Abstract
The pathophysiology of inflammatory bowel disease involves excessive immune effects of inflammatory cells against gut microbes. In genetically predisposed individuals, these effects are considered to contribute to the initiation and perpetuation of mucosal injury. Oxidative stress is a fundamental tissue-destructive mechanisms that can occur due to the reactive oxygen species and reactive nitrogen metabolites which are released in abundance from numerous inflammatory cells that have extravasated from lymphatics and blood vessels to the lamina propria. This extravasation is mediated by interactions between adhesion molecules including mucosal addressin cell adhesion molecule-1 and vascular cell adhesion molecule-1 on the surface of lymphocytes or neutrophils and their ligands on endothelial cells. Thus, reactive oxygen species and adhesion molecules play an important role in the development of inflammatory bowel disease. The present review focuses on the involvement of oxidative stress and adhesion molecules, in particular mucosal addressin cell adhesion molecule-1, in inflammatory bowel disease.
Keywords: ROS, oxidative stress, MAdCAM-1, IBD
Introduction
Although the etiology of inflammatory bowel diseases (IBD) such as ulcerative colitis (UC) and Crohn’s disease (CD) remains uncertain, the interplay of environmental, genetic and immunological factors against bacterial flora is believed to underlie the generation of IBD.(1) Uncontrolled and excessive host immune responses, during which oxidative stress-like reactive oxygen species (ROS) and free radicals are produced from inflammatory cell infiltrates in the gut mucosa, are known to trigger mucosal injury and induce inflammation.
IBD is characterized by the extravasation of numerous inflammatory cells, including neutrophils and lymphocytes. Adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) mediate a series of immune responses and gut inflammation. Among the adhesion molecules that are upregulated in IBD, MAdCAM-1 is considered to be preeminent for the development of chronic gut inflammation. MAdCAM-1 expression is induced on the surface of lymphatic vessels by ROS and by inflammatory cytokines such as tumor necrotic factor (TNF)-α and interleukin (IL)-1β. MAdCAM-1 has been implicated in the selective recruitment of lymphocytes to sites of inflammation in the gut. Thus, oxidative stress and MAdCAM-1 play important roles in IBD development by mediating the movement and accumulation of lymphocytes into gut interstitium and by causing mucosal injury.
The present review focuses on the involvement of oxidative stress and MAdCAM-1 during the development of IBD.
Oxidative Stress
Oxidative stress primarily arises and causes tissue injury when the cytotoxic effects of ROS and free radicals overwhelm elimination of their cytotoxic effects by antioxidants. ROS, which are comprised of singlet oxygen, superoxide anions, hydroxyl radicals, and hydrogen peroxide including free radicals, are all generated as by-products of the normal metabolism of molecular oxygen. A broad definition of ROS includes hydroperoxyl, peroxyl and alkoxyl radicals, hydroperoxide, hypochlorous acid, ozone, nitric monoxide, and nitrogen dioxide. ROS can directly impair any oxidizable molecule.
