Abstract
Aims
Type 1 diabetes mellitus increases the risk for sudden unexplained death (SUD), generating concern that diabetes processes and/or treatments underlie these deaths. Young (<50 yrs) and otherwise healthy patients who are found dead in bed have been classified as experiencing “dead in bed” (DIB) syndrome.
Methods
We thus identified all un-witnessed deaths in two related registries (Children’s Hospital of Pittsburgh and Allegheny County) yielding 1,319 persons with childhood-onset (age<18 yrs) Type 1 DM diagnosed between 1965 and 1979. Cause of death was determined by a mortality classification committee (MCC) of at least 2 physician epidemiologists, based on the death certificate and additional records surrounding the death.
Results
Of the 329 participants who had died, the MCC has so far reviewed and assigned a final cause of death to 255 (78%). Nineteen (8%) of these were SUDs (13 male), and 7 met DIB criteria. The MCC adjudicated cause of death in the 7 DIB persons as: diabetic coma (n=4), unknown (n=2), and cardiomyopathy (n=1, found on autopsy). The 3 DIB individuals who participated in a clinical study had higher HbA1c, lower BMI, and higher daily insulin dose compared to both those dying from other causes and those surviving.
Conclusions
SUD in Type 1 DM seems to be increased 10-fold and associated with male sex, while DIB individuals have a high HbA1c and insulin dose, and low BMI. Though sample size is too small for definitive conclusions, these results suggest specific sex and metabolic factors predispose to SUD and DIB.
Keywords: Type 1 diabetes, mortality, dead-in-bed syndrome, sudden death
Introduction
In 1991, Tattersall and Gill published a report on 22 young (12–43 years old), apparently healthy individuals with Type 1 diabetes mellitus (DM) who had been found dead in their beds.[1] In nearly all cases, there was no evidence of sweating or a terminal struggle, nor was a clear cause of death found on autopsy. These characteristic features were later coined “dead in bed syndrome”. The authors postulated that, due to their timing, the deaths were in some way associated with nocturnal hypoglycaemia.[1]
Since this report, more than 100 sudden unexplained deaths (SUDs) that fit the dead in bed syndrome criteria have been reported in Sweden, Denmark, Norway, and Australia.[2–9] Some of these individuals were found to be taking multiple daily insulin doses and having frequent episodes of hypoglycaemia prior to death.[5] Two studies have reported that sudden deaths occur in >20% of all young Type 1 DM deaths (age < 50), compared to 1–5% of similar general populations.[2, 6]
Most recently, a review of this topic estimated that 5–6% of all Type 1 DM deaths fit the criteria of dead in bed syndrome;[8] however, the underlying cause(s) of death remain unclear. Plausible theories include hypoglycaemia, malignant cardiac arrhythmias, cardiac autonomic neuropathy, hypoglycaemia-associated autonomic failure, or a combination of these.[10–16]
Reports often describe other Type 1 DM deaths where the individual is found dead and the cause of death in not determinable, yet the death does not fit the criteria for dead in bed syndrome (e.g., the person is > 50 years old or they are found dead elsewhere).[1, 3, 6] The current report explores all SUDs occurring in two long-term observational studies of childhood-onset Type 1 DM in Allegheny County (Pittsburgh), Pennsylvania, namely, the hospital-based Children’s Hospital of Pittsburgh registry and a population-based Allegheny County Type 1 DM registry. Specific objectives include: 1) determining the incidences of both SUD and its subgroup, dead in bed syndrome; 2) comparing these rates to those reported in the general literature; and 3) examining potential risk factors.
Participants and Methods
Study populations
Sudden deaths were identified from two related incidence registries of childhood-onset Type 1 DM, the Children’s Hospital of Pittsburgh registry and the Allegheny County registry, which have been described previously.[17, 18] Eligibility criteria for the current investigation were: 1) a diagnosis of Type 1 DM between 1 January 1965 and 31 December 1979; 2) receiving insulin therapy at discharge from the diagnosis admission; 3) age<18 years at diagnosis; and 4) residing in Allegheny County, Pennsylvania or living within 100 miles from Pittsburgh at diagnosis. These studies were approved by the University of Pittsburgh Institutional Review Board, and all participants or relatives of deceased participants provided informed consent.
Two hundred and forty-three participants came from the Children’s Hospital registry alone, 805 from only the Allegheny County registry, while another 271 individuals were part of both registries, yielding a total of 1319 individuals.
Mortality Data
Vital status was determined as of 1 January 2008, using ascertainment methods described previously.[19] Deaths not identified through mail or phone contact with participants were discovered by searching both the Social Security Death Index and the National Death Index (NDI). Death certificates (or NDI data) were obtained to confirm each death. Vital status was determined for 1247 (95%) patients of whom 329 have died as of 1 January 2008. In addition, whenever appropriate, the following sources of information were sought: 1) medical records surrounding the death; 2) autopsy/coroner’s reports; and 3) interview with next-of-kin. The underlying cause of death, and rank order for all contributing causes, for each decedent was determined by a Mortality Classification Committee (MCC) consisting of at least 2 physician epidemiologists using standardised protocols.[18] Only deaths that have been reviewed and classified by the MCC were included in this analysis (255 deaths (77.5% of all deaths)).
