Abstract
Iron is an essential metallic microelement for life. However, iron overload is toxic. The liver serves an important role as a storehouse for iron in the body. About 20–25 mg of iron is required each day for hemoglobin synthesis. To maintain iron homeostasis, transferrin and transferrin receptors are primarily involved in the uptake of iron into hepatocytes, ferritin in its storage, and ferroportin in its export. Moreover, hepcidin controls ferroportin and plays a central role in the iron metabolism. Excess “free” reactive iron produces damaging free radicals via Fenton or Harber-Weiss reactions. Produced free radicals attack cellular proteins, lipids and nucleic acid. Several detoxification system and anti-oxidant defense mechanisms exist to prevent cellular damage by free radicals. Excessive free radicals can lead to hepatocellular damage, liver fibrosis, and hepatocarcinogenesis.
Keywords: iron, hepatocyte, transferrin, hepcidin, free radicals
Introduction
Iron is the most abundant metallic microelement in the body, and it performs important actions in the body; being essential in hemoglobin synthesis for new erythrocytes (oxygen transport and delivery),(1) enzymes involving electron transport, iron-sulfur proteins,(2,3) and so on. The total amount of iron in the bodies of adult human males is about 5 g, of which approximately 65% is hemoglobin iron in erythrocytes.(1) The remainder is stored in ferritin in the liver and other organs.(4,5) Some iron is also present as myoglobin iron in muscles, heme iron in enzymes involved in respiration in all cells and drug metabolism. Daily food intake contains approximately 10 mg of iron, of which 1–2 mg is absorbed by the mucosa of the upper small intestine, bound to transferrin (Tf) in blood, and transported throughout the body as diferric transferrin (Fe2-Tf). Iron homeostasis is maintained by the loss of about 1 mg of iron per day through the sloughing of intestinal mucosa and skin. However, 20–25 mg of iron is required each day for the hemoglobinization of new erythrocytes, for which iron in senescent erythrocytes, largely processed in reticuloendothelial cells, such as macrophages, is reused. As there appears to be no developed mechanism to evacuate accumulated iron from the body, excess iron intake can easily lead to iron overload in tissues, producing free radicals. This article provides an overview of iron metabolism in the liver, particularly in hepatocytes, and the production of free radicals caused by iron overload in the liver.
Iron in Hepatocytes
Iron absorbed from food in the upper small intestine is first transported to the liver via the portal vein. The liver not only stores iron, but also plays a crucial role in iron metabolism, such as in the production of Tf, iron carrier protein, and hepcidin known as a hormone that functions in the regulation of iron metabolism.
Mechanisms of iron transport into hepatocytes
Extracellular circulating iron in the plasma is present as soluble Tf-bound iron, and when there is excess iron, as non-transferrin-bound iron (NTBI), which is bound to serum proteins, such as albumin or citric acid.(6,7) Hepatocytes express both transferrin receptor (TfR) 1 and a homolog, TfR2.(8) Under neutral pH conditions, Tf binds two iron atoms with high affinity, and are circulated in the blood as Fe2-Tf.(9) Known routes for the import of Fe2-Tf into hepatocytes are via TfR1(10,11) and TfR2,(8) and a TfR-independent route.(12,13)
Fe2-Tf binds with TfR1 on the cell membrane surface and forms a complex that is then taken up into hepatocytes by endocytosis.(14) Mice lacking TfR1 die during mid-gestation from reduction in erythrocyte sell size and hemoglobin content, an indication of its physiological essentiality.(15) TfR1 expression is controlled by the excess or deficiency of iron within cells, and is regulated by interactions between trans-acting iron regulatory proteins (IRP1 and IRP2) and multiple iron-responsive elements (IREs) present in the 3'-untranslated regions (UTRs) of TfR1 mRNA (The IRP-IRE regulatory system).(16–18) When there is insufficient iron, IRP binds with multiple IREs in the 3' UTR of TfR1 mRNA, and TfR1 protein levels increase via stabilization of the mRNA. In a state of excess iron, on the other hand, IRP is released from multiple IREs.(19) Thus, IRP acts as a sensor protein for intracellular iron and regulates the expression of IRE genes (Fig. 1A).
Fig. 1.
