 2007). Isoform-specific activation of Ras was assessed using the same pulldown technique followed by immunoblot with isoform-specific antibodies. Rac1 activity was assessed by pulldown using PBD-GST (Wu et al., 2007). Two-thirds of each sample was used for the pulldown assay and the remaining sample acetone-precipitated for assessment of total GTPase.
Cell culture
Phoenix-293 and Lewis lung carcinoma (LLC1) cells were obtained from American Type Culture Collection (Manassas, VA, USA). NHBE and human umbilical vein endothelial cells were from Lonza (Basel, Switzerland), and were routinely used at passages four and five. H209 and H69 SCLC cells were gifts of John Minna.
LLC metastasis
Retrovirus containing p66Shc or p66(S36E) were used to infect LLC cells, which were subsequently treated with 1 µg/ml puromycin. Puromycin-resistant cells were then selected by cell sorting for GFP expression (FACS Vantage, BD). 5 × 105 cells of each group in 100 µl saline were injected into the tail vein of 6-week-old female C57BL/6 mice. At 45 days following injection, surviving mice were killed. GFP-positive lung metastases were observed with the CRi MaestroTM imaging system (CRi, Woburn, MA, USA). Lungs were blotted, weighed and inflation fixed with formalin at 15cm H2O pressure.
Cell death and proliferation assays
Cells were plated on either cell culture-treated or low-attachment 24-well plates (Corning, Wilkes Barre, PA, USA) for 16 h. Cell death was assessed as DNA fragmentation using a cell death enzyme-linked immunosorbent assay (Roche, Basel, Switzerland). Absorbance was normalized for cell number. Proliferation was assessed using a 5-bromodeoxyuridine incorporation enzyme-linked immunosorbent assay (Roche), again normalizing for cell number.
Statistical analysis
Statistical analysis of cell death and proliferation end points was performed using analysis of variance with Tukey’s post hoc testing. Kapaln–Meier survival curves were analyzed with a two-tailed log-rank test.
Acknowledgements
We are grateful to Dr John Shelton for assistance with histology and Drs Ralph P Mason and Li Liu for technical support with in vivo GFP imaging. This work was supported by grants to LST by the NHLBI (R01-HL067256 and R01-HL061897).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Attwell S, Roskelley C, Dedhar S. The integrin-linked kinase (ILK) suppresses anoikis. Oncogene. 2000;19:3811–3815. doi: 10.1038/sj.onc.1203711. [DOI] [PubMed] [Google Scholar]
- Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–1039. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Gonzalez SL, Stremlau M, He X, Basile JR, Munger K. Degradation of the retinoblastoma tumor suppressor by the human papillomavirus type 16 E7 oncoprotein is important for functional inactivation and is separable from proteasomal degradation of E7. J Virol. 2001;75:7583–7591. doi: 10.1128/JVI.75.16.7583-7591.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignani F, Kinsella T, Mencarelli A, Valtieri M, Riganelli D, Lanfrancone L, et al. High-efficiency gene transfer and selection of human hematopoietic progenitor cells with a hybrid EBV/retroviral vector expressing the green fluorescence protein. Cancer Res. 1998;58:14–19. [PubMed] [Google Scholar]
- Ho VM, Schaffer BE, Karnezis AN, Park KS, Sage J. The retinoblastoma gene Rb and its family member p130 suppress lung adenocarcinoma induced by oncogenic K-Ras. Oncogene. 2009;28:1393–1399. doi: 10.1038/onc.2008.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanday FA, Santhanam L, Kasuno K, Yamamori T, Naqvi A, Dericco J, et al. Sos-mediated activation of rac1 by p66shc. J Cell Biol. 2006;172:817–822. doi: 10.1083/jcb.200506001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg AS, Hahn WC, Gupta P, Weinberg RA. Genes involved in senescence and immortalization. Curr Opin Cell Biol. 2000;12:705–709. doi: 10.1016/s0955-0674(00)00155-1. [DOI] [PubMed] [Google Scholar]
- Ma Z, Myers DP, Wu RF, Nwariaku FE, Terada LS. p66Shc mediates anoikis through RhoA. J Cell Biol. 2007;179:23–31. doi: 10.1083/jcb.200706097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabry M, Nakagawa T, Nelkin BD, McDowell E, Gesell M, Eggleston JC, et al. v-Ha-ras oncogene insertion: a model for tumor progression of human small cell lung cancer. Proc Natl Acad Sci USA. 1988;85:6523–6527. doi: 10.1073/pnas.85.17.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall A, Ulku A, Lambert QT, Kusa A, Rogers-Graham K, Der CJ. Oncogenic Ras blocks anoikis by activation of a novel effector pathway independent of phosphatidylinositol 3-kinase. Mol Cell Biol. 2001;21:5488–5499. doi: 10.1128/MCB.21.16.5488-5499.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettouchi A, Klein S, Guo W, Lopez-Lago M, Lemichez E, Westwick JK, et al. Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol Cell. 2001;8:115–127. doi: 10.1016/s1097-2765(01)00285-4. [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Mele S, Salcini AE, Pelicci G, Lai KM, Superti-Furga G, et al. Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J. 1997;16:706–716. doi: 10.1093/emboj/16.4.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittnacht S, Paterson H, Olson MF, Marshall CJ. Ras signalling is required for inactivation of the tumour suppressor pRb cell-cycle control protein. Curr Biol. 1997;7:219–221. doi: 10.1016/s0960-9822(97)70094-0. [DOI] [PubMed] [Google Scholar]
- Okada S, Kao AW, Ceresa BP, Blaikie P, Margolis B, Pessin JE. The 66-kDa Shc isoform is a negative regulator of the epidermal growth factor-stimulated mitogen-activated protein kinase pathway. J Biol Chem. 1997;272:28042–28049. doi: 10.1074/jbc.272.44.28042. [DOI] [PubMed] [Google Scholar]
- Peeper DS, Upton TM, Ladha MH, Neuman E, Zalvide J, Bernards R, et al. Ras signalling linked to the cell-cycle machinery by the retinoblastoma protein. Nature. 1997;386:177–181. doi: 10.1038/386177a0. [DOI] [PubMed] [Google Scholar]
- Phelps WC, Yee CL, Munger K, Howley PM. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988;53:539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Qiu RG, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- Sellers WR, Novitch BG, Miyake S, Heith A, Otterson GA, Kaye FJ, et al. Stable binding to E2F is not required for the retinoblastoma protein to activate transcription, promote differentiation, and suppress tumor cell growth. Genes Dev. 1998;12:95–106. doi: 10.1101/gad.12.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Takahashi C, Contreras B, Bronson RT, Loda M, Ewen ME. Genetic interaction between Rb and K-ras in the control of differentiation and tumor suppression. Mol Cell Biol. 2004;24:10406–10415. doi: 10.1128/MCB.24.23.10406-10415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi C, Contreras B, Iwanaga T, Takegami Y, Bakker A, Bronson RT, et al. Nras loss induces metastatic conversion of Rb1-deficient neuroendocrine thyroid tumor. Nat Genet. 2006;38:118–123. doi: 10.1038/ng1703. [DOI] [PubMed] [Google Scholar]
- Wistuba II, Gazdar AF, Minna JD. Molecular genetics of small cell lung carcinoma. Semin Oncol. 2001;28:3–13. [PubMed] [Google Scholar]
- Wu RF, Ma Z, Myers DP, Terada LS. HIV-1 Tat activates dual Nox pathways leading to independent activation of ERK and JNK MAP kinases. J Biol Chem. 2007;282:37412–37419. doi: 10.1074/jbc.M704481200. [DOI] [PubMed] [Google Scholar]