Key variables
The primary variable of interest was “sudden unexplained death” (SUD). This was defined as an unwitnessed death with no obvious cause (e.g., not a car accident or suicide) and not being treated in the hospital for a potentially fatal condition. This is a broader definition than “dead-in-bed,” as it included healthy individuals with Type 1 DM over age 50, as well as individuals who were found dead at home in places other than the bed. Other variables of interest were obtained either from baseline survey data or from death certificates.
Subgroup analysis
A portion (n=414) of the Children’s Hospital of Pittsburgh registry participated in the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study, which has previously been described in detail and includes individuals diagnosed with childhood-onset (age<17 yrs) Type 1 DM and first evaluated between 1986 and 1988.[20] Participants have been followed biennially by survey and for the first 10 years and again at 18 years by examination. Three participants in the EDC cohort met the dead-in-bed (DIB) criteria. Data from living EDC participants (n=363) were matched to each of these 3 DIB cases based on sex and 5-year intervals surrounding both age and diabetes duration at death for the DIB cases, for a total of 82 (22.6%) matched living participants.
EDC participants completed surveys regarding medical history and demographic information prior to a clinical examination. Resting blood pressure was measured in the sitting position according to the Hypertension Detection and Follow-Up Program protocol with a random-zero sphygmomanometer. Height and weight were measured for body mass index, and a 12-lead electrocardiogram (ECG) was obtained. The QT interval was derived from a single waveform in lead II and heart rate (HR) from an average of five R-R distances on the ECG. The QT interval was corrected for HR according to Hodges formula: QTc=QT+1.75×(HR–60).[21] Prolonged QTc interval was defined as QTc≥440 ms.
Glycated haemoglobin (HbA1), lipids, and lipoproteins were measured using fasting blood samples. Original HbA1 values were converted to Diabetes Complications and Control Trial (DCCT)-aligned HbA1c using the following regression equation derived from duplicate assays: DCCT HbA1c = 0.14 + 0.83(EDC HbA1). Total cholesterol was measured enzymatically, and HDL cholesterol was determined after a heparin and manganese chloride precipitation method.[22] Non-HDL cholesterol was calculated by subtracting HDL from total cholesterol.
Hard coronary artery disease (CAD) events included myocardial infarction, revascularization procedure, or angiographic stenosis ≥50%, confirmed by hospital records. Renal damage status was defined on the basis of urinary albumin excretion rates (AER) in at least two of three timed urine collections as: normoalbuminuria (<20 µg/min); microalbuminuria (MA, 20–200 µg/min), overt nephropathy (ON, >200 µg/min). Proliferative retinopathy was assessed by the University of Wisconsin-Madison Fundus Photography Reading Center using stereoscopic fundus photographs. Severe hypoglycaemia was defined as ≥1 episode of unconsciousness or seizure due to hypoglycaemia, as determined by an EDC-study physician during clinical examination. An ever smoker was defined as having smoked ≥100 cigarettes over their lifetime. Moderate alcohol use was defined as ≥5 drinks/week based on self-report data.
Statistical analysis
Distributional characteristics for each variable were assessed for normality. Univariate significance was tested using the Student’s t-test, one-way ANOVA or the χ2 (or Fisher’s exact) test, as appropriate. When comparing DIB to matched living participants, paired t-tests were used. Due to limited sample size, statistical significance was considered as p < 0.10. All analyses were performed using either SPSS 17.0 (SPSS, Chicago, IL) or SAS 9.2 (SAS, Cary, NC).
Results
As of 1 January 2008, there were 329 (24.9%) deaths in the two childhood-onset Type 1 diabetes registries. Of these, 255 (77.5%) deaths have been reviewed and classified by the Mortality Classification Committee (MCC). Deaths yet to be classified by the MCC were more recent deaths (calendar year of death = 1999.6 ± 6.8 vs. 1993.3 ± 8.8, p<0.001) and occurred in older individuals (p<0.001) with a longer diabetes duration (p<0.001) (Supplemental Table 1). However, whether or not the deaths have been classified did not differ by sex, race, age at diabetes onset, location of death (inpatient vs. other) or whether an autopsy was performed.