The IRP-IRE regulatory system. A: Regulation of TfR1; at the high level of iron, the IRPs binding with multiple IREs in the 3' UTRs of TfR1 mRNA promotes TfR1 mRNA’s translation via mRNA stabilization. B: Regulation of ferritin; the IRP binding with the single IRE in the 5' UTRs of ferritin mRNA blocks the translation of ferritin message. In iron overload, the IRP contained the Fe-S cluster cannot bind with the IRE in the 5' UTRs of ferritin mRNA, resulting in the increased translation of ferritin message.
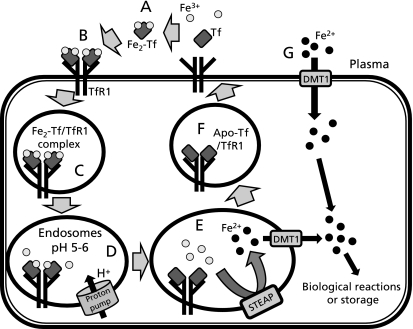
Once the Fe2-Tf/TfR1 complex is endocytosed, the pH of endosomes is lowered to 5–6 by a proton pump ATPase.(20) The Fe2-Tf bond is broken within acidic endosomes, and Fe3+ (ferric iron) is released within the endosome.(21) The free Fe3+ in endosomes then becomes Fe2+ (ferrous iron) by enzymes of the six-transmembrane epithelial antigen of the prostate (STEAP) family,(22,23) and subsequently transported to the cytoplasm by divalent metal transporter 1 (DMT1, also known as Nramp2 or DCT1), a member of the NRAMP (natural resistance-associated macrophage protein) family of metal iron transporters.(24,25) Much of the iron transported into the cytoplasm is employed in reactions for essential physiological reactions, such as mitochondrial adenosine triphosphate (ATP) generation, TCA cycle, and DNA damage repair.(26) Meanwhile, unused iron is stored in the iron-binding protein, ferritin. After dissociating from iron, apo-Tf and TfR1 return to the cell membrane surface, and are reused for further iron binding and uptake (Fig. 2 A–F).(21)
Fig. 2.
Iron metabolism in hepatocytes. A–F: TfR-dependent route (the Tf cycle). A: Two ferric irons (Fe3+) in the plasma bind to Tf with high affinity. B: Fe2-Tf binds to TfR1 on the surface of hepatocytes. C: Fe2-Tf/TfR1 complex is taken up into hepatocytes by endocytosis. D: The pH of endosomes is lowered to 5–6 by an action of proton pumps, and then Fe3+ is released at this acidic pH. E: Released Fe3+ becomes ferrous iron (Fe2+) by the enzymes of STEAP family, and then Fe2+ moves across the endsomal membrane through the DMT1 transporter. F: Apo-Tf still binds to TfR1 at the lower pH of endosomes. Apo-Tf/TfR1 is returned to the surface of the hepatocyte for further iron binding and uptake. G: TfR-independent route. At the high level of iron in plasma, NTBI is reduced to Fe2+ by ferric reductase. Fe2+ is rapidly transported into the hepatocytes through DMT1.
TfR2 was discovered in 1999 as a molecule highly homologous with the TfR1 gene,(8) and it was later shown that mutations in human TfR2 gene induced iron overload syndrome,(27) consistent with its important role in iron metabolism. TfR2 protein is distributed on the basolateral membrane of hepatocytes, and like the TfR1 protein, binds specifically to Tf.(28) IRE is not present in TfR2 mRNA; thus, its mRNA expression is almost completely unaffected by iron levels.(29) Nevertheless, when there is excess iron, Fe2-Tf stabilizes the TfR2 protein, resulting in increased TfR2 protein levels.(30,31)
There are also reports of a route for the import of iron into hepatocytes that is not mediated by TfRs (Fig. 2G),(12,32) and the involvement of DMT1(13) and the zinc transporter ZIP14 (Zrt-Irt-like protein 14)(33) molecules in the uptake of NTBI. In especially cardiomyocytes, it has been reported that L-type voltage-dependent calcium channels involved in the uptake of NTBI under iron overload condition.(34)
Iron storage in hepatocytes
Hepatocytes have a large capacity for iron storage. After iron’s entering hepatocytes, the portion that is not used is stored in the cores of ferritin shells, in order to prevent subsequent toxicity.(35) When hepatocytes become heavily iron overload, an insoluble hemosiderin is derived from iron-rich ferritin.(5) Ferritin is an iron-storage protein comprising heavy (H) and light (L) subunits.(36) Fe2+ is oxidized to Fe3+ by ferroxidase in the H subunit, and then Fe3+ is stored in spaces within ferritin.(37) The synthesis of ferritin, like the TfR protein, is also regulated by IRP protein binding in the mRNA IRE region, and when the concentration of iron in cells increases, the amount of ferritin protein increases.(38,39) As some of the ferritin in the cell cytoplasm is secreted into extracellular medium, it can be measured in clinical tests to gauge the amount of iron stored in the body under non-inflammation conditions.
