Abstract
Lipopolysaccharide-stimulated leukocytes secrete proinflammatory cytokines including tumor necrosis factor-α and interleukin-12. Over-activation of host defense systems may result in severe tissue damage and requires regulation. Granulocyte colony-stimulating factor and interleukin-10 are candidate cytokines for inducing tolerance to lipopolysaccharide re-stimulation. We compared cytokines secreted by lipopolysaccharide-stimulated blood cells from patients who had survived gram negative bacterial pneumonia (Pseudomonas aeruginosa, Escherichia coli or Proteus mirabilis, n = 26) and age-matched healthy volunteers (n = 18). Interleukin-12p70 and tumor necrosis factor-α expression was significantly lower in patients (p = 0.0039 and p<0.001) compared to healthy controls, while granulocyte colony-stimulating factor production was markedly higher in patients (p<0.001). Levels of interleukin-10 were comparable. Granulocyte colony-stimulating factor expression was inversely correlated with interleukin-12p70 (R = −0.71, p<0.001) and tumor necrosis factor-α (R = −0.64, p<0.001) expression; interleukin-10 showed no significant correlation. In unstimulated leukocytes from patients, cAMP levels were significantly raised (p = 0.020) and were correlated inversely with interleukin-12p70 levels (R = −0.81, p<0.001) and directly with granulocyte colony-stimulating factor (R = 0.72, p = 0.0020), matrix metalloproteinase-9 (R = 0.67, p = 0.0067) and interleukin-10 (R = 0.54, p = 0.039) levels. Our results demonstrate that granulocyte colony-stimulating factor production by lipopolysaccharide-stimulated leukocytes is a useful indicator of tolerance induction in surviving pneumonia patients and that measuring cAMP in freshly isolated leukocytes may also be clinically significant.
Keywords: cAMP, granulocyte colony-stimulating factor, tolerance, lipopolysaccharide, interleukin-12
Introduction
Lipopolysaccharides (LPS) in the outer membrane of gram negative bacteria are endotoxins that elicit strong immune responses and act as well-recognized alarm signals in human hosts.(1) Stimulating leukocytes with LPS in vitro induces the secretion of proinflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-12 which kill the bacteria but may also cause severe tissue damage in the host. The regulation of over-activation of this defense system is therefore very important to protect the host, especially when exposed to prolonged or recurrent alarm signals such as LPS.
Critical roles for IL-12 have been identified not only in the T helper type 1 immune response and host defenses against intracellular microorganisms(2,3) but also in combating extracellular microorganisms. Yamamoto et al. showed that IL-12 plays a critical role in the early phase of bacterial pneumonia by promoting the recruitment of neutrophils to infected lung tissues, where TNF-α production is dependent on IL-12, using IL-12p40-knockout mice.(4) IL-12 is a chemotactic factor for both neutrophils and NK cells and induces their adherence to vascular endothelial cells.(5) Preoperative levels of IL-12 secretion by monocytes stimulated in vitro with LPS were significantly lower in patients who developed lethal postoperative sepsis, compared with the survivors.(6) However, IL-12 has been closely associated with lung tissue injury, as the development of bleomycin-induced pneumopathy is prevented by treating normal mice(7) and IL-12p40-knockout mice with anti-IL-12 antibody.(8) IL-12 also reduces the expression of matrix metalloproteinase (MMP)-9, which is associated with angiogenesis.(9)
Granulocyte colony-stimulating factor (G-CSF)(10) and IL-10(11,12) are likely candidates for cytokines that induce tolerance to repeated LPS stimulation. In vivo treatment with recombinant G-CSF not only results in the mobilization of hematopoietic precursor cells(13) and neutrophils(14) but also results in the appearance of immunoregulatory cells, such as type 2 dendritic cells(15) and type 2 helper T cells,(16) in human blood. Pretreatment with G-CSF attenuates the LPS-stimulated secretion of proinflammatory cytokines, such as IL-12 and TNF-α, and simultaneously enhances IL-10 secretion.(17) G-CSF treatment also increases serum protein levels of hepatocyte growth factor(18) and plasma protein levels of MMP-9.(19) In contrast, in neutrophils G-CSF is known to inhibit apoptosis(20) and IL-10 reverses the anti-apoptotic effect of LPS.(21) Taken together, these observations suggest that G-CSF acts not only as an anti-inflammatory cytokine but also as a defense against infections and a promoter of tissue repair.