Sudden unexplained deaths and Dead-in-bed
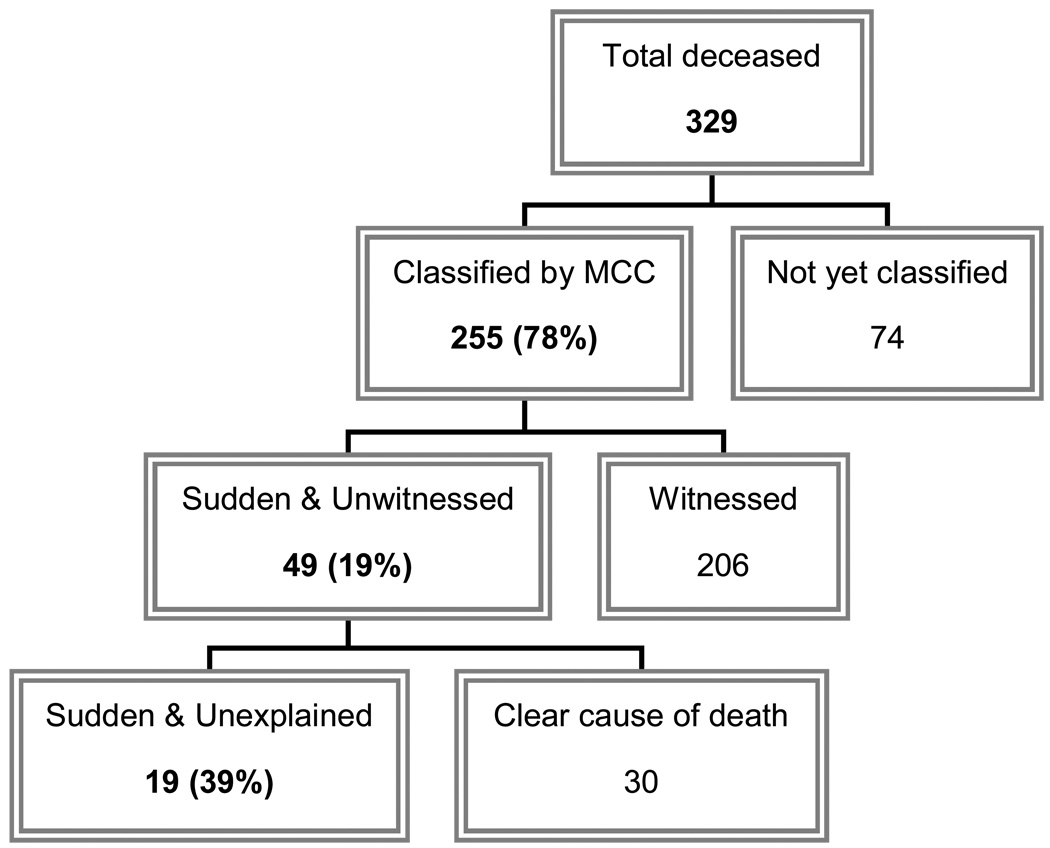
Of the 255 MCC-classified deaths, 47 (19%) were sudden, unwitnessed deaths (Figure 1), which were further categorised based on whether a clear cause of death could be determined by the MCC. Nineteen (40%) of these sudden, unwitnessed deaths were further classified as “sudden unexplained deaths” (SUDs) and are summarised in Table 1. The incidence density of SUD for this study population was thus 45.4/100,000 person-yrs (95% CI 25.0–65.8) and may be even higher given the currently unclassified deaths (48.0/100,000 person-yrs with 74 unclassified deaths excluded from denominator) (Table 2). The SUDs occurred primarily in males and in Caucasians between 1983 and 2007. Nine (47.3%) SUDs had no history of diabetes complications, although two of these had a history of alcohol abuse. Thirteen were found dead in bed, 2 on the bedroom floor, 1 on the living room floor, 1 in the bathroom, and 1 in a chair. Only 6 (31.6%) had an autopsy performed. The underlying cause of death on the death certificate was most often listed as diabetes (n=11) and matched the MCC-classified underlying cause of death in just 4 cases: CVD (n=2), pneumonia (n=1), and diabetic ketoacidosis (n=1). Based on the classic definition of dead-in-bed syndrome (i.e., age<50, no major diabetes complications, and found dead in bed), only 7 individuals in this study met dead-in-bed criteria (incidence density of 16.7/100,000 person-yrs, Table 2).
Figure 1.
Flow diagram showing the breakdown by cause of death.
Table 1.
Characteristics of individuals with sudden unexplained deaths (n=19) in this Type 1 diabetes study population.a
| Age (yr) |
Race | Sex | Diabetes Duration (yr) |
Major Diabetes Complications |
Where Found |
Autopsy Performed |
Underlying Cause on DC |
Underlying Cause by MCC |
Dead- in-bedb |
|---|---|---|---|---|---|---|---|---|---|
| 19 | Caucasian | Female | 7.6 | None | Bed | Yes | Diabetes mellitus | Diabetic coma | Yes |
| 22 | Caucasian | Female | 11.9 | CHF, MI | Bed | No | Arteriosclerotic heart disease |
Acute MI | No |
| 33 | Caucasian | Male | 17.6 | None | Bed | Yes | Diabetes mellitus | Diabetic cardiomyopathy |
Yes |
| 34 | Caucasian | Male | 23.7 | ESRD, silent MI | Bathroom* | No | Diabetes mellitus | ESRD | No |
| 23 | Caucasian | Male | 17.0 | None | Chair* | Yes | Focal myocardial fibrosis |
Presumed hypoglycaemia |
No |
| 37 | Caucasian | Male | 24.5 | None | Bed | No | Unstable diabetes mellitus |
Diabetic coma | Yes |
| 31 | Caucasian | Female | 21.0 | CAD | Bed | No | Cardiac failure | Unknown | No |
| 36 | Caucasian | Male | 26.4 | CAD | Bed | No | Arteriosclerotic heart disease |
Presumed CAD | No |
| 35 | Caucasian | Male | 27.0 | ESRD, hypoglycaemic brain damage |
Bed | No | Diabetes mellitus | ESRD | No |
| 37 | African- American |
Male | 21.6 | None | Floor* | Yes | Bronchopneumonia | Pneumonia | No |
| 37 | Caucasian | Female | 31.0 | None | Bed | No | COPD | Unknown | Yes |
| 36 | Caucasian | Male | 32.6 | Brain damage | Floor near bed* |
No | Diabetes mellitus | Unknown | No |
| 29 | Caucasian | Male | 23.7 | None | Bed | No | DKA | DKA | Yes |
| 37 | Caucasian | Male | 27.0 | None | Bed | No | Diabetes mellitus | Unknown | Yes |
| 39 | Caucasian | Male | 33.4 | ESRD, MI, PR | Floor near bed* |
No | Probable insulin shock |
CAD | No |
| 26 | Caucasian | Male | 25.4 | None | Bed | Yes | Diabetes mellitus | Hypoglycaemia | Yes |
| 52 | Caucasian | Female | 38.4 | PR | Bed | No | Diabetes mellitus | Unknown | No |
| 56 | Caucasian | Male | 39.1 | CAD, PR | Bed | No | Diabetes mellitus | Unknown | No |
