Abstract
Ischemia damages the mitochondrial electron transport chain (ETC), mediated in part by damage generated by the mitochondria themselves. Mitochondrial damage resulting from ischemia, in turn, leads to cardiac injury during reperfusion. The goal of the present study was to localize the segment of the ETC that produces the ischemic mitochondrial damage. We tested if blockade of the proximal ETC at complex I differed from blockade distal in the chain at cytochrome oxidase. Isolated rabbit hearts were perfused for 15 min followed by 30 min stop-flow ischemia at 37°C. Amobarbital (2.5 mM) or azide (5 mM) was used to block proximal (complex I) or distal (cytochrome oxidase) sites in the ETC. Time control hearts were buffer-perfused for 45 min. Subsarcolemmal mitochondria (SSM) and interfibrillar mitochondria (IFM) were isolated. Ischemia decreased cytochrome c content in SSM but not in IFM compared to time control. Blockade of electron transport at complex I preserved the cytochrome c content in SSM. In contrast, blockade of electron transport at cytochrome oxidase with azide did not retain cytochrome c in SSM during ischemia. Since blockade of electron transport at complex III also prevented cytochrome c loss during ischemia, the specific site that elicits mitochondrial damage during ischemia is likely located in the segment between complex III and cytochrome oxidase.
Keywords: electron transport chain, reactive oxygen species, ischemia, cytochrome c, cardiolipin
INTRODUCTION
The mitochondrial electron transport chain (ETC) is the key component for energy production in the cell [1], yet it also contributes to mitochondrial injury during stressed conditions [2–4]. Cardiac mitochondria exist in two populations: subsarcolemmal mitochondria (SSM), located beneath the plasma membrane, and interfibrillar mitochondria (IFM) present between the myofibrils [5–7]. Ischemia decreases respiration through cytochrome oxidase in SSM after 30 min of global ischemia [8]. Blockade of electron transport at complex I preserves respiration in SSM during ischemia, indicating that the ETC itself is the source for mitochondrial damage during ischemia and the ETC site that generates the damage is distal to complex I [9, 10]. In agreement with this finding, complex I inhibition by nitrosylation protects mitochondria during hypoxia and reoxygenation [2] and protects cardiomyocytes during ischemia and reperfusion [11]. These data support that blockade of electron transport at complex I decreases myocardial injury. However, nitric oxide not only inhibits complex I, it is also known to inhibit cytochrome oxidase [12]. Inhibition of cytochrome oxidase with potassium cyanide does not protect the heart during ischemia and reperfusion [13], nor cardiac myocytes during hypoxia and reoxygenation [14]. Carbon monoxide, another cytochrome oxidase inhibitor, decreases myocardial injury [15], indicating that blockade of electron transport at cytochrome oxidase may be cardioprotective. Taken together, there are inconsistent results regarding potential cardioprotection provided by cytochrome oxidase inhibition. The effect of reversible cytochrome oxidase inhibition on mitochondrial function during cardiac ischemia is unknown. In this study, we asked if blockade of electron transport at cytochrome oxidase before ischemia protected cardiac mitochondria during ischemia. If blockade of cytochrome oxidase does not protect mitochondria during ischemia, this will localize the site for mitochondrial damage during ischemia to the middle segment of the ETC. Further identification of the components of this site and modulation of its function during ischemia will provide a novel strategy to protect mitochondria and thus the heart during ischemia and reperfusion.
METHODS
The Institutional Animal Care and Use Committee of the Louis Stokes Department of Veterans Affairs Medical Center approved this study. The isolated, buffer-perfused rabbit heart model was used as previously described [6]. Groups of hearts underwent either 45 minutes of perfusion as non-ischemic time controls, or 15 minutes of perfusion followed with 30 min ischemia. Amobarbital (2.5 mM) or azide (5 mM) was given for 1 min immediately before ischemia.