Regulation of iron export from hepatocytes
There is only 4 mg of iron in blood, and stored iron must be sent quickly to the blood in order to maintain serum iron concentrations. Some kinds of cells, hepatocytes, intestinal epithelial cells and macrophages, have mechanisms to export iron. Iron is exported from cells by the ferroportin membrane protein (also known as IREG1 or MTP1),(40) and it is reported that in mice or Zebrafish lacking ferroportin, the supply of iron to the blood is almost entirely lost.(41) Iron sent into the blood is again transferred to Tf and is used in erythrocyte production in bone marrow. The expression of ferroportin is also regulated by the IRP-IRE regulatory system and hepcidin,(42) which is currently thought to play a central role in the iron metabolism.(43–46)
Hepcidin is synthesized in hepatocytes, secreted from hepatocytes, and excreted through the kidneys.(43,47) Hepcidin in its mature form is a 25-amino acid-peptide, hepcidin-25.(47) Hepcidin-25 binds with ferroportin and both are broken down in lysosomes by endocytosis.(46,48) Hepcidin-25 expression decreases and ferroportin iron export is promoted, when the body requires more serum iron, such as iron deficiency, hypoxia, and pregnancy.(47,49–50) However, in iron deficiency anemia in states of chronic inflammation, the supply of iron to the blood is inhibited by the reduced ferroportin function. It is thought that the inflammatory cytokine, interleukin-6 (IL-6), induces the expression of hepcidin-25 in hepatocytes, resulting in decreased ferroportin.(49,51) And when serum iron is not needed, hepcidin-25 expression increases and ferroportin is broken down, restricting the supply of iron.(47,49)
Iron Overload and Free Radicals
In healthy individuals, almost all of the iron in circulating blood is bound to Tf, while those in hepatocytes is bound to ferritin and isolated.(35) Ferritin has the capacity to hold 2,000–5,000 iron ions per a molecule, and as mentioned above, the amount of ferritin protein expressed increases with elevated intracellular levels of iron by the IRP-IRE regulatory system (Fig. 1B).(39) Ferritin can therefore handle even high amounts of iron imported into hepatocytes.(52) However, during iron overload, ferritin that is bound with iron undergoes denaturation in lysosomes to become hemosiderin, and Fe2+ is released from NTBI that is not bound to ferritin. When this occurs, Fe2+ reacts with hydrogen peroxide (H2O2) to generate hydroxyl radical (•OH) which have the highest toxicity among reactive oxygen species (ROS), via the Fenton reaction or Harber-Weiss reaction. And Fe2+ also reacts with lipid peroxides to generate lipid radicals. These radicals oxidize various cell components; including lipid membranes, proteins, and DNA, causing apoptosis or other cell damage.(53) The solubility of iron ions in the cytoplasm is very low; thus, they are assumed to be bound with other molecules, such as adenosine diphosphate (ADP), rather than be present as single ions. This notion is supported by the fact that administration of the iron chelating agent deferoxamine almost completely suppresses the cell damage from free radicals.(54,55) The risk of hepatocellular carcinoma (HCC) from hereditary hemochromatosis (HH) is about 200 times higher than that in healthy individuals. Interestingly, excess deposits of iron are also seen in hepatic tissues in chronic hepatitis C patients. It has been reported that chronic hepatitis C patients and hepatitis C virus (HCV) transgenic mice have lower levels of hepcidin, consistent with iron accumulation in the liver.(56–58) In such chronic hepatitis C patients, 8-hydroxydeoxy-guanosine (8-OHdG), an indicator of DNA damage by ROS, is significantly higher.(59) On the other hand, the rate of HCC from chronic hepatitis C patients treated with phlebotomy is significantly lower.(60) Moreover, it has been reported that the supplementation of exogenous antioxidants (vitamins A and E) reduced 8-OHdG levels in dietary iron overloaded rats.(61) These evidences in vivo and in vitro studies suggest that iron overload in the cytoplasm contributes, through the production of ROS, to both cellular damage and hepatocarcinogenesis.