| 45 | Caucasian | Female | 31.2 | PVD, PR | Bed | Yes | Diabetes mellitus | Presumed DKA | No |
Death occurred at home
Cases ordered by calendar year of death (range 1983–2007)
Meets criteria for “dead-in-bed” classification: under age 50, no major complications, found dead in bed
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; DC, death certificate; DKA, diabetic ketoacidosis; ESRD, end stage renal disease; MCC, Mortality Classification Committee; MI, myocardial infarction; PR, proliferative retinopathy; PVD, peripheral vascular disease
Table 2.
Comparison of incidence densities of sudden deaths, sudden unexplained deaths (SUDs), and dead-in-bed in young Type 1 diabetes and general populations
| Study | Location | # of deaths (definition used) |
Age Range (yr) |
Incidence Density (per 100,000 person-yrs) |
|---|---|---|---|---|
| Type 1 Diabetes Mellitus | ||||
| Current study | Allegheny County, PA | 19 (SUD) | 19–56 | 45.4 (25.0–65.8)a |
| Current study | Allegheny County, PA | 7 (Dead-in-bed) | 19–37 | 16.7 (4.3–29.1)a |
| Koltin et al, 2008[8] | Review of literature | Not stated (Dead-in-bed) |
14–49 | 20–60 (estimated) |
| General Population | ||||
| Shen et al, 1995[28] | Olmsted County, MN | 54 (Sudden deathsb) |
20–40 | 4.1 (Female); 8.7 (Male) |
| Eckart et al, 2004[29] | U.S. Military recruits | 44 (SUD) | 18–35 | 4.5 (calculated) |
| Vaartjes et al, 2009[30] |
Netherlands | 172 (SUD) | 1–40 | 0.2 (calculated) |
| Vaartjes et al, 2009[30] |
Netherlands | 1,908 (Sudden deathsb) |
1–40 | 1.2 (Female); 2.9 (Male) |
95% Confidence Interval
Includes all sudden deaths (both known and unknown causes)
Comparisons between the 19 SUD individuals with the other MCC-classified deaths (n=230) are reported in Table 3. SUDs were divided into two groups: those who met the dead-in-bed (DIB) criteria (n=7) and other SUDs (n=12). Compared to deaths from other causes, DIB and SUDs occurred more in males (71% and 67%, respectively, vs. 49% of other deaths) and were not as likely to attend college or be married at death. Diabetes was listed on the death certificate of all DIB (100%) and all but one SUD (92%) compared to only 72% of death certificates for all other deaths (p=0.09). Notably, DIB and SUDs were no more likely to have an autopsy performed (p=0.50). The MCC classified the DIB deaths as: 4 (57%) from hyper-/hypoglycaemia, 1 (14%) from cardiomyopathy (found on autopsy), and 2 (29%) from an unknown cause. Other SUDs were classified as: 2 (17% from hyper-/hypoglycaemia, 6 (50%) from a chronic diabetes complication (3 cardiovascular deaths, 2 end-stage renal disease, and 1 infection), and 4 (33%) from an unknown cause.
Table 3.
Characteristics (% (n) or mean ± SD) of the deceased Type 1 diabetes study population (n=249)a by sudden unexplained death (SUD) classification
| Characteristics | Dead-in-bed | Other SUD | All other deaths | Overall p-value |
|---|---|---|---|---|
| N | 7 | 12 | 230 | --- |
| Male sex | 71.4 (5) | 66.7 (8) | 49.1 (113) | 0.26 |
| African-American | 0.0 (0) | 8.3 (1) | 12.6 (29) | 0.54 |
| Education > 12 yrs (n=206) | 16.7 (1) | 36.4 (4) | 47.4 (89) | 0.26 |
| Married at Death (n=248) | 14.3 (1) | 25.0 (3) | 38.0 (87) | 0.30 |
| Autopsy Performed (n=248) | 42.9 (3) | 25.0 (3) | 28.8 (66) | 0.84 |
| Diabetes on Death Certificate | 100.0 (7) | 91.7 (11) | 72.2 (166) | 0.09 |
| Age at Diabetes Onset (yr) | 9.0 ± 5.1 | 10.8 ± 4.1 | 11.4 ± 3.9 | 0.26 |
| Diabetes Duration at Death (yr) | 22.4 ± 7.7 | 27.0 ± 8.4 | 23.1 ± 9.0 | 0.33 |
| Age at Death (yr) | 31.4 ± 6.9 | 37.7 ± 10.2 | 34.5 ± 9.2 | 0.34 |
p≤0.10.