Oxidative Damage by ROS
Excessive levels of ROS attack and impair almost all cellular components, including cell membranes, lipids, proteins, enzymes and DNA, and consequently cause apoptotic cell death. Regarding the effect of ROS on the cell membrane, it is known that the polyunsaturated fatty acids in the cell membrane lipid bi-layer have two or more carbon double bonds within their structure susceptible to oxidative attack.(2) Sequential attack against these bonds by hydroxyl radicals (•OH) converts the membrane lipids into oxidized phospholipids (lipid peroxidation). The accumulation of peroxidized lipids accelerates disruption of cell membrane integrity that occurs when the ability of the cell to remove excessive products of hydroxyl radicals and their precursors, in particular the products of hydrogen peroxide (H2O2), fails. This failure and the subsequent increase in ROS results in decreased function of transmembrane enzymes, transporters, receptors and other membrane proteins, which are consequently degraded.(3,4) Moreover, colonic epithelia disintegrate because of the ROS-induced increase in mucosal permeability.(5,6)
Next, proteins and enzymes, which are predominant constituents of the cells, are also the target of ROS and oxidative stress. Thus, the •OH radical also attacks, and abrogates many proteins and enzymes. The toxic oxidative effects of •OH include the induction of protein conformational change, which is a major cause of the partial or complete loss of protein function.(7)
Peroxynitrite (ONOO–) is a potent oxidant and nitrating species that is formed from a rapid reaction between the superoxide anion (O2•–) and nitric oxide (NO).(8) ONOO– easily crosses biological membranes, and, despite a relatively short half-life (within 10 ms), it can interact with target molecules in an adjacent cell within one or two cell diameters.(9) Interestingly, exposure to ONOO– promotes the conversion of tyrosine residues into 3-nitrotyrosine, which cannot be readily phosphorylated. ONOO– thus interferes with cellular signaling that is dependent on tyrosine phosphorylation by protein tyrosine kinases.(10) Tyrosine nitration by ONOO– can either prevent a protein from functioning as the phosphorylated form and/or can mimic the structural change induced by phosphorylation and thereby imitate the consequences of phosphorylation.(11) In contrast to •OH, ONOO– can up- or downregulate signaling cascades by controlling the activities of protein kinases. This control is achieved by nitration of tyrosine residues, thereby resulting in gain- or loss of function of kinase activity.(12)
Finally, regarding the effect of ROS on DNA, both nuclear DNA(13) and mitochondrial DNA(14) are also known to be targets of oxidative attack, particularly from •OH and ONOO–, which cause base and sugar hydroxylation(15) as well as breaks in the double strand, leading to adenosine triphosphate depletion, gene mutations and mitochondrial DNA deletions.(16,17) These changes ultimately induce malignant transformation and apoptotic cell death. Thus, oxidative stress thus harms almost all cellular components.
Noxious Involvement of ROS in IBD
Direct measurement of ROS in cells and tissues is quite difficult because of their short biological half-lives.(18) However, direct quantification of ROS levels in colon biopsy specimens from UC and CD patients using chemiluminescence assays showed that ROS levels in these tissues are considerably increased compared to those in normal mucosa and positively correlate with IBD.(19–22) Mounting evidences indicate that there are increased levels of reactive nitrogen metabolites (RNM) such as NO in the inflamed IBD mucosa based on analysis of nitric oxide synthase activity.(23–26) Thus, increased levels of both ROS and RNM are closely correlated with the clinical development of IBD.
Relationship between Adhesive Molecules and Cytokines, and Inflammatory Cells Infiltration and Immune Responses
The consecutive events involved in the extravasation of inflammatory cells from lymphatics and blood vessels to the extravascular space include the following steps: 1, tethering and rolling of the inflammatory cells on the endothelial cell surface; 2, firm attachment to endothelial cells followed by transendothelial migration; and 3, migration toward chemoattractants produced in the lamina propria, which is mediated by the interaction between adhesion molecules on the surface of lymphocytes or neutrophils and their receptors on endothelial cells and vice versa. Various cytokines induce the tethering and rolling of neutrophils on vascular endothelial cells through modulation of the interactions between L-selectin and carbohydrate antigen on neutrophils, and P- and E-selectin on endothelial cells.(27–29) On the other hand interactions between adhesion molecules on the surface of lymphocytes and the adhesion molecule MAdCAM-1 on lymphatic endothelial cells are responsible for lymphocyte tethering.(30) At a later stage, neutrophils and lymphocytes strongly adhere to endothelial cells through other adhesion molecules including ICAM-1, VCAM-1 and MAdCAM-1, and consequently transmigrate into lamina propria mucosae. ROS, lipopolysaccharide (LPS) and inflammatory cytokines such as IL-1β and TNF-α induce the translocation of P-selectin, L-selectin and MAdCAM-1 from intracellular locations to the cell surface. Increased surface expression of P-selectin, ICAM, and MAdCAM-1 is observed in the colon mucosa of patients with IBD.(31,32) Interaction between inflammatory cells and endothelial cells through these adhesion molecules is thus involved in the development of IBD.
