Abstract
Oxidative stress is a major cause of the gastrointestinal damage under physical or psychological stress. Ghrelin exhibits gastroprotective effects and they are supposed to be derived from antioxidant effects. In gastroduodenal mucosal injury, the plasma ghrelin levels increase in response to the demand for gastroduodenal cytoprotection. However, in the condition of Helicobacter pylori-induced gastric mucosal severe atrophy, the plasma ghrelin concentration shifted to lower levels. In diabetic gastroparesis, the regulation of ghrelin secretion is impaired with vagal nerve dysfunction. Selective ghrelin agonist is expected to represent a new class of prokinetic agent. In addition, the plasma ghrelin levels are also enhanced by systemic oxidative stress, and ghrelin exhibits antioxidant effects in many organs, such as heart, pancreas, and lung. This suggests that ghrelin would be an important player as a sensor of systemic oxidative stress.
Keywords: oxidative stress, ghrelin, peptic ulcer, gastroparesis
Introduction
The physiological response to stressor includes an increased activity of the hypothalamic-pituitary-adrenal axis as well as changes in gastrointestinal damage. According to Selye’s formulation of the general adaptation syndrome, an increase in adrenocortical activity should be related to an increase in the incidence of gastric ulceration.(1) The strong candidate for the cause of stress ulcer would be oxidative stress. There are some evidences that not only physical stress, such as surgery and infection, but also psychological stress leads to oxidative stress.(2,3)
Oxidative stress, which refers to a state of elevated levels of reactive oxygen species (ROS), forms a variety of conditions that stimulate either ROS production or a decline in antioxidant defenses. Oxidative stress is involved in the pathogenesis of lifestyle-related diseases, including atherosclerosis, hypertension, diabetes mellitus, ischemic diseases, and malignancies.(4) Several gastrointestinal diseases, such as peptic ulcer disease and gastroparesis, are known to be related with the dysfunction of the anti-oxidative properties.(5)
Ghrelin, produced and secreted by the A-like cells of the oxyntic glands of the stomach, stimulates growth hormone (GH) secretion, gastric motility, and food intake.(6) Many researchers reported the relationship between oxidative stress and the expression or function of ghrelin.(7,8) Moreover, ghrelin administration is expected to reduce oxidative stress and control diseases.(9) Previous studies have reported that ghrelin has an anti-inflammatory action on the oxidative injury of the diverse organs, such as the heart, liver and pancreas.(10–14) In the present article, we discuss the association of oxidative gastrointestinal damages with the potential role of ghrelin.
Effects of Ghrelin against Gastric Mucosal Injury
Recent studies have shown that ghrelin exhibits gastroprotective effects.(15–19) Ghrelin administration reduced ethanol-induced gastric ulceration,(15,17,18) acetic acid-induced chronic gastric and duodenal ulceration,(16) and ischemia-reperfusion (I/R)-induced gastric ulceration(18,19) in rats. In addition, ghrelin administration increased mucosal cell proliferation(16) and mucosal microvascular flow(16,18,20) in rats. These effects could be observed by intracerebroventricular,(15,17,18) subcutaneous,(15) intraperitoneal,(18) and peripheral intravascular (19) administration of synthetic ghrelin.
The mechanism of the gastroprotective effects of ghrelin remains unclear. Sibilia et al. reported that such effects of ghrelin are mediated by endogenous nitric oxide (NO) release and requires the integrity of sensory nerve fibers.(15) Sibilia et al.(17) also reported that cyclooxygenase-1-derived prostaglandins (PGs) are mainly involved in ghrelin-associated gastroprotection and that NO derived from constitutive source, together with PGE2, are involved in its activity. Ceranowicz et al.(16) reported that the gastroprotective effects of ghrelin are mediated by the release of endogenous GH and insulin-like growth factor-1. Brzozowski et al.(18) reported that these effects involved vagal nerve integrity, partially depending upon afferent nerves and hyperemia mediated by sensory neuropeptides such as calcitonin gene-related peptide released from these nerves.
The most remarkable gastroprotective effects of ghrelin are supposed to be derived from its antioxidant effects. Eter et al.(19) reported that peripheral administration of ghrelin attenuated I/R-induced gastric mucosal injury by reducing ulceration, tissue congestion, cellular infiltration and vascular permeability in rats. Serum level of LDH and tissue content of tumor necrosis factor α (TNFα) were markedly reduced by the ghrelin administration. In their study, a decrement of thiobarbituric acid reactive substance (TBARS) and an increment in glutathione were observed, which suggested that ghrelin has an antioxidant activity. In vitro studies, using human polymorphonuclear cells incubated with ghrelin, showed that ghrelin inhibited ROS generation as measured by chemiluminecence.(19) Iseri et al.(21) reported that although alendronate induces oxidative gastric damage by a local irritant effect, ghrelin ameliorates this damage by its possible antioxidant and anti-inflammatory powers.