Of the 255 deaths classified by the Mortality Classification Committee, 6 deaths lacked sufficient data to determine whether the death was a sudden unexplained death
Subgroup analysis from Pittsburgh EDC Study
To discover distinguishing features of individuals with DIB deaths, 133 Pittsburgh EDC Study participants (51 deceased and 82 living) were assessed (Table 4). Eight (16%) of the deaths were classified as SUDs, of which three (6%) met the DIB criteria. No DIB individuals and only 1 SUD person were married at death compared to nearly half of the other deaths and more than half of the matched living participants (0% and 13% vs. 49% and 62%, respectively). The mean follow-up time between the last EDC Study visit and death did not differ greatly between groups. Clinically, there were no major differences in systolic or diastolic blood pressure, pulse, or HDL or non-HDL cholesterol levels. HbA1c was much higher in the DIB individuals compared to other SUDs, all other deaths, and matched living participants (11.2 ± 2.2 % vs. 8.8 ± 1.1 vs. 9.8 ± 1.9 vs. 8.5 ± 1.4, respectively), as was daily insulin dose. Also, individuals with DIB had a very low BMI (19.3 ± 1.0 kg/m2) compared to other SUDs (22.3 ± 5.2), which was also much lower than all other deaths or those still alive (24.9 ± 4.9 and 25.6 ± 3.6, resp.). However, no other differences between DIB and other groups were apparent regarding prevalent diabetes complications (CAD, renal disease, or retinopathy) or other contributing factors (history of smoking or moderate alcohol consumption). Of note, none of the individuals with DIB or SUD had evidence of prolonged (≥440 ms) QTc interval on exam.
Table 4.
Characteristics (% (n) or mean ± SD) of deceased participants based on data from the most recent study visit in the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study (n=133) by sudden unexplained death (SUD) classification
| Characteristics | Dead-in-bed | Other SUD | All other deaths | Matched livinga |
|---|---|---|---|---|
| N | 3 | 5 | 43 | 82 |
| Demographic | ||||
| Male sex | 66.7 (2) | 80.0 (4) | 53.5 (23) | 69.5 (57) |
| African-American | 0.0 (0) | 0.0 (0) | 7.0 (3) | 1.2 (1) |
| Education > 12 yrs (n=130)* | 33.3 (1) | 60.0 (3) | 52.4 (22) | 72.5 (58) |
| Married (n=130)* | 0.0 (0) | 20.0 (1) | 48.8 (21) | 62.0 (49)** |
| Autopsy Performed | 33.3 (1) | 40.0 (2) | 32.6 (14) | --- |
| Diabetes on Death Certificate | 100.0 (3) | 100.0 (5) | 72.1 (31) | --- |
| Clinical | ||||
| HbA1c (%)* | 11.2 ± 2.2 | 8.8 ± 1.1** | 9.8 ± 1.9 | 8.5 ± 1.4** |
| HbA1c (mmol/mol)* | 98 ± 25 | 72 ± 12 | 83 ± 21 | 70 ± 16 |
| Systolic BP (mmHg)* | 126.0 ± 26.9 | 130.0 ± 19.1 | 126.6 ± 20.5 | 114.1 ± 14.3 |
| Diastolic BP (mmHg)* | 73.7 ± 2.9 | 79.6 ± 22.6 | 79.6 ± 10.8 | 71.5 ± 9.4 |
| Pulse (bpm) | 81.3 ± 9.2 | 80.0 ± 11.4 | 79.3 ± 13.0 | 74.2 ± 12.9 |
| Body Mass Index (kg/m2)* | 19.3 ± 1.0 | 22.3 ± 5.2 | 24.9 ± 4.9** | 25.6 ± 3.6** |
| HDL cholesterol (mmol/L) | 1.26 ± 0.09 | 1.35 ± 0.36 | 1.23 ± 0.42 | 1.36 ± 0.41 |
| Non-HDL cholesterol (mmol/L)* | 3.85 ± 1.03 | 4.38 ± 1.84 | 4.18 ± 1.19 | 3.45 ± 0.74 |
| Insulin dose (IU kg–1 day–1) | 0.98 ± 0.39 | 0.73 ± 0.24 | 0.66 ± 0.25** | 0.67 ± 0.23** |
| Intensive Insulin Therapy (≥3 inj/d) | 0.0 (0) | 60.0 (3) | 34.9 (15) | 39.0 (32) |
| Last exam to death (yr) | 2.99 ± 2.89 | 1.37 ± 0.66 | 1.47 ± 1.64 | --- |
| Prevalent Complication | ||||
| Hard Coronary Artery Disease* | 0.0 (0) | 60.0 (3) | 46.5 (20) | 11.0 (9) |
| Microalbuminuria (AER ≥ 20 µg/min)* | 66.7 (2) | 80.0 (4) | 88.4 (38) | 36.6 (30) |
| Overt Nephropathy (AER ≥ 200 µg/min)* | 33.3 (1) | 60.0 (3) | 72.1 (31) | 14.6 (12) |
| Proliferative Retinopathy* | 33.3 (1) | 80.0 (4) | 76.2 (32) | 56.1 (46) |
| Severe Hypoglycaemia | 100.0 (3) | 80.0 (4) | 55.8 (24) | 67.1 (55) |
| Hypoglycaemia Unawareness | 33.3 (1) | 60.0 (3) | 32.6 (14) | 43.9 (36) |
| Prolonged QTc interval (≥ 440 ms) | 0.0 (0) | 0.0 (0) | 7.0 (3) | 0.0 (0) |
| Ever Smoker* | 66.7 (2) | 80.0 (4) | 55.8 (24) | 31.7 (26) |
| Moderate Alcohol Use (≥ 5 drinks/wk) | 33.3 (1) | 40.0 (2) | 34.8 (15) | 28.0 (23) |
p≤0.10 for overall χ2 or ANOVA, as appropriate
p≤0.10 compared to “Dead-in-bed”.