Conclusions
Various molecules are involved in iron transport, storage, and export in hepatocytes, and because iron intake tends to be insufficient, despite being essential for life activity, systems for the reuse of iron have evolved. However, in states of iron overload from excess iron intake or chronic inflammation, harmful ROS are produced, and these may be able to cause hepatocellular damage and hepatocarcinogenesis.
Acknowledgment
This manuscript was partially supported by Grand-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 21790667).
Abbreviations
- Tf
transferrin
- Fe2-Tf
diferric transferrin
- NTBI
non-transferrin-bound iron
- TfR
transferrin receptor
- IRP
iron regulatory proteins
- IRE
iron-responsive element
- UTRs
untranslated regions
- STEAP
six-transmembrane epithelial antigen of the prostate
- DMT1
divalent metal transporter 1
- NRAMP
natural resistance-associated macrophage protein
- ATP
adenosine triphosphate
- ZIP
Zrt-Irt-like protein
- IL
interleukin
- H2O2
hydrogen peroxide
- ROS
reactive oxygen species
- ADP
adenosine diphosphate
- HCC
hepatocellular carcinoma
- HH
hereditary hemochromatosis
- HCV
hepatitis C virus
- 8-OHdG
8-hydroxydeoxy-guanosine
References
- 1.Wilson MT, Reeder BJ. Oxygen-binding haem proteins. Exp Physiol. 2008;93:128–132. doi: 10.1113/expphysiol.2007.039735. [DOI] [PubMed] [Google Scholar]
- 2.Rouault TA, Tong WH. Iron-sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat Rev Mol Cell Biol. 2005;6:345–351. doi: 10.1038/nrm1620. [DOI] [PubMed] [Google Scholar]
- 3.Lill R, Mühlenhoff U. Iron-sulfur protein biogenesis in eukaryotes: components and mechanisms. Annu Rev Cell Dev Biol. 2006;22:457–486. doi: 10.1146/annurev.cellbio.22.010305.104538. [DOI] [PubMed] [Google Scholar]
- 4.Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 5.Koorts AM, Viljoen M. Ferritin and ferritin isoforms I: structure-function relationships, synthesis, degradation and secretion. Arch Physiol Biochem. 2007;113:30–54. doi: 10.1080/13813450701318583. [DOI] [PubMed] [Google Scholar]
- 6.Silva AM, Hider RC. Influence of non-enzymatic post-translation modifications on the ability of human serum albumin to bind iron. Implications for non-transferrin-bound iron speciation. Biochim Biophys Acta. 2009;1794:1449–1458. doi: 10.1016/j.bbapap.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Silva AM, Kong X, Parkin MC, Cammack R, Hider RC. Iron(III) citrate speciation in aqueous solution. Dalton Trans. 2009;28:8616–8625. doi: 10.1039/b910970f. [DOI] [PubMed] [Google Scholar]
- 8.Kawabata H, Yang R, Hirama T, et al. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999;274:20826–20832. doi: 10.1074/jbc.274.30.20826. [DOI] [PubMed] [Google Scholar]
- 9.Sahlstedt L, von Bonsdorff L, Ebeling F, Ruutu T, Parkkinen J. Effective binding of free iron by a single intravenous dose of human apotransferrin in haematological stem cell transplant patients. Br J Haematol. 2002;119:547–553. doi: 10.1046/j.1365-2141.2002.03836.x. [DOI] [PubMed] [Google Scholar]
- 10.Aisen P. Transferrin receptor 1. Int J Biochem Cell Biol. 2004;36:2137–2143. doi: 10.1016/j.biocel.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 12.Ikuta K, Zak O, Aisen P. Recycling, degradation and sensitivity to the synergistic anion of transferrin in the receptor-independent route of iron uptake by human hepatoma (HuH-7) cells. Int J Biochem Cell Biol. 2004;36:340–352. doi: 10.1016/s1357-2725(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 13.Shindo M, Torimoto Y, Saito H, et al. Functional role of DMT1 in transferrin-independent iron uptake by human hepatocyte and hepatocellular carcinoma cell, HLF. Hepatol Res. 2006;35:152–162. doi: 10.1016/j.hepres.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Ciechanover A, Schwartz AL, Dautry-Varsat A, Lodish HF. Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J Biol Chem. 1983;258:9681–9689. [PubMed] [Google Scholar]
- 15.Levy JE, Jin O, Fujiwara Y, Kuo F, Andrews NC. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat Genet. 1999;21:396–399. doi: 10.1038/7727. [DOI] [PubMed] [Google Scholar]