Ghrelin Secretion and Gastric Mucosal Injury
The most common causes of gastric mucosal injury and peptic ulceration are Helicobacter pylori (H. pylori) infection and the consumption of non-steroidal anti-inflammatory drugs (NSAIDs). H. pylori induces a strong inflammatory response, generating large amounts of ROS during the process of colonizing the host.(20,22–29) In the pathogenesis of NSAID-induced gastric mucosal injury, oxygen radicals also play an important role.(30)
Although ghrelin secretion would be required to protect gastric mucosa against ROS-induced injury, the number of the gastric A-like cells is decreased simultaneously by gastric mucosal injury.(31,32) Therefore, the status of ghrelin secretion in gastric mucosal injury is complicated. The plasma total and active ghrelin levels are known to be increased by the formation of duodenal ulcers, which induced by administration of cysteamine, a somatostatin inhibitor, in rats.(33) In a human study, enhanced plasma ghrelin levels were observed in patients, not only with duodenal ulcers, but also with gastric ulcers.(34) According to the report by Isomoto et al.,(35) amomg plasma ghrelin levels of the patients with chronic gastritis, gastric ulcer, duodenal ulcer, acute gastritis, and normal mucosa, the levels of acute gastritis group were the highest, and then that of chronic gastritis group were the lowest. Within the H. pylori-positive population, the plasma ghrelin levels of duodenal ulcer group were higher than gastric ulcer group or chronic gastritis group.(35) The plasma total and active ghrelin levels correlated with the serum pepsinogen I levels, as well as the serum pepsinogen I/II ratio, and decreased with increasing extent of gastric mucosal atrophy.(36,37) These results suggest that the plasma ghrelin levels increase in response to the severe gastric mucosal oxidative stress. However, in the condition of H. pylori-induced gastric mucosal severe atrophy, the number of A like cells as well as the plasma ghrelin concentration shifted to lower levels with the reduction of other component cells in gastric fundic gland due to inflammatory cell infiltrations.
Gastric Motility Dysfunction and Ghrelin
Oxidative stress induces not only gastric mucosal injury, but also gastric motility dysfunction, such as diabetic gastroparesis. Gastroparesis are thought to be caused by ROS-induced damage of the networks of the interstitial cells of Cajal.(38) The authors reported that the plasma ghrelin levels and gastric preproghrelin mRNA expression levels are increased in rats with streptozotocin-induced diabetes, that is known for hyperphagia.(39) In a human study, however, the fasting plasma ghrelin level was significantly lower in diabetes mellitus with diabetic gastroparesis than in healthy controls.(40) The change in the plasma ghrelin levels with sham feeding in diabetic gastroparesis patients and postsurgical gastroparesis patients were significantly lower than in normal subjects, although the plasma ghrelin levels increased in idiopathic gastroparesis.(41) Impaired regulation of the plasma ghrelin levels in diabetic gastroparesis would be caused by vagal nerve dysfunction.(41)
Ghrelin administration accelerated gastric emptying of a meal in humans even in the presence of a neural dysregulation by diabetes or surgical vagotomy.(42) Also in idiopathic gastroparesis, administration of ghrelin enhances gastric emptying and improves meal-related symptoms.(43) Therefore, analogues of ghrelin are expected to represent a new class of prokinetic agents.(44) TZP-101, the synthetic, selective ghrelin agonist, is now tested in clinical trials. This new agent was well-tolerated in diabetes patients with moderate-to-severe chronic gastroparesis and showed statistically significant improvements in gastric emptying.(45,46)
Systemic Oxidative Stress and Ghrelin
Systemic oxidative stress is induced by various reasons. In diabetes mellitus, NADPH oxidases, endothelial NO synthase uncoupling, and protein kinase C signaling plays an important roles for mediating increased vascular superoxide production and endothelial dysfunction.(47) Smoking stimulates pulmonary alveolar macrophages and increased superoxide production.(48) During sepsis, multiple intracellular sites, such as mitochondrial, NADPH oxidase, and Rac1 pathways, are responsible to the superoxide production.(49)
Several studies suggested that systemic oxidative stress enhance the plasma ghrelin levels. The plasma ghrelin levels were correlated with vascular super oxide. production and NADPH oxidase activity in patients with atherosclerosis.(50) Plasma ghrelin was elevated in cachectic patients with chronic heart failure, associated with increases in GH and TNFα.(51) Smoking acutely increased the plasma ghrelin levels.(52) On the other hand, in the early stage of sepsis, ghrelin levels decreased, although the activity of its receptor was markedly elevated in rats.(53) In patients with acute pancreatitis, the plasma ghrelin levels increased after patients’ recovery, as compared with the levels before therapy.(54) Decreased ghrelin levels in the early phase of sepsis or pancreatitis would be caused by the damage of gastric A like cells. With repairment of the A like cells, the plasma ghrelin levels would recover after sepsis or pancreatitis.