Living individuals matched to dead-in-bed cases based on sex and 5-year interval surrounding age and diabetes duration at death for cases.
Abbreviations: AER, albumin excretion rate; BP, blood pressure; QTc, corrected QT-interval
Discussion
This study identifies and characterises the unfortunate occurrence of sudden unexplained deaths (SUDs), including dead-in-bed syndrome, in two related cohorts in Southwestern Pennsylvania, one hospital-based and one population-based, of individuals diagnosed with Type 1 diabetes between 1965 and 1979. SUDs and DIB deaths accounted for 7.8% and 2.8% of all deaths so far classified, respectively, in this cohort. Individuals meeting dead-in-bed criteria had a higher HbA1c and daily insulin dose, and lower BMI compared to similar Type 1 DM individuals.
Two-thirds of the SUDs did not have an autopsy, and an underlying cause of death could not be determined by the MCC for nearly one-third of the SUDs, despite interviews with next-of-kin and hospital or autopsy records, when appropriate. Without (and sometimes even with) autopsies, post-mortem diagnoses are highly speculative and often inaccurate or non-specific (e.g., 32% of these SUDs listed diabetes mellitus or sudden death as the only cause of death on the death certificate).
Although the contribution of SUDs (and DIB syndrome) to early mortality in Type 1 DM is not insignificant (5–10% of all Type 1 DM deaths),[8] it has been unclear whether SUDs occur more frequently in Type 1 DM than in the similarly-aged general population. Table 2 compares data from a review of the literature on the incidence density of sudden deaths, SUDs, and DIB syndrome. The incidence densities found in the present study are quite comparable to the range reported in a recent review of DIB syndrome in Type 1 DM.[8] However, compared to incidence densities for all sudden deaths or for SUDs alone in the young (age<50) general population, SUDs appear to occur up to 10 times more often in Type 1 DM.
These results confirm findings from previous studies showing that SUDs and dead-in-bed syndrome tend to occur more in males (Thordarson et al[5]: 10 M, 6 F, Tu et al[6]: 8 M, 2 F). While one U.K. report (n=128 deaths) on Type 1 DM children aged 1–19, reported more female DIB deaths (7 F, 2 M),[9] they also reported that a significant proportion of deaths overall occurred in young males with poorly controlled diabetes who died at home (often of hypo- or hyperglycaemia).
Potential mechanisms for DIB syndrome in Type 1 DM include both nocturnal hypoglycaemia and undiagnosed cardiac autonomic neuropathy (AN).[10, 11, 16, 23] A plausible theory for the etiology of DIB syndrome has been proposed and developed over the last decade.[10–16] Although nocturnal hypoglycaemia rarely itself results in sudden death,[11] those at risk of DIB syndrome may have reduced parasympathetic activity (and, thus, relative sympathetic predominance), due to both long-standing diabetes and early stages of cardiac AN,[10, 15] which leads to ventricular arrhythmias.[15] Also, both AN and severe hypoglycaemia have also been associated with abnormal cardiac repolarisation, as evidenced by prolonged corrected QT (QTc) intervals.[14] However, none of the 3 DIB or 5 other SUDs in our subgroup analysis had any clinical evidence of prolonged QTc interval in any EDC exam visits. Nonetheless, this does not preclude the possibility that severe nocturnal hypoglycaemia might have caused acute QT prolongation, as has been demonstrated recently.[24]
Nocturnal hypoglycaemia has a higher propensity to persist for hours due to long-standing diabetes causing “hypoglycaemia-associated autonomic failure” (HAAF), where the body produces an inadequate response of glucagon and adrenaline when it senses hypoglycaemia. Woodward et al. very recently showed that nocturnal hypoglycaemia (<3.5 mmol/l) is still very common in individuals with long-standing Type 1 DM,[25] where nearly 50% experienced nocturnal hypoglycaemia, a rate which has not dramatically changed in over 20 years despite the current wide usage of long-acting insulin analogs.[26] A 2009 case report describes a 23-yr old man with recurrent severe hypoglycaemia placed on continuous glucose monitoring (CGM) and then found dead in an undisturbed bed the next day.[27] The CGM revealed glucose levels below 1.7 mmol/L around the time of his death with only minimal counterregulatory response.