Subsarcolemmal (SSM) and interfibrillar (IFM) populations of cardiac mitochondria were isolated as previously described [6, 8]. Protein concentrations were measured using the biuret method with bovine serum albumin as standard [6]. Oxygen consumption in intact mitochondria was measured using a Clark-type oxygen electrode at 30°C [6]. Cytochromes were quantified using the difference of dithionite-reduced minus air-oxidized absorption spectra [6].
Statistical Analysis
Data are expressed as the mean ± standard error of the mean. Differences among groups were compared by one-way analysis of variance with post hoc comparisons performed using the Student-Newman-Keuls test of multiple comparisons (Sigmastat 3.5, ProgramPaketet, Gothenburg, Sweden). A difference of p<0.05 was considered significant.
RESULTS
Isolated rabbit heart model of ischemia
There were no differences in developed pressure (mmHg) between groups at 15 min equilibration (Control 88±4, n=9; Ischemia 91±4, n=9; Amobarbital + Ischemia 93±2, n=9; Azide + Ischemia 99±4, n=5 p=NS). The developed pressure (mmHg) was maintained during 45 minutes perfusion in the time control group (15 min equilibration 88±4 vs. 92±1 at 45 min perfusion, n=9; P=NS). Compared to the time control, ischemia markedly increased diastolic pressure. Amobarbital, but not azide, treatment significantly decreased myocardial diastolic pressure during ischemia (Time control 7±1, n=9; Ischemia 67±5*, n=9; Amobarbital + ischemia 18±4, n=9; Azide + ischemia 73±7*, n=5; *P<0.05 vs. time control or amobarbital + ischemia).
Oxidative phosphorylation
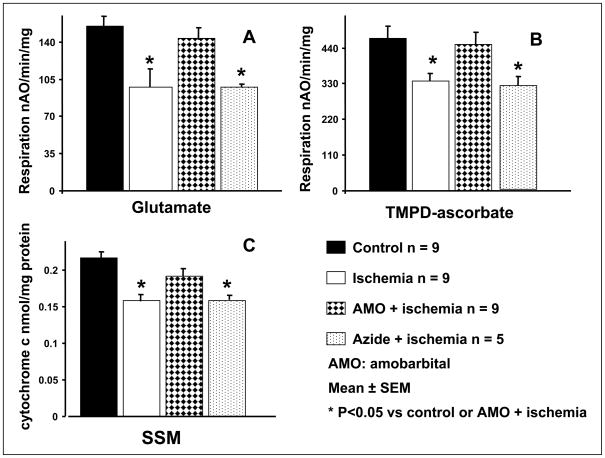
Ischemia decreased the oxidation of glutamate in SSM (Figure 1, panel A) and IFM (Table 1), consistent with previous results [6]. Amobarbital given before ischemia preserved respiration with glutamate as complex I substrate, whereas azide inhibition did not protect SSM (Figure 1, panel A) nor IFM (Table 1). The oxidation of TMPD-ascorbate, an electron donor to cytochrome oxidase via cytochrome c, was decreased following 30 min of ischemia in SSM [6, 8]. Amobarbital treatment preserved the oxidation through cytochrome oxidase in SSM during ischemia, whereas azide treatment did not (Figure 1, panel B). There were no differences in TMPD oxidation between groups in IFM following ischemia (Table 1). High concentration of azide (15 mM) administrated before ischemia also did not protect SSM (TMPD oxidation: control 489±38, n=9 vs. ischemia 303±39, n=3, P<0.05), whereas the rate of TMPD oxidation is similar in control IFM and azide treated IFM following ischemia (control 807±131, n=9 vs. ischemia 661±51, n=3, P=NS).
Figure 1. Blockade of electron transport before ischemia at cytochrome oxidase using azide does not protect oxidative phosphorylation nor preserve the cytochrome c content.
The rate of uncoupled respiration stimulated using dinitrophenol (DNP, 0.3 mM) and the cytochrome c content in subsarcolemmal mitochondria (SSM) was decreased at the end of 30 min. of ischemia compared to the time control. Amobarbital, a selective inhibitor of complex I at the rotenone site, treatment immediately before ischemia markedly attenuated the decrease in the rate of oxidation and cytochrome c content in SSM. In contrast, azide, a selective inhibitor of cytochrome oxidase, did not protect. Panel A; glutamate was used as complex I substrate; Panel B; TMPD-ascorbate was used as a complex IV substrate; Panel C; cytochrome c content.