A response to LPS requires its presentation by CD14 to Toll-like receptor (TLR) 4.(22) Among circulating blood cells, monocytes express relatively high levels of cell surface TLR4 and CD14, while neutrophils also express them both but at low levels. Basophils express TLR4 but not CD14 and eosinophils and most lymphocytes express neither molecule.(23,24) Monocytes are an important source of both the proinflammatory cytokines IL-12 and TNF-α and the anti-inflammatory cytokines G-CSF and IL-10. However, human neutrophils have also been reported to produce physiologically relevant levels of IL-12(24) and IL-10.(25)
In this study, we investigated the production of TNF-α, IL-12, CSF and IL-10 in LPS-tolerant patients who had survived gram negative bacterial pneumonia with no severe inflammatory symptoms. We also measured cyclic adenosine monophosphate (cAMP) in unstimulated leukocytes from these patients to elucidate the relationship between cAMP levels and the profile of cytokines secreted in response to LPS, because recent in vitro studies have shown that intracellular cAMP concentrations in monocytes can determine the secretion of these cytokines(26–28) although LPS itself does not affect cAMP levels.(29)
Materials and Methods
Patients
We enrolled patients over 50 years of age who had survived aspiration pneumonia due to gram negative rods as Pseudomonas aeruginosa (P. aeruginosa), Escherichia coli (E. coli) or Proteus mirabilis (P. mirabilis). All patients had been diagnosed with gram negative bacterial pneumonia from chest X-rays, computed tomography and sputum cultures. Their informed consent was obtained and blood samples were collected at least one week after withdrawal of antibiotics and/or anti-inflammatory drugs. Control blood samples were collected from healthy volunteers who had experienced no episodes of pneumonia within the last 20 years or any infectious diseases within the last 3 months.
Quantifying cAMP levels in leukocytes
Blood (10 ml) was collected using a syringe containing 0.5 ml of heparin (Mochida Pharmaceutical, Tokyo, Japan), mixed with 5 ml 6% dextran and incubated for 30 min at room temperature. The enriched leukocyte fraction was collected and centrifuged at 400 g for 5 min, and any residual erythrocytes were then lysed by adding 3 ml ACK lysing buffer (BioWhittaker, Walkersville, MD) for 7 min at room temperature. After washing three times with PBS, the leukocytes were adjusted to 1 × 106 cells/ml. 200 µl aliquots were lysed with ACK lysing buffer according to the manufacturer’s instructions and used to measure cAMP concentrations with an enzyme-linked immunosorbent assay (ELISA) kit (RPN225, GE Healthcare, Buckinghamshire, UK).
Cell culture
LPS-stimulated supernatants were obtained from 40 µl aliquots of heparinized whole blood, which were mixed with 160 µl DMEM (Life Technologies, Rockville, MD) in the presence of 10 µg/ml LPS (Sigma, St. Louis, MO), in triplicate in flat bottomed 96-well microplates (Becton Dickinson Labware, NJ). After incubation for 24 h at 37°C in humidified air containing 5% CO2, the cells were centrifuged at 400 g for 5 min and the supernatants were collected and stored at −80°C until their use in assays.
Quantifying cytokines secreted by LPS-stimulated leukocytes
The concentrations of IL-12p70 (the active heterodimer of IL-12p40 and p35), TNF-α, G-CSF, IL-10 and MMP-9 in the culture supernatants were quantified using commercial ELISA kits (Biosource International, CA).
Statistical analysis
Data were imported into Statview 5.0 (SAS Institute, Cary, NC) and plotted using log-log, semi-log or linear coordinates. Regression analyses were performed to assess the correlation coefficients (R) and Fisher’s protected least significant difference was used to evaluate the degree of fit of the data to power-law distributions. The Mann-Whitney U test was used to determine the statistical significance of differences between two experimental groups.
Results
Effect of aging on the production of pro-inflammatory and anti-inflammatory cytokines
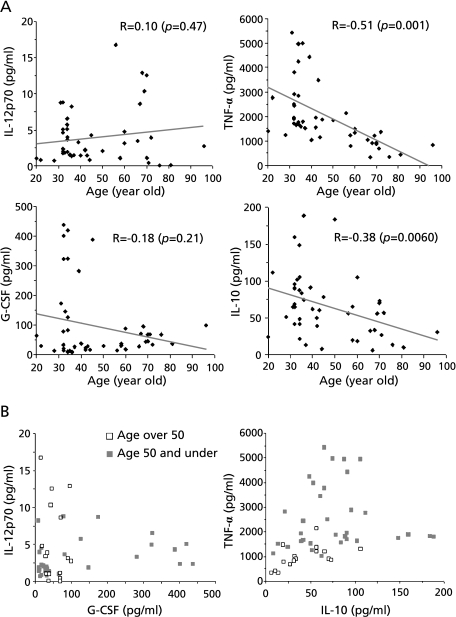
Blood from 47 healthy volunteers between 20 and 96 years of age were stimulated in vitro with LPS and the cytokines secreted were measured. Fig. 1A shows the relationship between the age of the volunteers and the concentration of the pro-inflammatory and anti-inflammatory cytokines secreted. TNF-α production was inversely correlated with age (R = −0.51, p<0.001). IL-10 secretion also dropped with age, but with a lower correlation (R = −0.38, p = 0.0060). In contrast, production of IL-12p70 and G-CSF was not significantly correlated with age, although amongst individuals aged over 50 there were no outliers showing high production of these cytokines. The plots shown in Fig. 1B compare cytokine production in volunteers over 50 (open squares) with those 50 years or younger (closed squares). Interestingly, no older volunteers showed extraordinarily high levels of production of any cytokine, compared to younger volunteers, and there were no individuals who showed exceptionally high levels of both IL-12p70 and G-CSF production in response to LPS.