The findings in this study are preliminary as several limitations exist. Nearly 25% of all deaths have yet to be classified by the MCC. However, of the deaths not yet classified, at present only three deaths potentially meet SUD criteria. As the number of SUDs might increase slightly, the SUD incidence density reported herein might be underestimated. Also, this report is primarily descriptive in nature, because clinical and diabetes complication data were not available for the majority of the study population, since the Allegheny County cohort has only been contacted three times since the cohort was developed (late 1970s), primarily to determine population-based mortality rates. Few associations reach statistical significance; however, there is limited sample size. Generalising these finding to other Type 1 DM populations should be done with caution.
In conclusion, these results suggest patterns predictive of sudden unexplained deaths and dead-in-bed syndrome in Type 1 diabetes. However, since SUD and DIB comprise a limited proportion of all Type 1 DM deaths, large epidemiologic studies combining data from multiple centers or studies are necessary to determine predictors and work to prevent these devastating deaths in Type 1 diabetes.
Supplementary Material
Acknowledgments
AMS was supported by a training grant from the National Institute of Diabetes and Digestive and Kidney Diseases (F30-DK082137). The authors also acknowledge the long term help of participants in the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study and the Allegheny County Type 1 Diabetes Registry.
Abbreviations
- AER
albumin excretion rate
- BMI
body mass index
- CAD
coronary artery disease
- CGMS
continuous glucose monitoring systems
- DIB
dead-in-bed
- DM
diabetes mellitus
- EDC
Epidemiology of Diabetes Complications
- HAAF
hypoglycaemia-associated autonomic failure
- HDL
high density lipoprotein
- MA
microalbuminuria
- MCC
Mortality Classification Committee
- NDI
National Death Index
- ON
overt nephropathy
- SUD
sudden unexplained death
Footnotes
Declaration of Competing Interests: Nothing to declare.
References
- 1.Tattersall RB, Gill GV. Unexplained deaths of type 1 diabetic patients. Diabet Med. 1991;8:49–58. doi: 10.1111/j.1464-5491.1991.tb01516.x. [DOI] [PubMed] [Google Scholar]
- 2.Dahlquist G, Kallen B. Mortality in childhood-onset type 1 diabetes: a population-based study. Diabetes Care. 2005;28:2384–2387. doi: 10.2337/diacare.28.10.2384. [DOI] [PubMed] [Google Scholar]
- 3.Borch-Johnsen K, Helweg-Larsen K. Sudden death and human insulin: is there a link? Diabet Med. 1993;10:255–259. doi: 10.1111/j.1464-5491.1993.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 4.Sartor G, Dahlquist G. Short-term mortality in childhood onset insulin-dependent diabetes mellitus: a high frequency of unexpected deaths in bed. Diabet Med. 1995;12:607–611. doi: 10.1111/j.1464-5491.1995.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 5.Thordarson H, Sovik O. Dead in bed syndrome in young diabetic patients in Norway. Diabet Med. 1995;12:782–787. doi: 10.1111/j.1464-5491.1995.tb02080.x. [DOI] [PubMed] [Google Scholar]
- 6.Tu E, Twigg SM, Duflou J, Semsarian C. Causes of death in young Australians with type 1 diabetes: a review of coronial postmortem examinations. Med J Aust. 2008;188:699–702. doi: 10.5694/j.1326-5377.2008.tb01849.x. [DOI] [PubMed] [Google Scholar]
- 7.Wibell L, Nystrom L, Ostman J, Arnqvist H, Blohme G, Lithner F, et al. Increased mortality in diabetes during the first 10 years of the disease. A population-based study (DISS) in Swedish adults 15–34 years old at diagnosis. J Intern Med. 2001;249:263–270. doi: 10.1046/j.1365-2796.2001.00802.x. [DOI] [PubMed] [Google Scholar]
- 8.Koltin D, Daneman D. Dead-in-bed syndrome - a diabetes nightmare. Pediatr Diabetes. 2008;9:504–507. doi: 10.1111/j.1399-5448.2008.00404.x. [DOI] [PubMed] [Google Scholar]
- 9.Edge JA, Ford-Adams ME, Dunger DB. Causes of death in children with insulin dependent diabetes 1990–96. Arch Dis Child. 1999;81:318–323. doi: 10.1136/adc.81.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weston PJ, Gill GV. Is undetected autonomic dysfunction responsible for sudden death in Type 1 diabetes mellitus? The 'dead in bed' syndrome revisited. Diabet Med. 1999;16:626–631. doi: 10.1046/j.1464-5491.1999.00121.x. [DOI] [PubMed] [Google Scholar]