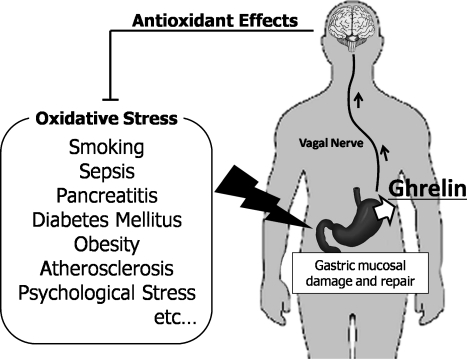
Taken together, it is considered that gastric mucosa would play an important role for sensing a systemic oxidative stress (Fig. 1). Exposure to oxidative stress could lead to gastric mucosal injury, and ghrelin would be released when the A like cells were damaged or repaired. Secreted ghrelin would have an anti-inflammatory action on the oxidative injury of the several organs, such as increasing cardiac output,(55) vasorelaxation,(56) attenuation of acute pancreatic damage,(57) and attenuation of acute lung injury,(58) as well as rapid repairment of gastric epithelial cells. Ghrelin would be also secreted from the stomach as an anti-inflammatory player for the systemic oxidative injury.
Fig. 1.
Ghrelin has antioxidant effects on systemic oxidative stress. Many kinds of systemic oxidative stress could lead to gastric mucosal injury. Ghrelin would be released when the A-like cells were damaged or repaired. Secreted ghrelin send a signal to the brain through the vagal nerve, and enhance the antioxidant reaction in the body.
Conclusions
Ghrelin has the possible antioxidant and anti-inflammatory effects. Selective ghrelin agonist would be expected as a new agent to treat not only gastrointestinal motility dysfunction, but also gastric mucosal injury, cardiovascular disease, and various systemic diseases induced by oxidative stress. The stomach would be an important organ as a sensor of systemic oxidative stress.
Abbreviations
- ROS
reactive oxygen species
- I/R
ischemia-reperfusion
- GH
growth hormone
- TBARS
thiobarbituric acid reactive substance
- NSAIDs
non-steroidal anti-inflammatory drugs
References
- 1.Selye H. The general adaptation syndrome and the diseases of adaptation. Practitioner. 1949;163:393–405. [PubMed] [Google Scholar]
- 2.Schiavone S, Sorce S, Dubois-Dauphin M, et al. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry. 2009;66:384–392. doi: 10.1016/j.biopsych.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Nagahashi S, Suzuki H, Miyazawa M, Nagata H, Suzuki M, Miura S, Ishii H. Ammonia aggravates stress-induced gastric mucosal oxidative injury through the cancellation of cytoprotective heat shock protein 70. Free Radic Biol Med. 2002;33:1073–1081. doi: 10.1016/s0891-5849(02)00998-x. [DOI] [PubMed] [Google Scholar]
- 4.Naito Y, Yoshikawa T. Oxidative stress-induced posttranslational modification of proteins as a target of functional food. Forum Nutr. 2009;61:39–54. doi: 10.1159/000212737. [DOI] [PubMed] [Google Scholar]
- 5.Ohashi Y, Aihara E, Takasuka H, Takahashi K, Takeuchi K. Antral ulcers induced by alendronate, a nitrogen-containing biphophonate, in rat stomachs - prophylactic effect of rebamipide. J Physiol Pharmacol. 2009;60:85–93. [PubMed] [Google Scholar]
- 6.Murray CD, Kamm MA, Bloom SR, Emmanuel AV. Ghrelin for the gastroenterologist: history and potential. Gastroenterology. 2003;125:1492–1502. doi: 10.1016/j.gastro.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Suematsu M, Katsuki A, Sumida Y, et al. Decreased circulating levels of active ghrelin are associated with increased oxidative stress in obese subjects. Eur J Endocrinol. 2005;153:403–407. doi: 10.1530/eje.1.01977. [DOI] [PubMed] [Google Scholar]
- 8.Zwirska-Korczala K, Adamczyk-Sowa M, Sowa P, et al. Role of leptin, ghrelin, angiotensin II and orexins in 3T3 L1 preadipocyte cells proliferation and oxidative metabolism. J Physiol Pharmacol. 2007;58 Suppl 1:53–64. [PubMed] [Google Scholar]
- 9.Kyoraku I, Shiomi K, Kangawa K, Nakazato M. Ghrelin reverses experimental diabetic neuropathy in mice. Biochem Biophys Res Commun. 2009;389:405–408. doi: 10.1016/j.bbrc.2009.08.171. [DOI] [PubMed] [Google Scholar]