MAdCAM-1 and Its Receptor, α4β7 Integrin
MAdCAM-1 is a 58–66 kDa type 1 transmembrane glycoprotein belonging to the immunoglobulin (Ig) superfamily, which is comprised of two amino-terminal Ig-like domains and shares a conserved amino acid sequence homology with VCAM-1.(33) The interaction of integrin α4β7 and MAdCAM-1 is involved in cell homing, firm adhesion, and transendothelial cell migration when lymphocytes are recirculated to peripheral lymph nodes under normal conditions and when lymphocytes are recruited to sites of gut inflammation.(30) The homing of murine lymphocytes to intestinal mucosa was first discovered to be mediated by a molecule that bound to Peyer’s patches high endothelial venules (HEV).(34) This molecule is known as lymphocyte Peyer’s patches HEV adhesion molecule (LPAM)-1 and was ultimately identified as an integrin, which is a heterodimer of α- and β-subunits. The α4-subunit of murine LPAM-2 has 84% homology at the amino acid level with the human integrin α4-subunit.(35) An anti-rat α4 blocking antibody inhibited lymphocyte migration to Peyer’s patches, indicating that α4 integrin plays an important role in mucosal homing.(36)
Initially, the murine integrin β-subunit was believed to be a novel molecule. However, it is currently known that the human β7 integrin subunit is the human homolog of the murine integrin β-subunit.(37,38)
MAdCAM-1 is expressed in Peyer’s patches HEV and in mesenteric lymph nodes, intestinal mucosal venules in the lamina propria, and lymphoid follicles in the normal murine gut. MAdCAM-1 directly binds to its receptor, α4β7 integrin. Blocking antibodies against either α4- or β7-subunits abrogate the binding of lymphocytes to MAdCAM-1 in vivo and in vitro.(39) Therefore, MAdCAM-1 binds to both α4- and β7-subunits.
IBD and MAdCAM-1
Under normal conditions, MAdCAM-1 expression is limited to the endothelium of venules within the lamina propria and submucosa, and to the HEVs of Peyer’s patches and mesenteric lymph nodes. In mouse models of IBD in which experimental colitis was induced with dextran sulfate sodium (DSS) or trinitrobenzene sulfonic acid (TNB), and also in the inflamed colon of human patients with UC and CD, MAdCAM-1 expression was reported to be increased in the lamina propria and submucosal venules within the inflamed sites of the colon compared to its expression in non-inflamed tissues.(40–42) MAdCAM-1 transcription is activated through translocation of the activated p50/p65 nuclear factor kappa-B (NF-κB) complex into the nucleus following proteosomal degradation of phosphorylated IκB (inhibitor of κB) in response to several cytokines including TNF-α and IL-1β.(43,44) Moreover, experimental studies using a SVEC cell line derived from axillary lymph nodes, and a colon-derived endothelial cell line established from transgenic mice bearing a temperature-sensitive SV40 large T antigen, have shown that TNF-α stimulates MAdCAM-1 expression through activation of tyrosine kinase, p38 and p42/p44 mitogen-activated protein kinase (MAPK), and NF-κB/poly-ADP ribose polymerase (PARP) signaling cascades.(45,46)
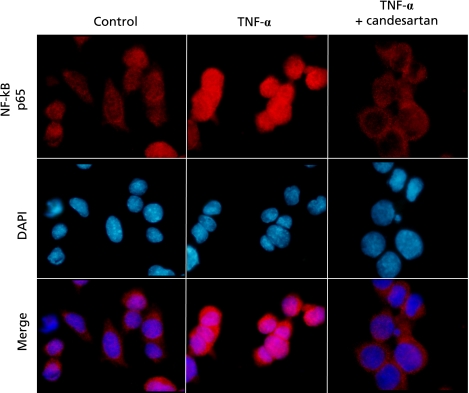
The addition of cytochrome P-450 (CYP450) 3A4 inhibitors such as bergamottin and 6',7'-dihydroxybergamottin (DHB) to cultured SVEC endothelial cells demonstrated that these CYP450 inhibitors blocked TNF-α-induced MAdCAM-1 expression and lymphocyte adhesion in vitro.(47) Interestingly, our very recent study showed that the angiotensin II type 1 receptor antagonist (AT1R antagonist), candesartan, can be used as a novel therapy for IBD. We demonstrated that this AT1R antagonist blocked intranuclear translocation of the activated p50/p65 NF-κB complex in a p38 MAPK independent manner and thereby downregulated TNF-α-induced MAdCAM-1 expression, resulting in the amelioration of colitis (Fig. 1).(48) It has also shown that there is a significant attenuation of MAdCAM-1 expression, inflammatory cell infiltration and mucosal damages during DSS-induced colitis in mice lacking AT1R gene compared to these parameters in wild-type mice.(48,49) This result suggests that TNF-α can induce MAdCAM-1 expression by three different pathways.