Fig. 1.
Effect of aging on cytokine production by LPS-stimulated leukocytes. (A) Blood from healthy volunteers (n = 47) ranging from 20 to 96 y of age was incubated in the presence of 10 µg/ml LPS for 24 h and cytokine concentrations in the supernatants were then measured by ELISA. Correlation coefficients (R) were calculated by linear regression analysis. (B) Comparison of data from volunteers aged over 50 y (open squares) and 50 y or under (closed squares).
Cytokine production in patients surviving gram negative bacterial pneumonia
Table 1 shows the clinical data for the patients enrolled in this study. The 26 patients were aged between 54 and 95 years with a median age of 78 years. Sputum cultures identified P. aeruginosa in 17 patients, E. coli in 5 patients, and P. mirabilis in 4 patients. Methicillin-resistant Staphylococcus aureus (MRSA) was also detected in 3 patients. The mean CRP level was 2.6 ± 1.5 mg/dl and the mean leukocyte count in freshly drawn blood was 6,885 ± 2,109 cells/µl. The proportion of neutrophils and monocytes was more than 50% of total leukocytes in all patients. Percutaneous endoscopic gastrostomy tube (PEG) were placed into 19 patient’s stomach and the mean level of total protein (TP) of all the patients was 6.8 ± 0.5 g/dl.
Table 1.
Clinical data for the enrolled patients
| Patient | Age | Sex | Bacteria in sputum cultures | Leukocyte (cells/µl) | CRP (mg/dl) | TP (g/dl) | DM | CVD |
|---|---|---|---|---|---|---|---|---|
| 1 | 54 | Female | E. coli | 2,600 | 1.2 | 7.4 | + | + |
| 2 | 55 | Male | P. aeruginosa | 8,000 | 3.3 | 7.6 | + | + |
| 3 | 67 | Male | P. aeruginosa | 8,400 | 5.8 | 6.7 | + | + |
| 4 | 68 | Male | P. aeruginosa | 8,000 | 3.5 | 6.5 | + | + |
| 5 | 68 | Female | P. aeruginosa | 5,000 | 3.1 | 7.7 | + | + |
| 6 | 70 | Male | P. mirabillis | 6,300 | 1.5 | 6.8 | + | + |
| 7 | 70 | Female | P. aeruginosa | 5,000 | 0.7 | 7.7 | – | + |
| 8 | 72 | Male | P. aeruginosa | 6,500 | 1.4 | 7.7 | + | + |
| 9 | 72 | Male | P. aeruginosa | 6,900 | 1 | 7.1 | + | + |
| 10 | 74 | Male | P. aeruginosa | 9,800 | 4.2 | 6.5 | - | + |
| 11 | 74 | Female | P. aeruginosa | 4,000 | 1 | 6.8 | - | - |
| 12 | 74 | Female | P. aeruginosa | 8,500 | 1.5 | 6.8 | + | + |
| 13 | 77 | Female | P. mirabillis | 7,400 | 2.3 | 6.7 | + | + |
| 14 | 79 | Male | E. coli | 7,400 | 3.7 | 7.1 | – | – |
| 15 | 79 | Female | P. mirabillis, MRSA | 4,300 | 4.3 | 5.9 | – | + |
| 16 | 82 | Female | P. aeruginosa | 8,700 | 4.1 | 6.6 | – | + |
| 17 | 82 | Female | P. aeruginosa | 4,500 | 0.8 | 6.9 | – | + |
| 18 | 84 | Male | P. aeruginosa, MRSA | 11,000 | 3.9 | 7.1 | – | + |
| 19 | 88 | Male | P. aeruginosa | 9,100 | 1.9 | 7.1 | + | + |
| 20 | 88 | Male | P. aeruginosa | 7,600 | 2.8 | 7 | + | – |
| 21 | 90 | Female | E. coli | 8,700 | 1.5 | 5.7 | – | + |
| 22 | 92 | Male | E. coli, MRSA | 7,900 | 4.7 | 6.8 | – | + |
| 23 | 93 | Female | P. aeruginosa | 8,400 | 1.1 | 6.3 | + | + |
| 24 | 94 | Female | P. mirabillis | 5,200 | 3.4 | 7.1 | – | – |
| 25 | 94 | Female | E. coli | 6,500 | 1.2 | 6.3 | – | + |
| 26 | 95 | Female | P. aeruginosa | 3,300 | 4.1 | 6.1 | – | + |
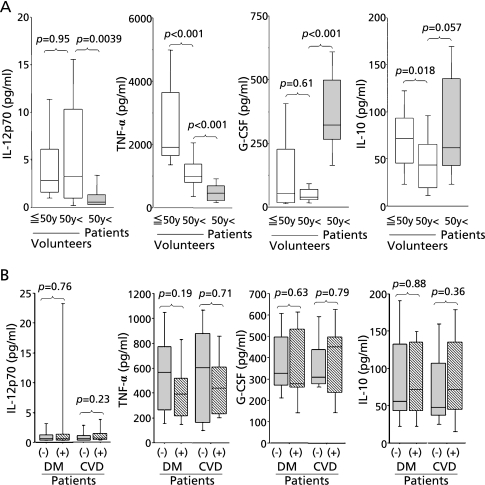
Fig. 2A shows a comparison of the cytokines secreted by LPS-stimulated blood from patients (n = 26), age-matched healthy volunteers over 50 years old (n = 18) and healthy volunteers who were 50 y old or younger (n = 29). In the patient group, production of IL-12p70 was significantly lower (p = 0.0039) than in age-matched healthy volunteers. TNF-α production was also lower in the older, compared to younger, volunteer groups and lower still in the patient group (p<0.001). G-CSF production was markedly higher in patients compared to both healthy volunteer groups (p<0.001), while IL-10 production was similar in all three groups. Amongst the patient group, 13 people suffered from diabetes mellitus (DM) and 22 people suffered from cerebrovascular disease (CVD). However, these possible confounding factors did not significantly affect the cytokine production in these patients, as shown in Fig. 2B.