- 10.Hou Y, An J, Hu XR, et al. Ghrelin inhibits interleukin-8 production induced by hydrogen peroxide in A549 cells via NF-kappaB pathway. Int Immunopharmacol. 2009;9:120–126. doi: 10.1016/j.intimp.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Hedayati N, Annambhotla S, Jiang J, et al. Growth hormone-releasing peptide ghrelin inhibits homocysteine-induced endothelial dysfunction in porcine coronary arteries and human endothelial cells. J Vasc Surg. 2009;49:199–207. doi: 10.1016/j.jvs.2008.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Z, Lin S, Wu W, et al. Ghrelin prevents doxorubicin-induced cardiotoxicity through TNF-alpha/NF-kappaB pathways and mitochondrial protective mechanisms. Toxicology. 2008;247:133–138. doi: 10.1016/j.tox.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Huang CX, Yuan MJ, Huang H, et al. Ghrelin inhibits post-infarct myocardial remodeling and improves cardiac function through anti-inflammation effect. Peptides. 2009;30:2286–2291. doi: 10.1016/j.peptides.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Dembinski A, Warzecha Z, Ceranowicz P, et al. Role of growth hormone and insulin-like growth factor-1 in the protective effect of ghrelin in ischemia/reperfusion-induced acute pancreatitis. Growth Horm IGF Res. 2006;16:348–356. doi: 10.1016/j.ghir.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Sibilia V, Rindi G, Pagani F, et al. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology. 2003;144:353–359. doi: 10.1210/en.2002-220756. [DOI] [PubMed] [Google Scholar]
- 16.Ceranowicz P, Warzecha Z, Dembinski A, et al. Treatment with ghrelin accelerates the healing of acetic acid-induced gastric and duodenal ulcers in rats. J Physiol Pharmacol. 2009;60:87–98. [PubMed] [Google Scholar]
- 17.Sibilia V, Pagani F, Rindi G, et al. Central ghrelin gastroprotection involves nitric oxide/prostaglandin cross-talk. Br J Pharmacol. 2008;154:688–697. doi: 10.1038/bjp.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brzozowski T, Konturek PC, Sliwowski Z, et al. Neural aspects of ghrelin-induced gastroprotection against mucosal injury induced by noxious agents. J Physiol Pharmacol. 2006;57 Suppl 6:63–76. [PubMed] [Google Scholar]
- 19.El Eter E, Al Tuwaijiri A, Hagar H, Arafa M. In vivo and in vitro antioxidant activity of ghrelin: Attenuation of gastric ischemic injury in the rat. J Gastroenterol Hepatol. 2007;22:1791–1799. doi: 10.1111/j.1440-1746.2006.04696.x. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki H, Suzuki M, Imaeda H, Hibi T. Helicobacter pylori and Microcirculation. Microcirculation. 2009:1–12. doi: 10.1080/10739680902949953. [DOI] [PubMed] [Google Scholar]
- 21.Iseri SO, Sener G, Yuksel M, et al. Ghrelin against alendronate-induced gastric damage in rats. J Endocrinol. 2005;187:399–406. doi: 10.1677/joe.1.06432. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki H, Masaoka T, Miyazawa M, Suzuki M, Miura S, Ishii H. Gastric mucosal response to Helicobacter pylori. Keio J Med. 2002;51 Suppl 2:40–44. doi: 10.2302/kjm.51.supplement2_40. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki H, Miura S, Imaeda H, et al. Enhanced levels of chemiluminescence and platelet activating factor in urease-positive gastric ulcers. Free Radic Biol Med. 1996;20:449–454. doi: 10.1016/0891-5849(96)02048-5. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki H, Mori M, Suzuki M, Sakurai K, Miura S, Ishii H. Extensive DNA damage induced by monochloramine in gastric cells. Cancer Lett. 1997;115:243–248. doi: 10.1016/s0304-3835(97)04745-9. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki H, Seto K, Mori M, Suzuki M, Miura S, Ishii H. Monochloramine induced DNA fragmentation in gastric cell line MKN45. Am J Physiol. 