Fig. 1.
Localization of NF-κB p65protein in the presence and the absence of Candesartan during TNF-α stimulation. Cells were pretreated with or without Candesartan and then stimulated with TNF-α. Immunofluorescent co-staining of NF-κB p65 protein and cell nuclei stained using DAPI was subsequently performed. Quoted from ref. 48.
Blocking the pathway by which TNF-α induces MAdCAM-1 expression is thus considered to be useful for IBD treatment. Several studies have been carried out to search for potent candidate blockers of TNF-α-induced MAdCAM-1 expression that could be used for IBD treatment. Such candidates include a component present in grapefruit and grapefruit peel oil, its derivatives; bergamottin and DHB,(47) CYP450 inhibitor, troglitazone,(50) which is a γ-subtype of a peroxisome proliferator-activated receptor (PPAR-γ) ligand that blocks phosphorylation of p65 NF-κB, and candesartan,(48) which, as mentioned above, is an agent that blocks translocation of the activated p50/p65 NF-κB complex into the nucleus.
Conclusion and Perspectives
It has been established that ROS and MAdCAM-1 play a critical role in the development of IBD by mediating enhanced extravasation of lymphocytes. Future development and study of neutralizing or blocking antibodies, and chemicals that target molecules involved in the development of IBD, (Table 1), will ensure that detailed molecular mechanisms that underlie the occurrence and perpetuation of gut inflammation will be elucidated in the future.
Table 1.
Therapuetic molecular target for IBD under clinical application and investigation
| molecular targets and name | drug product | disease | efficacy for human | references | |
|---|---|---|---|---|---|
| TNF-α | Infliximab | antibody | UC, CD | effective | 51, 52, 53 |
| Adalimumab | antibody | UC, CD | effective | 54, 55 | |
| Certolizumab pegol | antibody | CD | effective | 56 | |
| Etanercept | antibody | CD | invalid | 57 | |
| Golimumab | antibody | UC, CD | effective | 58 | |
| IFN-γ | Fontlizumab | antibody | CD | invalid | 59 |
| IL-6 receptor | Tocilizumab | antibody | CD | effective # | 60 |
| IL-12/23 | Ustekinumab | antibody | CD | effective # | 61 |
| CD20 | Rituximab | antibody | UC, CD | underway | |
| α1β1 integrin | no name | antibody | DSS colitis | N/A for human | 62 |
| α4 integrin | AJM-300 | chemical | CD | effective # | 63 |
| Natalizumab | antibody | UC, CD | effective | 64 | |
| α4β7 integrin | Vedolizumab | antibody | UC, CD | effective | 65, 66 |
| β7 integrin | rhuMab b7 | antibody | UC | underway | |
| PSGL-1 | no name | antibody | DSS colitis | N/A for human | 67 |
| ICAM-1 | no name | antibody | DSS colitis | N/A for human | 68 |
| VCAM-1 | no name | antibody | DSS colitis | N/A for human | 69 |
| MAdCAM-1 | PF-00547659 | antibody | UC | underway | |
#: result in phase II trial, N/A: not applicable, DSScolitis: dextran sodium sulfate-induced experimental colitis
PSGL-1: P-selectin glycoprotein ligand-1
Acknowledgments
Satoshi Tanida, Tsutomu Mizoshita, Takashi Mizushima, Takaya Shimura, Takeshi Kamiya and Hiromi Kataoka are all members of digestive disease group in Nagoya City University, Department of Gastroenterology and Metabolism and Professor Takashi Joh heads up our department. Makoto Sasaki is one of the members of digestive disease group in Aichi Medical University. We are going forward on the advanced basic researches in order to elucidate how persistent inflammation induces the occurrence of cancer, and how IBD inflammation occurs, and are trying to clarify these difficult problems in the future.