Fig. 2.
Changes in cytokine production in patients with gram negative bacterial pneumonia. (A) Blood from volunteers aged 50 years and under (n = 29), volunteers aged over 50 years (n = 18) and patients with gram negative bacterial pneumonia (n = 26) was incubated in the presence of 10 µg/ml LPS for 24 h and cytokine concentrations in the supernatants were compared statistically between different pairs of groups. (B) Comparison of cytokine production in patients with or without the additional complications of diabetes mellitus (DM) and cerebrovascular disease (CVD).
Correlation between production of pro-inflammatory and anti-inflammatory cytokines
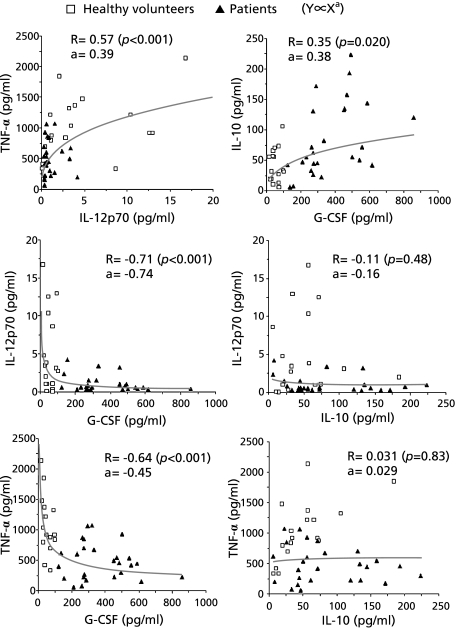
When we analyzed the production of cytokines, we found that the correlation coefficients between any pair of cytokines we measured were higher in log-log (power-law) distributions than in semi-log or linear distributions. Fig. 3 shows curves modeled for the production of cytokine X and cytokine Y using a power-law distribution in which Y is proportional to Xa, where a is the exponent of the power law. The production of IL-12p70 and TNF-α showed a strong direct correlation (R = 0.57, p<0.001, a = 0.39). The production of G-CSF was inversely correlated with the production of both IL-12p70 (R = −0.71, p<0.001, a = −0.74) and also TNF-α (R = −0.64, p<0.001, a = −0.45). These results cannot be fully explained by secondary effects of secreted G-CSF, by an autocrine or paracrine mechanism, because we found that the addition of even a high concentration of recombinant G-CSF to the cultures reduced the secretion of IL-12 by at most 34% and TNF-α by at most 28%. The degree of correlation between G-CSF and IL-10 production was lower (R = 0.35, p = 0.020, a = 0.38) and IL-10 production showed no significant correlation with either IL-12p70 or TNF-α production.
Fig. 3.
Relationship between pro-inflammatory and anti-inflammatory cytokines secreted by LPS-stimulated leukocytes. Blood cells were collected from patients (n = 26, closed triangle) or age-matched healthy volunteers (n = 18, open square), and stimulated with 10 µg/ml LPS for 24 h. Correlations between pairs (X and Y) of the secreted cytokines (TNF-α, IL-12p70, G-CSF, IL-10) were analyzed. The degree of fit to a power-law distribution in which Y was proportional to Xa was calculated. Correlation coefficient (R) between log X and log Y and the slope (a) of the regression line ((log Y)/(log X)) are shown.
Correlation between cytokine and MMP-9 production
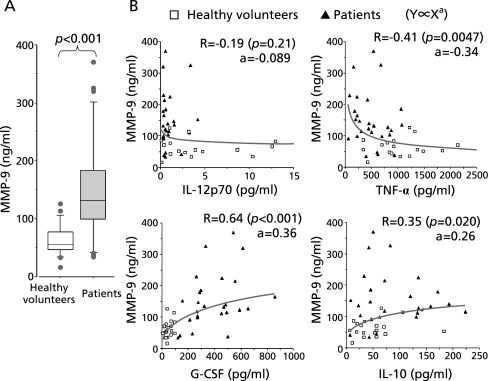
Secretion of MMP-9 by blood cells stimulated with LPS in vitro was significantly higher in the patient group compared to healthy volunteers (p = 0.011; Fig. 4A). As shown in Fig. 4B, MMP-9 production was very closely correlated with G-CSF (R = 0.64, p<0.001, a = 0.36) and IL-10 (R = 0.35, p = 0.020, a = 0.26) production. Conversely, MMP-9 levels were inversely correlated with TNF-α levels (R = −0.41, p = 0.0047, a = −0.34), but there was no significant correlation between MMP-9 and IL-12p70 production.