TABLE 1.
The function of IFM during ischemia with amobarbital or azide treatment
| Time control (n=9) | Ischemia (n=9) | Amobarbital + ischemia (n=9) | Azide + ischemia (n=5) | |
|---|---|---|---|---|
| Glutamate - Complex I substrate | ||||
| 0.3 mM DNP | 277±10 | 186±18* | 283±14 | 189±13* |
| TMPD-ascorbate – Complex IV substrate | ||||
| 0.3 mM DNP | 807±131 | 659±74 | 773±55 | 592±56 |
| Cytochrome c content | ||||
| (nmol/mg protein) | 0.276±0.016 | 0.255±0.018 | 0.301±0.016 | 0.264±0.012 |
Data are expressed as mean ± SEM;
p<0.05 vs. time control or amobarbital + ischemia.
Dinitrophenol (DNP) was used as an uncoupler to stimulate respiration.
Cytochrome c content in SSM and IFM during ischemia
The content of cytochrome c was decreased at 30 min of ischemia in SSM. Amobarbital treatment before ischemia blunted the decrease in cytochrome c content in SSM. Azide treatment before ischemia did not prevent cytochrome c loss in SSM following ischemia (Figure 1, panel C). There were no differences in cytochrome c content in IFM following ischemia (Table 1).
DISCUSSION
In the present study, we found that blockade of electron transport at cytochrome oxidase did not protect mitochondria during ischemia. In contrast, blockade of proximal electron transport with amobarbital protects mitochondria against ischemic damage. Blockade of electron transport at complex III with antimycin A also protects mitochondria during ischemia [10]. Thus, a specific region in the ETC leads to mitochondrial damage during ischemia. This site is located between complex III and cytochrome oxidase.
Blockade of electron transport with rotenone preserves respiration through cytochrome oxidase during ischemia [10]. However, the potential protection of complex I during ischemia cannot be assessed because rotenone is an irreversible inhibitor [10]. Thus, in the current study, we used amobarbital to inhibit complex I. Amobarbital is a reversible complex I inhibitor and blocks electron transport within complex I at the rotenone site [9, 16]. Preserved glutamate oxidation in SSM from amobarbital treated hearts following ischemia supports that complex I inhibition before ischemia protects both the proximal and distal electron transport chain. This also indicates that amobarbital can easily wash out during the mitochondrial isolation procedure [17]. Azide inhibition before ischemia does not improve the glutamate oxidation in SSM following ischemia compared to the untreated ischemic hearts. The oxidation through cytochrome oxidase is also decreased in azide treated SSM. Azide is a selective cytochrome oxidase inhibitor [18]. The decreased oxidation through cytochrome oxidase may be due to the incomplete wash out of azide during the mitochondrial isolation procedure. However, the restored oxidation through cytochrome oxidase in azide treated IFM does not support the presence of persistent azide effect in isolated mitochondria. High concentration azide treatment (15 mM) does not inhibit the oxidation through cytochrome oxidase in SSM nor in IFM, suggesting that azide is washed out during mitochondrial isolation.
Ischemia causes cytochrome c release from SSM [6, 8]. Amobarbital but not azide treatment prevents cytochrome c loss during ischemia, providing additional respiration-independent evidence that blockade of the proximal but not distal electron transport protects cardiac mitochondria during ischemia.