Abbreviations
- IBD
inflammatory bowel diseases
- UC
ulcerative colitis
- CD
Crohn’s disease
- ROS
reactive oxygen species
- ICAM-1
intercellular adhesion molecule 1
- VCAM-1
vascular cell adhesion molecule-1
- MAdCAM-1
mucosal addressin cell adhesion molecule-1
- TNF
tumor necrotic factor
- IL
interleukin
- NO
nitric oxygen
- RNM
reactive nitrogen metabolites
- LPS
lipopolysaccharide
- Ig
immunoglobulin
- HEV
high endothelial venules
- LPAM
lymphocyte Peyer’s patches HEV adhesion molecule
- DSS
dextran sulfate sodium
- TNB
trinitrobenzene sulfonic acid
- NF-κB
nuclear factor kappa-B
- MAPKs
mitogen-activated protein kinases
- PARP
poly-ADP ribose polymerase
- CYP450
cytochrome P-450
- DHB
6',7'-dihydroxybergamottin
- AT1R
angiotensin II type 1 receptor
- PPAR-γ
γ-subtype of peroxisome proliferator-activated receptor
References
- 1.Hoffmann JC, Pawlowski NN, Kühl AA, Höhne W, Zeitz M. Animal models of inflammatory bowel disease: an overview. Pathobiology. 2002;70:121–130. doi: 10.1159/000068143. [DOI] [PubMed] [Google Scholar]
- 2.Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–1828. [PubMed] [Google Scholar]
- 3.Ohyashiki T, Ohtsuka T, Mohri T. A change in the lipid fluidity of the porcine intestinal brush-border membranes by lipid peroxidation. Studies using pyrene and fluorescent stearic acid derivatives. Biochim Biophys Acta. 1986;861:311–318. doi: 10.1016/0005-2736(86)90433-5. [DOI] [PubMed] [Google Scholar]
- 4.Jourd’Heuil D, Vaananen P, Meddings JB. Lipid peroxidation of the brush-border membrane: membrane physical properties and glucose transport. Am J Physiol. 1993;264:G1009–G1015. doi: 10.1152/ajpgi.1993.264.6.G1009. [DOI] [PubMed] [Google Scholar]
- 5.Riedle B, Kerjaschk D. Reactive oxygen species cause direct damage of Engelbreth-Holm-Swarm matrix. Am J Pathol. 1997;151:215–231. [PMC free article] [PubMed] [Google Scholar]
- 6.Rao RK, Baker RD, Baker SS, Gupta A, Holycross M. Oxidant-induced disruption of the intestinal epithelial barrier function: role of protein tyrosine phosphorylation. Am J Physiol. 1997;273:G812–G823. doi: 10.1152/ajpgi.1997.273.4.G812. [DOI] [PubMed] [Google Scholar]
- 7.Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Chem Res Toxicol. 1997;10:485–494. doi: 10.1021/tx960133r. [DOI] [PubMed] [Google Scholar]
- 8.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 10.Kong SK, Yim MB, Stadtman ER, Chock PB. Peroxynitrite disables the tyrosine phosphorylation regulatory mechanism: lymphocyte-specific tyrosine kinase fails to phosphorylate nitrated cdc2(6-20)NH2 peptide. Proc Natl Acad Sci USA. 1996;93:3377–3382. doi: 10.1073/pnas.93.8.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacMillan-Crow LA, Greendorfer JS, Vickers SM, Thompson JA. Tyrosine nitration of c-SRC tyrosine kinase in human pancreatic ductal adenocarcinoma. Arch Biochem Biophys. 2000;377:350–356. doi: 10.1006/abbi.2000.1799. [DOI] [PubMed] [Google Scholar]
- 12.Peluffo G, Radi R. Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovasc Res. 2007;75:291–302. doi: 10.1016/j.cardiores.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 13.Henle ES, Linn S. Formation, prevention, and repair of DNA damage by iron/hydrogen peroxide. J Biol Chem. 1997;272:19095–19098. doi: 10.1074/jbc.272.31.19095. [DOI] [PubMed] [Google Scholar]
- 14.Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H. Free radical-induced damage to DNA: mechanisms and measurement. Free Radic Biol Med. 2002;32:1102–1115. doi: 10.1016/s0891-5849(02)00826-2. [DOI] [PubMed] [Google Scholar]