Fig. 4.
Relationship between MMP-9 and cytokines secreted by LPS-stimulated leukocytes. (A) Blood from age-matched healthy volunteers (n = 18) and patients with gram negative bacterial pneumonia (n = 26) was incubated in the presence of 10 µg/ml LPS for 24 h and cytokine concentrations in the supernatants were compared statistically. (B) Concentrations of cytokines and of MMP-9 secreted by LPS-stimulated blood cells from patients (n = 26, closed triangle) or age-matched healthy volunteers (n = 18, open square) were plotted as X and Y. Correlation coefficients (R) were calculated by fitting the data to a power-law distribution in which Y is proportional to Xa.
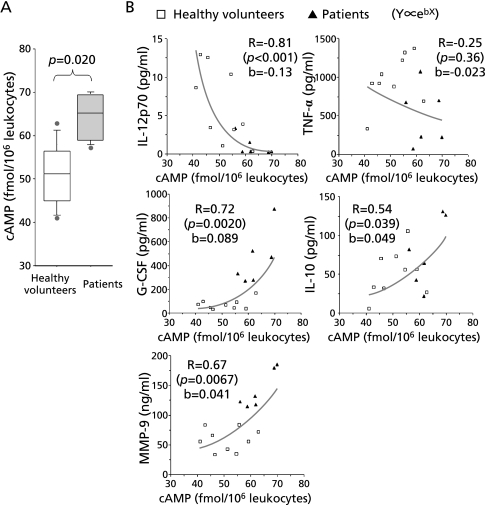
Correlation between cAMP levels in leukocytes and cytokine production
We next quantified and analyzed the cAMP content of freshly isolated leukocytes. As shown in Fig. 5A, cAMP levels were significantly higher in patients (p = 0.020) compared to healthy volunteers. Fig. 5B shows the relationship between cAMP (X) and cytokine production (Y), fitted to exponential curves in which Y is proportional to ebX, where e is the base of the natural logarithm and b is a constant. The levels of cAMP were inversely correlated with IL-12p70 production (R = −0.81, p<0.001, b = −0.13) and directly correlated with G-CSF (R = 0.72, p = 0.0020, b = 0.089), MMP-9 (R = 0.67, p = 0.0067, b = 0.041) and IL-10 (R = 0.54, p = 0.039, b = 0.049) production. These results helped to explain why the relationship between cytokine production follows a power-law relationship. No statistically significant correlation was detected between cAMP and TNF-α levels in this experiment. In particular, the production of IL-12p70 and G-CSF showed similar degrees of sensitivity, as expressed by the values of b, to intracellular cAMP, which explains the inverse relationship seen between IL-12p70 and G-CSF production seen in Fig. 3.
Fig. 5.
Relationship between intracellular cAMP levels and cytokines secreted by LPS-stimulated leukocytes. (A) cAMP level in freshly isolated leukocytes from age-matched healthy volunteers (n = 9) and patients with gram negative bacterial pneumonia (n = 6) were compared statistically. (B) cAMP levels and the concentrations of cytokines and MMP-9 from blood cells stimulated with LPS in vitro were plotted as X and Y and fitted to an exponential distribution in which Y is proportional to ebx. Correlation coefficients (R) between X and log Y and the slope of the regression line ((log Y)/X) (b) were calculated. Samples were from patients (n = 6, closed triangle) or age-matched healthy volunteers (n = 9, open square).
Discussion
LPS-stimulated monocytes from elderly humans have been reported to produce low amounts of cytokines, including TNF-α and G-CSF.(30) A significant association with age has also been reported for IL-12, but not IL-10, expression in feline monocytes.(31) In this study, using whole blood cells from 47 healthy human volunteers, we have demonstrated that the LPS-induced secretion of TNF-α and IL-10 is reduced with age, although we did not detect any statistically significant changes in IL-12 or G-CSF expression. Furthermore, we found that most leukocytes from people showing high IL-12 production secreted very small amounts of G-CSF and vice versa.