Protection of mitochondria during ischemia decreases myocardial injury during reperfusion [13, 17, 19]. In the current study, we found that blockade of electron transport at cytochrome oxidase did not protect mitochondria during ischemia. This highlights the concept that ischemia-mediated mitochondrial damage leads to myocardial injury during reperfusion [17, 20]. Carbon monoxide given before ischemia decreases myocardial reperfusion injury in the isolated heart [15, 21]. Carbon monoxide is known to inhibit cytochrome oxidase [22], and this may argue that inhibition of cytochrome oxidase can protect the heart during ischemia-reperfusion. However, carbon monoxide has multiple effects in addition to cytochrome oxidase inhibition [22]. Carbon monoxide has an antioxidant effect [23] and inhibits NAD(P)H oxidase and L-type calcium channels [24, 25]. Blockade of L-type calcium channels is known to protect the heart during ischemia-reperfusion by decreasing calcium loading [26]. These results suggest that the cardiac protection provided by carbon monoxide is unlikely to occur via cytochrome oxidase inhibition. Nitric oxide inhibits both complex I and cytochrome oxidase [12, 27, 28]. Inhibition of complex I by nitric oxide decreases myocardial injury, whereas inhibition of cytochrome oxide by nitric oxidase augments tissue injury [29]. Protective effect of NO via blockade of complex I is demonstrated in the intact heart [2]. Blockade of electron transport at complex III protects mitochondria during ischemia [10]. The present study localizes the site for mitochondrial damage in the ETC is between complex III and cytochrome oxidase.
Cytochrome c is traditionally recognized as an electron shuttle between complex III and cytochrome oxidase [30]. Cytochrome c and cardiolipin can also form a cardiolipin-cytochrome c peroxidase [31, 32]. When H2O2 is available as a substrate, cardiolipin-cytochrome c peroxidase will reduce H2O2 to H2O and simultaneously generate peroxidized cardiolipin [31–33]. The peroxidized cardiolipin further leads to cytochrome c delocalization from the inner mitochondrial membrane and subsequent release into cytosol. Inhibition of cytochrome c cardiolipin peroxidase by nitric oxide prevents cardiolipin oxidation and cytochrome c loss, suggesting that the peroxidase is a potential site that generates mitochondrial damage [33]. Ischemia damages the ETC and increases the production of reactive oxygen species in rat [34] and rabbit heart mitochondria [35]. This production may activate the cytochrome c-cardiolipin peroxidase that leads to the previously observed decrease in cardiolipin content in rabbit SSM following ischemia [8, 10].(Figure 2) Blockade of proximal electron transport decreases the production of ROS [17, 36] and prevents cardiolipin depletion [10]. This suggests that limitation of electron flow into the cytochrome c-cardiolipin peroxidase may provide an alternative way to inhibit this peroxidase.(Figure 2) Antimycin A blocks electron transport at the complex III Qi center, and this leads to the increased production of ROS from the Qo center of complex III [37–39]. Antimycin A inhibition protects cardiac mitochondria during ischemia, suggesting that complex III Qo center is not the key site that generates mitochondrial damage during ischemia [10, 40]. The observation that antimycin A inhibition protects mitochondria further supports that blockade of electron transport upstream of cytochrome c peroxidase exerts cardioprotection.
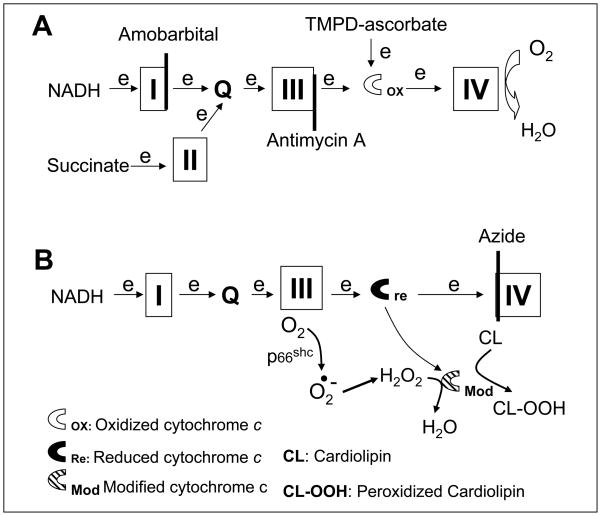
Figure 2. Depiction of the potential sites in the electron transport chain that lead to mitochondrial damage during ischemia.