- 16.Wanrooij S, Goffart S, Pohjoismäki JL, Yasukawa T, Spelbrink JN. Expression of catalytic mutants of the mtDNA helicase Twinkle and polymerase POLG causes distinct replication stalling phenotypes. Nucleic Acids Res. 2007;35:3238–3251. doi: 10.1093/nar/gkm215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieber MR, Karanjawala ZE. Ageing, repetitive genomes and DNA damage. Nat Rev Mol Cell Biol. 2004;5:69–75. doi: 10.1038/nrm1281. [DOI] [PubMed] [Google Scholar]
- 18.Rumley AG, Paterson JR. Analytical aspects of antioxidants and free radical activity in clinical biochemistry. Ann Clin Biochem. 1998;35:181–200. doi: 10.1177/000456329803500202. [DOI] [PubMed] [Google Scholar]
- 19.Keshavarzian A, Sedghi S, Kanofsky J, et al. Excessive production of reactive oxygen metabolites by inflamed colon: analysis by chemiluminescence probe. Gastroenterology. 1992;103:177–185. doi: 10.1016/0016-5085(92)91111-g. [DOI] [PubMed] [Google Scholar]
- 20.Simmonds NJ, Allen RE, Stevens TR, Van Someren RN, Blake DR, Rampton DS. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology. 1992;103:186–196. doi: 10.1016/0016-5085(92)91112-h. [DOI] [PubMed] [Google Scholar]
- 21.Sedghi S, Fields JZ, Klamut M, et al. Increased production of luminol enhanced chemiluminescence by the inflamed colonic mucosa in patients with ulcerative colitis. Gut. 1993;34:1191–1197. doi: 10.1136/gut.34.9.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naito Y, Takagi T, Yoshikawa T. Neutrophil-dependent oxidative stress in ulcerative colitis. J Clin Biochem Nutr. 2007;41:18–26. doi: 10.3164/jcbn.2007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boughton-Smith NK, Evans SM, Hawkey CJ, et al. Nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Lancet. 1993;342:338–340. doi: 10.1016/0140-6736(93)91476-3. [DOI] [PubMed] [Google Scholar]
- 24.Godkin AJ, De Belder AJ, Villa L, et al. Expression of nitric oxide synthase in ulcerative colitis. Eur J Clin Invest. 1996;26:867–872. doi: 10.1111/j.1365-2362.1996.tb02131.x. [DOI] [PubMed] [Google Scholar]
- 25.Singer II, Kawka DW, Scott S, et al. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996;111:871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki M, Joh T. Oxidative stress and ischemia-reperfusion injury in gastrointestinal tract and antioxidant, protective agents. J Clin Biochem Nutr. 2007;40:1–12. doi: 10.3164/jcbn.40.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kokura S, Wolf RE, Yoshikawa T, Granger DN, Aw TY. Molecular mechanisms of neutrophil-endothelial cell adhesion induced by redox imbalance. Circ Res. 1999;84:516–524. doi: 10.1161/01.res.84.5.516. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida N, Yamaguchi T, Nakagawa S, Nakamura Y, Naito Y, Yoshikawa T. Role of P-selectin and intercellular adhesion molecule-1 in TNB-induced colitis in rats. Digestion. 2001;63:81–86. doi: 10.1159/000051916. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura T, Andoh A, Hashimoto T, Kobori A, Tsujikawa T, Fujiyama Y. Cellobiose prevents the development of dextran sulfate sodium (DSS)-induced experimental colitis. J Clin Biochem Nutr. 2010;46:105–110. doi: 10.3164/jcbn.09-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berlin C, Berg EL, Briskin MJ, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura S, Ohtani H, Watanabe Y, et al. In situ expression of the cell adhesion molecules in inflammatory bowel disease. Evidence of immunologic activation of vascular endothelial cells. Lab Invest. 1993;69:77–85. [PubMed] [Google Scholar]
- 32.Schürmann GM, Bishop AE, Facer P, et al. Increased expression of cell adhesion molecule P-selectin in active inflammatory bowel disease. Gut. 1995;36:411–418. doi: 10.1136/gut.36.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Briskin MJ, McEvoy LM, Butcher EC. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature. 1993;363:461–464. doi: 10.1038/363461a0. [DOI] [PubMed] [Google Scholar]