Chen et al. reported that patients with acute bacterial infections who had low serum G-CSF concentrations on hospital admission were more likely to die.(32) Indeed maintaining high levels of G-CSF is known to be important for sustaining hematopoiesis in emergencies and for neutrophil differentiation, although it should be emphasized that G-CSF also has a regulatory role in controlling over-activation of host defense systems. TNF-α has an essential role in the pathogenesis associated with septic shock and in disseminated intravascular coagulation (DIC).(33) However, Weiss et al. reported that in vitro, the LPS-inducible secretion of TNF-α is downregulated and the secretion of G-CSF is upregulated in patients with septic shock.(34) Since G-CSF upregulation occurs earlier in the survivors than in the non-survivors, a rapidly elevated and sustained G-CSF response might contribute to the regulation of inflammation and so facilitate survival in endotoxin shock.(30) It is well known that G-CSF administration improves survival in animal models of sepsis and also endotoxin shock even when administered therapeutically after the onset of sepsis.(35)
In terms of anti-inflammatory effects, IL-10, as well as G-CSF, is a candidate cytokine for inducing tolerance to alarm signals such as LPS. In vitro the inhibitory effects of IL-10 on the production of IL-12 and TNF-α are much greater than the effects of G-CSF, on a molar basis. Nevertheless, our study of gram negative bacterial pneumonia has clearly demonstrated that the production of IL-12 and TNF-α by the patients’ leukocytes was inversely related to G-CSF production and not significantly related to IL-10 production. These results showed the significance of G-CSF in protecting hosts from tissue injury resulting from prolonged or repetitive alarm signals. G-CSF has been widely accepted to enhance the healing process in skin wounds(36,37) as well as in cardiac infarction,(38) but there has been no evidence for a direct role for IL-10 in tissue repair. MMP-9 has been shown to have an active role in tissue repair in the liver.(39) In the lung, Choi et al. have reported a role for MMP-9 in tissue repair in cryptogenic organizing pneumonia.(40) In the more common interstitial pneumonia, pulmonary structure is extensively remodeled, whereas in cryptogenic organizing pneumonia architectural changes are minimal; levels of MMP-9 in broncho-alveolar lavage fluid are also higher in these patients. MMP-9 production was high in the patients recovering from pneumonia in our study and was more closely correlated with G-CSF production than IL-10 production.
The results of this study cannot in our view be adequately explained by a mild regulatory effect of secreted G-CSF in vivo. There is increasing evidence that intracellular cAMP levels determine cell functions such as patterns of cytokine production. For example, cAMP-elevating agents have been shown to enhanced G-CSF promoter activity and attenuation of TNF-α production in a human monocytic cell line,(26) upregulate IL-10 mRNA expression in human peripheral blood mononuclear cells(27) and downregulate IL-12p40 mRNA levels in murine peritoneal macrophages.(28) Taken together, the upregulation of intracellular cAMP levels results in increased G-CSF and IL-10 expression and reduced IL-12 and TNF-α expression in vitro. Notably, recombinant G-CSF has been shown to increase intracellular cAMP levels in human PBMC in a dose-dependent fashion.(41) These reports provide a molecular basis, supporting our in vivo results, for the production of G-CSF and proinflammatory cytokines (IL-12, TNF-α) being inversely related in leukocytes, while also suggesting the importance of a positive feedback circuit in which secreted G-CSF enhances intracellular cAMP levels.
Chemical mediators synthesized during host inflammatory response, such as prostaglandin E2, histamine and the catecholamines, have been reported to increase intracellular cAMP levels and suppress IL-12 production in monocytes and monocyte-derived dendritic cells.(42–45) In addition, catecholamines potentiate the LPS-induced expression of MMP-9 in human monocytes.(46) Thus the host response to repeated alarm signals such as LPS involves elevating cAMP levels and promoting both anti-inflammatory and tissue repair systems. This mechanism could be a reasonable host adaptation for surviving severe acute inflammatory episodes, even though prolonged suppression of normal host responses might be expected to cause susceptibility to infection and carcinogenesis.
We have shown in this study that both G-CSF production by leukocytes stimulated with LPS in vitro and also the cAMP levels in freshly isolated leukocytes are useful indices of tolerance induction in patients who have survived gram negative bacterial pneumonia. From a clinical viewpoint, it is much more significant that we can predict the tolerant status of patients by simply measuring cAMP levels in leukocytes, as culturing cells with LPS is more time-consuming and costly.