- 11.Tu E, Twigg SM, Semsarian C. Sudden death in type 1 diabetes: The mystery of the 'dead in bed' syndrome. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Harris ND, Heller SR. Sudden death in young patients with Type 1 diabetes: a consequence of disease, treatment or both? Diabet Med. 1999;16:623–625. doi: 10.1046/j.1464-5491.1999.00046.x. [DOI] [PubMed] [Google Scholar]
- 13.Heller S. Dead in bed. Diabet Med. 1999;16:782–785. [PubMed] [Google Scholar]
- 14.Heller SR. Abnormalities of the electrocardiogram during hypoglycaemia: the cause of the dead in bed syndrome? Int J Clin Pract Suppl. 2002:27–32. [PubMed] [Google Scholar]
- 15.Lawrence IG, Weston PJ, Bennett MA, McNally PG, Burden AC, Thurston H. Is impaired baroreflex sensitivity a predictor or cause of sudden death in insulin-dependent diabetes mellitus? Diabet Med. 1997;14:82–85. doi: 10.1002/(SICI)1096-9136(199701)14:1<82::AID-DIA290>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.McNally PG, Lawrence IG, Panerai RB, Weston PJ, Thurston H. Sudden death in type 1 diabetes. Diabetes Obes Metab. 1999;1:151–158. doi: 10.1046/j.1463-1326.1999.00025.x. [DOI] [PubMed] [Google Scholar]
- 17.Dorman JS, LaPorte RE, Kuller LH, Cruickshanks KJ, Orchard TJ, Wagener DK, et al. The Pittsburgh insulin-dependent diabetes mellitus (IDDM) morbidity and mortality study. Mortality results. Diabetes. 1984;33:271–276. doi: 10.2337/diab.33.3.271. [DOI] [PubMed] [Google Scholar]
- 18.International evaluation of cause-specific mortality and IDDM. Diabetes Epidemiology Research International Mortality Study Group. Diabetes Care. 1991;14:55–60. doi: 10.2337/diacare.14.1.55. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura R, LaPorte RE, Dorman JS, Tajima N, Becker D, Orchard TJ. Mortality trends in type 1 diabetes. The Allegheny County (Pennsylvania) Registry 1965–1999. Diabetes Care. 2001;24:823–827. doi: 10.2337/diacare.24.5.823. [DOI] [PubMed] [Google Scholar]
- 20.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Ellis D, LaPorte RE, et al. Factors associated with avoidance of severe complications after 25 yr of IDDM. Pittsburgh Epidemiology of Diabetes Complications Study I. Diabetes Care. 1990;13:741–747. doi: 10.2337/diacare.13.7.741. [DOI] [PubMed] [Google Scholar]
- 21.Hodges M, Salerno D, Erlien D. Bazett’s QT correction reviewed-Evidence that a linear QT correction for heart is better. J Am Coll Cardiol. 1983;1:694. [Google Scholar]
- 22.Warnick GR, Albers JJ. Heparin--Mn2+ quantitation of high-density-lipoprotein cholesterol: an ultrafiltration procedure for lipemic samples. Clin Chem. 1978;24:900–904. [PubMed] [Google Scholar]
- 23.Start RD, Barber C, Kaschula RO, Robinson RT. The 'dead in bed syndrome'- a cause of sudden death in Type 1 diabetes mellitus. Histopathology. 2007 doi: 10.1111/j.1365-2559.2007.02829.x. [DOI] [PubMed] [Google Scholar]
- 24.Gill GV, Woodward A, Casson IF, Weston PJ. Cardiac arrhythmia and nocturnal hypoglycaemia in type 1 diabetes--the 'dead in bed' syndrome revisited. Diabetologia. 2009;52:42–45. doi: 10.1007/s00125-008-1177-7. [DOI] [PubMed] [Google Scholar]
- 25.Woodward A, Weston P, Casson IF, Gill GV. Nocturnal hypoglycaemia in type 1 diabetes--frequency and predictive factors. QJM. 2009;102:603–607. doi: 10.1093/qjmed/hcp082. [DOI] [PubMed] [Google Scholar]
- 26.Gale EA, Tattersall RB. Unrecognised nocturnal hypoglycaemia in insulin-treated diabetics. Lancet. 1979;1:1049–1052. doi: 10.1016/s0140-6736(79)92950-7. [DOI] [PubMed] [Google Scholar]
- 27.Tanenberg RJ, Newton CA, Drake AJ. Confirmation of Hypoglycemia in the 'Dead-in-Bed' Syndrome as Captured by a Retrospective Continuous Glucose Monitoring System. Endocr Pract. 2009:1–13. doi: 10.4158/EP09260.CR. [DOI] [PubMed] [Google Scholar]
- 28.Shen WK, Edwards WD, Hammill SC, Bailey KR, Ballard DJ, Gersh BJ. Sudden unexpected nontraumatic death in 54 young adults: a 30-year population-based study. Am J Cardiol. 1995;76:148–152. doi: 10.1016/s0002-9149(99)80047-2. [DOI] [PubMed] [Google Scholar]
- 29.Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN, et al. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141:829–834. doi: 10.7326/0003-4819-141-11-200412070-00005. [DOI] [PubMed] [Google Scholar]
- 30.Vaartjes I, Hendrix A, Hertogh EM, Grobbee DE, Doevendans PA, Mosterd A, et al. Sudden death in persons younger than 40 years of age: incidence and causes. Eur J Cardiovasc Prev Rehabil. 2009;16:592–596. doi: 10.1097/HJR.0b013e32832d555b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.