- 34.Holzmann B, Weissman IL. Peyer’s patch-specific lymphocyte homing receptors consist of a VLA-4-like alpha chain associated with either of two integrin beta chains, one of which is novel. EMBO J. 1989;8:1735–1741. doi: 10.1002/j.1460-2075.1989.tb03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neuhaus H, Hu MC, Hemler ME, Takada Y, Holzmann B, Weissman IL. Cloning and expression of cDNAs for the alpha subunit of the murine lymphocyte-Peyer’s patch adhesion molecule. J Cell Biol. 1991;115:1149–1158. doi: 10.1083/jcb.115.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Issekutz TB. Inhibition of in vivo lymphocyte migration to inflammation and homing to lymphoid tissues by the TA-2 monoclonal antibody. A likely role for VLA-4 in vivo. J Immunol. 1991;147:4178–4184. [PubMed] [Google Scholar]
- 37.Yuan QA, Jiang WM, Krissansen GW, Watson JD. Cloning and sequence analysis of a novel beta 2-related integrin transcript from T lymphocytes: homology of integrin cysteine-rich repeats to domain III of laminin B chains. Int Immunol. 1990;2:1097–1108. doi: 10.1093/intimm/2.11.1097. [DOI] [PubMed] [Google Scholar]
- 38.Hu MC, Crowe DT, Weissman IL, Holzmann B. Cloning and expression of mouse integrin beta p(beta 7): a functional role in Peyer’s patch-specific lymphocyte homing. Proc Natl Acad Sci USA. 1992;89:8254–8258. doi: 10.1073/pnas.89.17.8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erle DJ, Briskin MJ, Butcher EC, Garcia-Pardo A, Lazarovits AI, Tidswell M. Expression and function of the MAdCAM-1 receptor, integrin alpha 4 beta 7, on human leukocytes. J Immunol. 1994;153:517–528. [PubMed] [Google Scholar]
- 40.Viney JL, Jones S, Chiu HH, et al. Mucosal addressin cell adhesion molecule-1: a structural and functional analysis demarcates the integrin binding motif. J Immunol. 1996;157:2488–2497. [PubMed] [Google Scholar]
- 41.Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 42.Souza HS, Elia CC, Spencer J, MacDonald TT. Expression of lymphocyte-endothelial receptor-ligand pairs, alpha4beta7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 1999;45:856–863. doi: 10.1136/gut.45.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeuchi M, Baichwal VR. Induction of the gene encoding mucosal vascular addressin cell adhesion molecule 1 by tumor necrosis factor alpha is mediated by NF-kappa B proteins. Proc Natl Acad Sci USA. 1995;92:3561–3565. doi: 10.1073/pnas.92.8.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 45.Oshima T, Pavlick KP, Laroux FS, et al. Regulation and distribution of MAdCAM-1 in endothelial cells in vitro. Am J Physiol Cell Physiol. 2001;281:C1096–C1105. doi: 10.1152/ajpcell.2001.281.4.C1096. [DOI] [PubMed] [Google Scholar]
- 46.Ando T, Jordan P, Wang Y, et al. MAdCAM-1 expression and regulation in murine colonic endothelial cells in vitro. Inflamm Bowel Dis. 2005;11:258–264. doi: 10.1097/01.mib.0000160807.53858.1c. [DOI] [PubMed] [Google Scholar]
- 47.Sasaki M, Elrod JW, Jordan P, et al. CYP450 dietary inhibitors attenuate TNF-alpha-stimulated endothelial molecule expression and leukocyte adhesion. Am J Physiol Cell Physiol. 2004;286:C931–C939. doi: 10.1152/ajpcell.00351.2003. [DOI] [PubMed] [Google Scholar]
- 48.Mizushima T, Sasaki M, Ando T, et al. Blockage of angiotensin II type 1 receptor regulates TNF-alpha-induced MAdCAM-1 expression via inhibition of NF-kappaB translocation to the nucleus and ameliorates colitis. Am J Physiol Gastrointest Liver Physiol. 2010;298:G255–G266. doi: 10.1152/ajpgi.00264.2009. [DOI] [PubMed] [Google Scholar]