References
- 1.Glauser MP, Zanetti G, Baumgartner JD, Cohen J. Septic shock: pathogenesis. Lancet. 1991;338:732–736. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- 2.Decken K, Köhler G, Palmer-Lehmann K, et al. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper AM, Magram J, Ferrante J, Orme IM. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto N, Kawakami K, Kinjo Y, et al. Essential role for the p40 subunit of interleukin-12 in neutrophil-mediated early host defense against pulmonary infection with Streptococcus pneumoniae: involvement of interferon-gamma. Microbes Infect. 2004;6:1241–1249. doi: 10.1016/j.micinf.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Allavena P, Paganin C, Zhou D, Bianchi G, Sozzani S, Mantovani A. Interleukin-12 is chemotactic for natural killer cells and stimulates their interaction with vascular endothelium. Blood. 1994;84:2261–2268. [PubMed] [Google Scholar]
- 6.Weighardt H, Heidecke CD, Westerholt A, et al. Impaired monocyte IL-12 production before surgery as a predictive factor for the lethal outcome of postoperative sepsis. Ann Surg. 2002;235:560–567. doi: 10.1097/00000658-200204000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maeyama T, Kuwano K, Kawasaki M, Kunitake R, Hagimoto N, Hara N. Attenuation of bleomycin-induced pneumopathy in mice by monoclonal antibody to interleukin-12. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1128–L1137. doi: 10.1152/ajplung.2001.280.6.L1128. [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto H, Zhao LH, Jain F, Kradin R. IL-12p40(-/-) mice treated with intratracheal bleomycin exhibit decreased pulmonary inflammation and increased fibrosis. Exp Mol Pathol. 2002;72:1–9. doi: 10.1006/exmp.2001.2409. [DOI] [PubMed] [Google Scholar]
- 9.Mitola S, Strasly M, Prato M, Ghia P, Bussolino F. IL-12 regulates an endothelial cell-lymphocyte network: effect on metalloproteinase-9 production. J Immunol. 2003;171:3725–3733. doi: 10.4049/jimmunol.171.7.3725. [DOI] [PubMed] [Google Scholar]
- 10.Hartung T, Döcke WD, Gantner F, et al. Effect of granulocyte colony-stimulating factor treatment on ex vivo blood cytokine response in human volunteers. Blood. 1995;85:2482–2489. [PubMed] [Google Scholar]
- 11.D’Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiorentino DF, Zlotnik A, Mosmann TR, et al. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 13.Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 14.Morstyn G, Campbell L, Souza LM, et al. Effect of granulocyte colony stimulating factor on neutropenia induced by cytotoxic chemotherapy. Lancet. 1988;1:667–672. doi: 10.1016/s0140-6736(88)91475-4. [DOI] [PubMed] [Google Scholar]
- 15.Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 2000;95:2484–2490. [PubMed] [Google Scholar]
- 16.Pan L, Delmonte J, Jr., Jalonen CK, Ferrara JL. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86:4422–4429. [PubMed] [Google Scholar]
- 17.Saito M, Kiyokawa N, Taguchi T, et al. Granulocyte colony-stimulating factor directly affects human monocytes and modulates cytokine secretion. Exp Hematol. 2002;30:1115–1123. doi: 10.1016/s0301-472x(02)00889-5. [DOI] [PubMed] [Google Scholar]
- 18.Fujii K, Ishimaru F, Kozuka T, et al. Elevation of serum hepatocyte growth factor during granulocyte colony-stimulating factor-induced peripheral blood stem cell mobilization. Br J Haematol. 2004;124:190–194. doi: 10.1046/j.1365-2141.2003.04745.x. [DOI] [PubMed] [Google Scholar]
- 19.Pelus LM, Bian H, King AG, Fukuda S. Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4. Blood. 2004;103:110–119. doi: 10.1182/blood-2003-04-1115. [DOI] [PubMed] [Google Scholar]
- 20.Hareng L, Hartung T. Induction and regulation of endogenous granulocyte colony-stimulating factor formation. Biol Chem. 2002;383:1501–1517. doi: 10.1515/BC.2002.172. [DOI] [PubMed] [Google Scholar]
- 21.Ward C, Murray J, Clugston A, Dransfield I, Haslett C, Rossi AG. Interleukin-10 inhibits lipopolysaccharide-induced survival and extracellular signal-regulated kinase activation in human neutrophils. Eur J Immunol. 2005;35:2728–2737. doi: 10.1002/eji.200425561. [DOI] [PubMed] [Google Scholar]
- 22.Sabroe I, Jones EC, Usher LR, Whyte MK, Dower SK. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol. 2002;168:4701–4710. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- 23.Dasari P, Nicholson IC, Hodge G, Dandie GW, Zola H. Expression of toll-like receptors on B lymphocytes. Cell Immunol. 2005;236:140–145. doi: 10.1016/j.cellimm.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Cassatsella MA, Meda L, Gasperini S, D’Andrea A, Ma X, Trinchieri G. Interleukin-12 production by human polymorphonuclear leukocytes. Eur J Immunol. 1995;25:1–5. doi: 10.1002/eji.1830250102. [DOI] [PubMed] [Google Scholar]
- 25.Köller M, Clasbrummel B, Kollig E, Hahn MP, Muhr G. Major injury induces increased production of interleukin-10 in human granulocyte fractions. Langenbecks Arch Surg. 1998;383:460–465. doi: 10.1007/s004230050161. [DOI] [PubMed] [Google Scholar]