- 16.Pantopoulos K. Iron metabolism and the IRE/IRP regulatory system: an update. Ann NY Acad Sci. 2004;1012:1–13. doi: 10.1196/annals.1306.001. [DOI] [PubMed] [Google Scholar]
- 17.Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol. 2006;2:406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- 18.Cairo G, Recalcati S. Iron-regulatory proteins: molecular biology and pathophysiological implications. Expert Rev Mol Med. 2007;9:1–13. doi: 10.1017/S1462399407000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Di X, D’Agostino RB,, Jr., Torti SV, Torti FM. Excess capacity of the iron regulatory protein system. J Biol Chem. 2007;282:24650–24659. doi: 10.1074/jbc.M703167200. [DOI] [PubMed] [Google Scholar]
- 20.Klausner RD, Ashwell G, van Renswoude J, Harford JB, Bridges KR. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci USA. 1983;80:2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dautry-Varsat A, Ciechanover A, Lodish HF. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci USA. 1983;80:2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, Chen J. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sendamarai AK, Ohgami RS, Fleming MD, Lawrence CM. Structure of the membrane proximal oxidoreductase domain of human Steap3, the dominant ferrireductase of the erythroid transferrin cycle. Proc Natl Acad Sci USA. 2008;105:7410–7415. doi: 10.1073/pnas.0801318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci USA. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ludwiczek S, Theurl I, Muckenthaler MU, et al. Ca2+ channel blockers reverse iron overload by a new mechanism via divalent metal transporter-1. Nat Med. 2007;13:448–454. doi: 10.1038/nm1542. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto Y, Zhang QM, Takao M, Yasui A, Yonei S. Escherichia coli Nth and human hNTH1 DNA glycosylases are involved in removal of 8-oxoguanine from 8-oxoguanine/guanine mispairs in DNA. Nucleic Acids Res. 2001;29:1975–1981. doi: 10.1093/nar/29.9.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camaschella C, Roetto A, Cali A, et al. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet. 2000;25:14–15. doi: 10.1038/75534. [DOI] [PubMed] [Google Scholar]
- 28.Merle U, Theilig F, Fein E, et al. Localization of the iron-regulatory proteins hemojuvelin and transferrin receptor 2 to the basolateral membrane domain of hepatocytes. Histochem Cell Biol. 2007;127:221–226. doi: 10.1007/s00418-006-0229-7. [DOI] [PubMed] [Google Scholar]
- 29.Kawabata H, Germain RS, Vuong PT, Nakamaki T, Said JW, Koeffler HP. Transferrin receptor 2-alpha supports cell growth both in iron-chelated cultured cells and in vivo. J Biol Chem. 2000;275:16618–16625. doi: 10.1074/jbc.M908846199. [DOI] [PubMed] [Google Scholar]
- 30.Johnson MB, Enns CA. Diferric transferrin regulates transferrin receptor 2 protein stability. Blood. 2004;104:4287–4293. doi: 10.1182/blood-2004-06-2477. [DOI] [PubMed] [Google Scholar]
- 31.Johnson MB, Chen J, Murchison N, Green FA, Enns CA. Transferrin receptor 2: evidence for ligand-induced stabilization and redirection to a recycling pathway. Mol Biol Cell. 2007;18:743–754. doi: 10.1091/mbc.E06-09-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sturrock A, Alexander J, Lamb J, Craven CM, Kaplan J. Characterization of a transferrin-independent uptake system for iron in HeLa cells. J Biol Chem. 1990;265:3139–3145. [PubMed] [Google Scholar]
- 33.Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oudit GY, Sun H, Trivieri MG, et al. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med. 2003;9:1187–1194. doi: 10.1038/nm920. [DOI] [PubMed] [Google Scholar]
- 35.Munro HN. Iron regulation of ferritin gene expression. J Cell Biochem. 1990;44:107–115. doi: 10.1002/jcb.240440205. [DOI] [PubMed] [Google Scholar]
- 36.Boyd D, Jain SK, Crampton J, Barrett KJ, Drysdale J. Isolation and characterization of a cDNA clone for human ferritin heavy chain. Proc Natl Acad Sci USA. 1984;81:4751–4755. doi: 10.1073/pnas.81.15.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theil EC. Ferritin: at the crossroads of iron and oxygen metabolism. J Nutr. 2003;133:1549S–1553S. doi: 10.1093/jn/133.5.1549S. [DOI] [PubMed] [Google Scholar]