- 49.Katada K, Yoshida N, Suzuki T, et al. Dextran sulfate sodium-induced acute colonic inflammation in angiotensin II type 1a receptor deficient mice. Inflamm Res. 2008;57:84–91. doi: 10.1007/s00011-007-7098-y. [DOI] [PubMed] [Google Scholar]
- 50.Sasaki M, Jordan P, Welbourne T, et al. Troglitazone, a PPAR-gamma activator prevents endothelial cell adhesion molecule expression and lymphocyte adhesion mediated by TNF-alpha. BMC Physiol. 2005;5:3. doi: 10.1186/1472-6793-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 52.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 53.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 54.Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 55.Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239. doi: 10.1136/gut.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357:228–238. doi: 10.1056/NEJMoa067594. [DOI] [PubMed] [Google Scholar]
- 57.Marzo-Ortega H, McGonagle D, O’Connor P, Emery P. Efficacy of etanercept for treatment of Crohn’s related spondyloarthritis but not colitis. Ann Rheum Dis. 2003;62:74–76. doi: 10.1136/ard.62.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hutas G. Golimumab, a fully human monoclonal antibody against TNFalpha. Curr Opin Mol Ther. 2008;10:393–406. [PubMed] [Google Scholar]
- 59.Reinisch W, Hommes DW, Van Assche G. A dose escalating, placebo controlled, double blind, single dose and multidose, safety and tolerability study of fontolizumab, a humanised anti-interferon gamma antibody, in patients with moderate to severe Crohn’s disease. Gut. 2006;55:1138–1144. doi: 10.1136/gut.2005.079434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ito H, Takazoe M, Fukuda Y, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology. 2004;126:989–996. doi: 10.1053/j.gastro.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 61.Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135:1130–1141. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 62.Krieglstein CF, Cerwinka WH, Sprague AG, et al. Collagen-binding integrin alpha1beta1 regulates intestinal inflammation in experimental colitis. J Clin Invest. 2002;110:1773–1782. doi: 10.1172/JCI200215256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takazoe M, Watanabe M, Kawaguchi T, et al. Oral alpha-4 integrin Inhibitor (AJM300) in patients with active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2009;136(Suppl 1):1240. [Google Scholar]
- 64.Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353:1912–1925. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- 65.Feagan BG, Greenberg GR, Wild G, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499–2507. doi: 10.1056/NEJMoa042982. [DOI] [PubMed] [Google Scholar]
- 66.Feagan BG, Greenberg GR, Wild G, et al. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol. 2008;6:1370–1377. doi: 10.1016/j.cgh.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 67.Rijcken EM, Laukoetter MG, Anthoni C, et al. Immunoblockade of PSGL-1 attenuates established experimental murine colitis by reduction of leukocyte rolling. Am J Physiol Gastrointest Liver Physiol. 2004;287:G115–G124. doi: 10.1152/ajpgi.00207.2003. [DOI] [PubMed] [Google Scholar]
- 68.Taniguchi T, Tsukada H, Nakamura H, et al. Effects of the anti-ICAM-1 monoclonal antibody on dextran sodium sulphate-induced colitis in rats. J Gastroenterol Hepatol. 1998;13:945–949. doi: 10.1111/j.1440-1746.1998.tb00766.x. [DOI] [PubMed] [Google Scholar]
- 69.Soriano A, Salas A, Sans M, et al. VCAM-1, but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Lab Invest. 2000;80:1541–1551. doi: 10.1038/labinvest.3780164. [DOI] [PubMed] [Google Scholar]