Electron flux from NADH enters the electron transport chain (ETC) at complex I and proceeds complex I→III→cytochrome c→ IV. Succinate is oxidized by complex II and results in electron flow from complex II →III→ cytochorme c→cytochrome oxidase. Amobarbital blocks electron transport distal within complex I (Panel A) and protect against ischemia-mediated mitochondrial damage. Thus, the key site of ETC-driven injury must lie distal to complex I. Previous work shows that antimycin A inhibits at cytochrome b of complex III (Panel A) and it prevents cardiolipin depletion and the loss of cytochrome c from cardiac mitochondria during ischemia [9, 10]. This further indicates the ischemia-damaged site is distal to complex III (Panel A). Ischemia damages complex III and increases the production of reactive oxygen species [5, 34, 35]. The increased superoxide is converted to H2O2. H2O2 is reduced to H2O by cytochrome c-cardiolipin peroxidase (Modified cytochrome c), and simultaneously cardiolipin is oxidized to peroxidized cardiolipin (CL-OOH) that favors cytochrome c detachment from the inner mitochondrial membrane and release from mitochondria as observed in the present study (Panel B). Azide inhibits complex IV and maintains cytochrome c in the reduced state that favors H2O2 generation via the actions of p66shc(Panel B).
Blockade of electron transport at cytochrome oxidase does not protect the heart during reperfusion [13]. Cytochrome oxidase is distal to the cytochrome c peroxidase [31]. Blockade of distal electron transport will lead to the accumulation of electron at upstream complex and increase ROS generation [35, 37]. Blockade of electron transport at cytochrome oxidase may increase the production of ROS from upstream complexes that leads to mitochondrial damage and subsequent cardiac injury.(Figure 2)
p66shc is a mitochondrial intermembrane protein, also located in the ETC segment between complex III and cytochrome oxidase [41–43]. p66shc is usually bound to the mitochondrial protein import complexes (TOM and TIM) and dissociates from them with an increase in H2O2 generation during stressed conditions [41, 43]. Reduced cytochrome c is the substrate for H2O2 generation from p66shc [41, 43]. Consistent with this proposed mechanism, cardiac injury during ischemia and reperfusion is decreased in hearts devoid of p66shc[44]. Blockade of electron transport at cytochrome oxidase maintains the upstream complexes including cytochrome c in a reduced state and should increase H2O2 generation and mitochondrial damage during ischemia(Figure 2). This potential mechanism is actively under investigation in our laboratory.
In summary, the mitochondrial ETC is the source of mitochondrial damage during ischemia. The ETC locus that produces the damage is located between complex III and cytochrome oxidase. Blockade at cytochrome oxidase allows mitochondrial driven injury from this segment of the ETC.(Figure 2) The current study guides future work to further elucidate the action of this segment of the ETC in the pathogenesis of ischemic damage to cardiac mitochondria that in turn augment cardiac injury during reperfusion.
Acknowledgments
This work was supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs and program project 2PO1AG15885 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia-reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 2.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borutaite V, Budriunaite A, Brown GC. Reversal of nitric oxide-, peroxynitrite- and S-nitrosothiol-induced inhibition of mitochondrial respiration or complex I activity by light and thiols. Biochim Biophys Acta. 2000;1459:405–412. doi: 10.1016/s0005-2728(00)00178-x. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–147. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- 5.Lesnefsky EJ, Gudz TI, Migita CT, Ikeda-Saito M, Hassan MO, Turkaly PJ, Hoppel CL. Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron-sulfur protein subunit of electron transport complex III. Arch Biochem Biophys. 2001;385:117–128. doi: 10.1006/abbi.2000.2066. [DOI] [PubMed] [Google Scholar]