- 38.Hentze MW, Caughman SW, Rouault TA, et al. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987;238:1570–1573. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- 39.Hentze MW, Rouault TA, Caughman SW, et al. A cis-acting element is necessary and sufficient for translational regulation of human ferritin expression in response to iron. Proc Natl Acad Sci USA. 1987;84:6730–6734. doi: 10.1073/pnas.84.19.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donovan PJ, Gearhart J. The end of the beginning for pluripotent stem cells. Nature. 2001;414:92–97. doi: 10.1038/35102154. [DOI] [PubMed] [Google Scholar]
- 41.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Yeh KY, Yeh M, Glass J. Hepcidin regulation of ferroportin 1 expression in the liver and intestine of the rat. Am J Physiol Gastrointest Liver Physiol. 2004;286:G385–G394. doi: 10.1152/ajpgi.00246.2003. [DOI] [PubMed] [Google Scholar]
- 43.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 44.Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loréal O, Brissot P. Hepcidin: the Grail of iron metabolism. Gastroenterol Clin Biol. 2002;26:805–807. [PubMed] [Google Scholar]
- 46.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 47.Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 48.De Domenico I, Lo E, Ward DM, Kaplan J. Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proc Natl Acad Sci USA. 2009;106:3800–3805. doi: 10.1073/pnas.0900453106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Millard KN, Frazer DM, Wilkins SJ, Anderson GJ. Changes in the expression of intestinal iron transport and hepatic regulatory molecules explain the enhanced iron absorption associated with pregnancy in the rat. Gut. 2004;53:655–660. doi: 10.1136/gut.2003.031153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miyazaki E, Kato J, Kobune M, et al. Denatured H-ferritin subunit is a major constituent of haemosiderin in the liver of patients with iron overload. Gut. 2002;50:413–419. doi: 10.1136/gut.50.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Um HD, Orenstein JM, Wahl SM. Fas mediates apoptosis in human monocytes by a reactive oxygen intermediate dependent pathway. J Immunol. 1996;156:3469–3477. [PubMed] [Google Scholar]
- 54.Sakaida I, Kyle ME, Farber JL. Autophagic degradation of protein generates a pool of ferric iron required for the killing of cultured hepatocytes by an oxidative stress. Mol Pharmacol. 1990;37:435–442. [PubMed] [Google Scholar]
- 55.Sakaida I, Thomas AP, Farber JL. Increases in cytosolic calcium ion concentration can be dissociated from the killing of cultured hepatocytes by tert-butyl hydroperoxide. J Biol Chem. 1991;266:717–722. [PubMed] [Google Scholar]
- 56.Fujita N, Sugimoto R, Takeo M, et al. Hepcidin expression in the liver: relatively low level in patients with chronic hepatitis C. Mol Med. 2007;13:97–104. doi: 10.2119/2006-00057.Fujita. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagashima M, Kudo M, Chung H, et al. Regulatory failure of serum prohepcidin levels in patients with hepatitis C. Hepatol Res. 2006;36:288–293. doi: 10.1016/j.hepres.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Nishina S, Hino K, Korenaga M, et al. Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology. 2008;134:226–238. doi: 10.1053/j.gastro.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 59.Kato J, Kobune M, Nakamura T, et al. Normalization of elevated hepatic 8-hydroxy-2'-deoxyguanosine levels in chronic hepatitis C patients by phlebotomy and low iron diet. Cancer Res. 2001;61:8697–8702. [PubMed] [Google Scholar]
- 60.Kato J, Miyanishi K, Kobune M, et al. Long-term phlebotomy with low-iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J Gastroenterol. 2007;42:830–836. doi: 10.1007/s00535-007-2095-z. [DOI] [PubMed] [Google Scholar]
- 61.Asare GA, Kew MC, Mossanda KS, Paterson AC, Siziba K, Kahler-Venter CP. Effects of exogenous antioxidants on dietary iron overload. J Clin Biochem Nutr. 2009;44:85–94. doi: 10.3164/jcbn.08-184. [DOI] [PMC free article] [PubMed] [Google Scholar]