1998;275:G712–716. doi: 10.1152/ajpgi.1998.275.4.G712. [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Olczak A, Forsberg LS, Maier RJ. Oxidative stress-induced peptidoglycan deacetylase in Helicobacter pylori. J Biol Chem. 2009;284:6790–6800. doi: 10.1074/jbc.M808071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki H, Suzuki M, Mori M, et al. Augmented levels of gastric mucosal leucocyte activation by infection with cagA gene-positive Helicobacter pylori. J Gastroenterol Hepatol. 1998;13:294–300. doi: 10.1111/j.1440-1746.1998.01558.x. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki H, Hibi T. Oxidative stress in Helicobacter pylori-associated gastroduodenal disease. J Clin Biochem Nutri. 2006;39:56–63. [Google Scholar]
- 29.Suzuki H, Hibi T, Marshall BJ. Helicobacter pylori: present status and future prospects in Japan. J Gastroenterol. 2007;42:1–15. doi: 10.1007/s00535-006-1990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murata Y, Matsui H, Hirano KI, et al. Autofluorescence in indomethacin-induced gastric mucosal lesions in rats. J Gastroenterol. 2000;35:510–517. doi: 10.1007/s005350070073. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H, Masaoka T, Hosoda H, et al. Helicobacter pylori infection modifies gastric and plasma ghrelin dynamics in Mongolian gerbils. Gut. 2004;53:187–194. doi: 10.1136/gut.2003.021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki H, Hibi T. Does Helicobacter pylori attack ghrelin-producing cells? J Gastroenterol. 2005;40:437–439. doi: 10.1007/s00535-005-1568-1. [DOI] [PubMed] [Google Scholar]
- 33.Fukuhara S, Suzuki H, Masaoka T, et al. Enhanced ghrelin secretion in rats with cysteamine-induced duodenal ulcers. Am J Physiol Gastrointest Liver Physiol. 2005;289:G138–145. doi: 10.1152/ajpgi.00298.2004. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki H, Masaoka T, Nomoto Y, et al. Increased levels of plasma ghrelin in peptic ulcer disease. Aliment Pharmacol Ther. 2006;24 (S4):120–126. [Google Scholar]
- 35.Isomoto H, Ueno H, Nishi Y, et al. Circulating ghrelin levels in patients with various upper gastrointestinal diseases. Dig Dis Sci. 2005;50:833–838. doi: 10.1007/s10620-005-2648-z. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki H, Masaoka T, Hosoda H, et al. Plasma ghrelin concentration correlates with the levels of serum pepsinogen I and pepsinogen I/II ratio--a possible novel and non-invasive marker for gastric atrophy. Hepatogastroenterology. 2004;51:1249–1254. [PubMed] [Google Scholar]
- 37.Kawashima J, Ohno S, Sakurada T, et al. Circulating acylated ghrelin level decreases in accordance with the extent of atrophic gastritis. J Gastroenterol. 2009;44:1046–1054. doi: 10.1007/s00535-009-0120-0. [DOI] [PubMed] [Google Scholar]
- 38.Forster J, Damjanov I, Lin ZY, Sarosiek I, Wetzel P, McCallum RW. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. Gastroenterology. 2003;124:A788–A789. doi: 10.1016/j.gassur.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Masaoka T, Suzuki H, Hosoda H, et al. Enhanced plasma ghrelin levels in rats with streptozotocin-induced diabetes. FEBS Lett. 2003;541:64–68. doi: 10.1016/s0014-5793(03)00306-5. [DOI] [PubMed] [Google Scholar]
- 40.Asai S, Katabami T, Obi N, et al. No ghrelin response to oral glucose in diabetes mellitus with gastroparesis. Endocr J. 2009;56:79–87. doi: 10.1507/endocrj.k08e-169. [DOI] [PubMed] [Google Scholar]
- 41.Gaddipati KV, Simonian HP, Kresge KM, Boden GH, Parkman HP. Abnormal ghrelin and pancreatic polypeptide responses in gastroparesis. Dig Dis Sci. 2006;51:1339–1346. doi: 10.1007/s10620-005-9022-z. [DOI] [PubMed] [Google Scholar]