- 6.Lesnefsky EJ, Tandler B, Ye J, Slabe TJ, Turkaly J, Hoppel CL. Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am J Physiol. 1997;273:H1544–1554. doi: 10.1152/ajpheart.1997.273.3.H1544. [DOI] [PubMed] [Google Scholar]
- 7.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- 8.Lesnefsky EJ, Slabe TJ, Stoll MS, Minkler PE, Hoppel CL. Myocardial ischemia selectively depletes cardiolipin in rabbit heart subsarcolemmal mitochondria. Am J Physiol. 2001;280:H2770–2778. doi: 10.1152/ajpheart.2001.280.6.H2770. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Hoppel CL, Lesnefsky EJ. Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J Pharmacol Exp Ther. 2006;316:200–207. doi: 10.1124/jpet.105.091702. [DOI] [PubMed] [Google Scholar]
- 10.Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of electron transport during Ischemia protects cardiac mitochondria. J Biol Chem. 2004;279:47961–47967. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- 11.Nadtochiy SM, Burwell LS, Brookes PS. Cardioprotection and mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury. J Mol Cell Cardiol. 2007;42:812–825. doi: 10.1016/j.yjmcc.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antunes F, Cadenas E. The mechanism of cytochrome C oxidase inhibition by nitric oxide. Front Biosci. 2007;12:975–985. doi: 10.2741/2118. [DOI] [PubMed] [Google Scholar]
- 13.Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem. 1993;268:18532–18541. [PubMed] [Google Scholar]
- 14.Prabu SK, Anandatheerthavarada HK, Raza H, Srinivasan S, Spear JF, Avadhani NG. Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury. J Biol Chem. 2006;281:2061–2070. doi: 10.1074/jbc.M507741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavitrano M, Smolenski RT, Musumeci A, Maccherini M, Slominska E, Di Florio E, Bracco A, Mancini A, Stassi G, Patti M, Giovannoni R, Froio A, Simeone F, Forni M, Bacci ML, D’Alise G, Cozzi E, Otterbein LE, Yacoub MH, Bach FH, Calise F. Carbon monoxide improves cardiac energetics and safeguards the heart during reperfusion after cardiopulmonary bypass in pigs. FASEB J. 2004;18:1093–1095. doi: 10.1096/fj.03-0996fje. [DOI] [PubMed] [Google Scholar]
- 16.Chance B, Williams GR, Hollunger G. Inhibition of electron and energy transfer in mitochondria. I. Effects of Amytal, thiopental, rotenone, progesterone, and methylene glycol. J Biol Chem. 1963;238:418–431. [PubMed] [Google Scholar]
- 17.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther. 2006;319:1405–1412. doi: 10.1124/jpet.106.110262. [DOI] [PubMed] [Google Scholar]
- 18.Smith RP, Wilcox DE. Toxicology of selected nitric oxide-donating xenobiotics, with particular reference to azide. Crit Rev Toxicol. 1994;24:355–377. doi: 10.3109/10408449409017923. [DOI] [PubMed] [Google Scholar]
- 19.Park JW, Chun YS, Kim YH, Kim CH, Kim MS. Ischemic preconditioning reduces Op6 generation and prevents respiratory impairment in the mitochondria of post-ischemic reperfused heart of rat. Life Sci. 1997;60:2207–2219. doi: 10.1016/s0024-3205(97)00236-1. [DOI] [PubMed] [Google Scholar]
- 20.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musameh MD, Green CJ, Mann BE, Fuller BJ, Motterlini R. Improved myocardial function after cold storage with preservation solution supplemented with a carbon monoxide-releasing molecule (CORM-3) J Heart Lung Transplant. 2007;26:1192–1198. doi: 10.1016/j.healun.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 23.Muchova L, Wong RJ, Hsu M, Morioka I, Vitek L, Zelenka J, Schroder H, Stevenson DK. Statin treatment increases formation of carbon monoxide and bilirubin in mice: a novel mechanism of in vivo antioxidant protection. Can J Physiol Pharmacol. 2007;85:800–810. doi: 10.1139/y07-077. [DOI] [PubMed] [Google Scholar]
- 24.Scragg JL, Dallas ML, Wilkinson JA, Varadi G, Peers C. Carbon monoxide inhibits L-type Ca2+ channels via redox modulation of key cysteine residues by mitochondrial reactive oxygen species. J Biol Chem. 2008;283:24412–24419. doi: 10.1074/jbc.M803037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uemura K, Adachi-Akahane S, Shintani-Ishida K, Yoshida K. Carbon monoxide protects cardiomyogenic cells against ischemic death through L-type Ca2+ channel inhibition. Biochem Biophys Res Commun. 2005;334:661–668. doi: 10.1016/j.bbrc.2005.06.142. [DOI] [PubMed] [Google Scholar]
- 26.Parent de Curzon O, Ghaleh B, Hittinger L, Giudicelli JF, Berdeaux A. Beneficial effects of the T- and L-type calcium channel antagonist, mibefradil, against exercise-induced myocardial stunning in dogs. J Cardiovasc Pharmacol. 2000;35:240–248. doi: 10.1097/00005344-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Murphy E, Steenbergen C. Cardioprotection in females: a role for nitric oxide and altered gene expression. Heart Fail Rev. 2007;12:293–300. doi: 10.1007/s10741-007-9035-0. [DOI] [PubMed] [Google Scholar]
- 28.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr. 2008;40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 29.Cooper CE, Davies NA, Psychoulis M, Canevari L, Bates TE, Dobbie MS, Casley CS, Sharpe MA. Nitric oxide and peroxynitrite cause irreversible increases in the K(m) for oxygen of mitochondrial cytochrome oxidase: in vitro and in vivo studies. Biochim Biophys Acta. 2003;1607:27–34. doi: 10.1016/j.bbabio.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia--reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 31.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 32.Belikova NA, Vladimirov YA, Osipov AN, Kapralov AA, Tyurin VA, Potapovich MV, Basova LV, Peterson J, Kurnikov IV, Kagan VE. Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry. 2006;45:4998–5009. doi: 10.1021/bi0525573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vlasova, Tyurin VA, Kapralov AA, Kurnikov IV, Osipov AN, Potapovich MV, Stoyanovsky DA, Kagan VE. Nitric oxide inhibits peroxidase activity of cytochrome c.cardiolipin complex and blocks cardiolipin oxidation. J Biol Chem. 2006;281:14554–14562. doi: 10.1074/jbc.M509507200. [DOI] [PubMed] [Google Scholar]
- 34.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008;294:C460–466. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- 35.Chen Q, Lesnefsky EJ. Depletion of cardiolipin and cytochrome c during ischemia increases hydrogen peroxide production from the electron transport chain. Free Radic Biol Med. 2006;40:976–982. doi: 10.1016/j.freeradbiomed.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 36.Chen Q, Lesnefsky EJ. Blockade of electron transport preserves the contents of bcl-2 and cytochrome c in subsarcolemmal mitochondria during ischemia. Circulation. 2007;115 (abstratct 16690) [Google Scholar]
- 37.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: Central role of complex III. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 38.Han D, Antunes F, Canali R, Rettori D, Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J Biol Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 39.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aon MA, Cortassa S, Marban E, O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 41.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Orsini F, Moroni M, Contursi C, Yano M, Pelicci P, Giorgio M, Migliaccio E. Regulatory effects of the mitochondrial energetic status on mitochondrial p66Shc. Biol Chem. 2006;387:1405–1410. doi: 10.1515/BC.2006.176. [DOI] [PubMed] [Google Scholar]
- 43.Orsini F, Migliaccio E, Moroni M, Contursi C, Raker VA, Piccini D, Martin-Padura I, Pelliccia G, Trinei M, Bono M, Puri C, Tacchetti C, Ferrini M, Mannucci R, Nicoletti I, Lanfrancone L, Giorgio M, Pelicci PG. The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J Biol Chem. 2004;279:25689–25695. doi: 10.1074/jbc.M401844200. [DOI] [PubMed] [Google Scholar]
- 44.Carpi A, Menabo R, Kaludercic N, Pelicci P, Di Lisa F, Giorgio M. The cardioprotective effects elicited by p66(Shc) ablation demonstrate the crucial role of mitochondrial ROS formation in ischemia/reperfusion injury. Biochim Biophys Acta. 2009;1787:774–780. doi: 10.1016/j.bbabio.2009.04.001. [DOI] [PubMed] [Google Scholar]