- 42.Binn M, Albert C, Gougeon A, et al. Ghrelin gastrokinetic action in patients with neurogenic gastroparesis. Peptides. 2006;27:1603–1606. doi: 10.1016/j.peptides.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Tack J, Depoortere I, Bisschops R, Verbeke K, Janssens J, Peeters T. Influence of ghrelin on gastric emptying and meal-related symptoms in idiopathic gastroparesis. Aliment Pharmacol Ther. 2005;22:847–853. doi: 10.1111/j.1365-2036.2005.02658.x. [DOI] [PubMed] [Google Scholar]
- 44.Murray CD, Martin NM, Patterson M, et al. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut. 2005;54:1693–1698. doi: 10.1136/gut.2005.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ejskjaer N, Vestergaard ET, Hellstrom PM, et al. Ghrelin receptor agonist (TZP-101) accelerates gastric emptying in adults with diabetes and symptomatic gastroparesis. Aliment Pharmacol Ther. 2009;29:1179–1187. doi: 10.1111/j.1365-2036.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- 46.Wargin W, Thomas H, Clohs L, et al. Contribution of protein binding to the pharmacokinetics of the ghrelin receptor agonist TZP-101 in healthy volunteers and adults with symptomatic gastroparesis: two randomized, double-blind studies and a binding profile study. Clin Drug Investig. 2009;29:409–418. doi: 10.2165/00044011-200929060-00004. [DOI] [PubMed] [Google Scholar]
- 47.Guzik TJ, Mussa S, Gastaldi D, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus Role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 48.Richter AM, Abboud RT, Johal SS, Fera TA. Acute effect of smoking on superoxide production by pulmonary alveolar macrophages. Lung. 1986;164:233–242. doi: 10.1007/BF02713647. [DOI] [PubMed] [Google Scholar]
- 49.Ritter C, Andrades M, Moreira JC, Dal-Pizzol F, Hussain SN. Superoxide production during sepsis development. Am J Respir Crit Care Med. 2003;167:474–475. doi: 10.1164/ajrccm.167.3.297e. 474; author reply . [DOI] [PubMed] [Google Scholar]
- 50.Guzik TJ, Harrison DG. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov Today. 2006;11:524–533. doi: 10.1016/j.drudis.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Nagaya N, Uematsu M, Kojima M, et al. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation. 2001;104:2034–2038. doi: 10.1161/hc4201.097836. [DOI] [PubMed] [Google Scholar]
- 52.Bouros D, Tzouvelekis A, Anevlavis S, et al. Smoking acutely increases plasma ghrelin concentrations. Clin Chem. 2006;52:777–778. doi: 10.1373/clinchem.2005.065243. [DOI] [PubMed] [Google Scholar]
- 53.Wu R, Zhou M, Cui X, Simms HH, Wang P. Ghrelin clearance is reduced at the late stage of polymicrobial sepsis. Int J Mol Med. 2003;12:777–781. [PubMed] [Google Scholar]
- 54.Liu B, Liu X, Tang C. Change of plasma ghrelin level in acute pancreatitis. Pancreatology. 2006;6:531–535. doi: 10.1159/000096976. [DOI] [PubMed] [Google Scholar]
- 55.Nagaya N, Kangawa K. Ghrelin improves left ventricular dysfunction and cardiac cachexia in heart failure. Curr Opin Pharmacol. 2003;3:146–151. doi: 10.1016/s1471-4892(03)00013-4. [DOI] [PubMed] [Google Scholar]
- 56.Shimizu Y, Nagaya N, Teranishi Y, et al. Ghrelin improves endothelial dysfunction through growth hormone-independent mechanisms in rats. Biochem Biophys Res Commun. 2003;310:830–835. doi: 10.1016/j.bbrc.2003.09.085. [DOI] [PubMed] [Google Scholar]
- 57.Dembinski A, Warzecha Z, Ceranowicz P, et al. Ghrelin attenuates the development of acute pancreatitis in rat. J Physiol Pharmacol. 2003;54:561–573. [PubMed] [Google Scholar]
- 58.Chen J, Liu X, Shu Q, Li S, Luo F. Ghrelin attenuates lipopolysaccharide-induced acute lung injury through NO pathway. Med Sci Monit. 2008;14:BR141–146. [PubMed] [Google Scholar]