- 26.Hareng L, Meergans T, von Aulock S, Volk HD, Hartung T. Cyclic AMP increases endogenous granulocyte colony-stimulating factor formation in monocytes and THP-1 macrophages despite attenuated TNF-alpha formation. Eur J Immunol. 2003;33:2287–2296. doi: 10.1002/eji.200323923. [DOI] [PubMed] [Google Scholar]
- 27.Platzer C, Meisel C, Vogt K, Platzer M, Volk HD. Up-regulation of monocytic IL-10 by tumor necrosis factor-alpha and cAMP elevating drugs. Int Immunol. 1995;7:517–523. doi: 10.1093/intimm/7.4.517. [DOI] [PubMed] [Google Scholar]
- 28.Feng WG, Wang YB, Zhang JS, Wang XY, Li CL, Chang ZL. cAMP elevators inhibit LPS-induced IL-12 p40 expression by interfering with phosphorylation of p38 MAPK in murine peritoneal macrophages. Cell Res. 2002;12:331–337. doi: 10.1038/sj.cr.7290135. [DOI] [PubMed] [Google Scholar]
- 29.Katakami Y, Nakao Y, Koizumi T, Katakami N, Ogawa R, Fujita T. Regulation of tumour necrosis factor production by mouse peritoneal macrophages: the role of cellular cyclic AMP. Immunology. 1988;64:719–724. [PMC free article] [PubMed] [Google Scholar]
- 30.Gon Y, Hashimoto S, Hayashi S, Koura T, Matsumoto K, Horie T. Lower serum concentrations of cytokines in elderly patients with pneumonia and the impaired production of cytokines by peripheral blood monocytes in the elderly. Clin Exp Immunol. 1996;106:120–126. [PubMed] [Google Scholar]
- 31.Kipar A, Baptiste K, Meli ML, et al. Age-related dynamics of constitutive cytokine transcription levels of feline monocytes. Exp Gerontol. 2005;40:243–248. doi: 10.1016/j.exger.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen YM, Whang-Peng J, Chern CH, Kuo BI, Wang SY, Perng RP. The prognostic value of serum cytokine levels in patients with acute infections. Zhonghua Yi Xue Za Zhi (Taipei) 1995;56:75–79. [PubMed] [Google Scholar]
- 33.Wada H, Ohiwa M, Kaneko T, et al. Plasma level of tumor necrosis factor in disseminated intravascular coagulation. Am J Hematol. 1991;37:147–151. doi: 10.1002/ajh.2830370302. [DOI] [PubMed] [Google Scholar]
- 34.Weiss M, Fischer G, Barth E, et al. Dissociation of LPS-induced monocytic ex vivo production of granulocyte colony-stimulating factor (G-CSF) and TNF-alpha in patients with septic shock. Cytokine. 2001;13:51–54. doi: 10.1006/cyto.2000.0796. [DOI] [PubMed] [Google Scholar]
- 35.Lundblad R, Nesland JM, Giercksky KE. Granulocyte colony-stimulating factor improves survival rate and reduces concentrations of bacteria, endotoxin, tumor necrosis factor, and endothelin-1 in fulminant intra-abdominal sepsis in rats. Crit Care Med. 1996;24:820–826. doi: 10.1097/00003246-199605000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Peter FW, Schuschke DA, Barker JH, et al. The effect of severe burn injury on proinflammatory cytokines and leukocyte behavior: its modulation with granulocyte colony stimulating factor. Burns. 1999;25:477–486. doi: 10.1016/s0305-4179(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 37.Gough A, Clapperton M, Rolando N, Foster AV, Philpott-Howard J, Edmonds ME. Randomised placebo-controlled trial of granulocyte-colony stimulating factor in diabetic foot infection. Lancet. 1997;350:855–859. doi: 10.1016/S0140-6736(97)04495-4. [DOI] [PubMed] [Google Scholar]
- 38.Minatoguchi S, Takemura G, Chen XH, et al. Acceleration of the healing process and myocardial regeneration may be important as a mechanism of improvement of cardiac function and remodeling by postinfarction granulocyte colony–stimulating factor treatment. Circulation. 2004;109:2572–2580. doi: 10.1161/01.CIR.0000129770.93985.3E. [DOI] [PubMed] [Google Scholar]
- 39.Haruyama T, Ajioka I, Akaike T, Watanabe Y. Regulation and significance of hepatocyte-derived matrix metalloproteinases in liver remodeling. Biochem Biophys Res Commun. 2000;272:681–686. doi: 10.1006/bbrc.2000.2837. [DOI] [PubMed] [Google Scholar]
- 40.Choi KH, Lee HB, Jeong MY, et al. The role of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in cryptogenic organizing pneumonia. Chest. 2002;121:1478–1485. doi: 10.1378/chest.121.5.1478. [DOI] [PubMed] [Google Scholar]
- 41.Kitabayashi A, Hirokawa M, Hatano Y, et al. Granulocyte colony-stimulating factor downregulates allogeneic immune responses by posttranscriptional inhibition of tumor necrosis factor-alpha production. Blood. 1995;86:2220–2227. [PubMed] [Google Scholar]
- 42.Iwasaki K, Noguchi K, Endo H, Kondo H, Ishikawa I. Prostaglandin E2 downregulates interleukin-12 production through EP4 receptors in human monocytes stimulated with lipopolysaccharide from Actinobacillus actinomycetemcomitans and interferon-gamma. Oral Microbiol Immunol. 2003;18:150–155. doi: 10.1034/j.1399-302x.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 43.Gutzmer R, Diestel C, Mommert S, et al. Histamine H4 receptor stimulation suppresses IL-12p70 production and mediates chemotaxis in human monocyte-derived dendritic cells. J Immunol. 2005;174:5224–5232. doi: 10.4049/jimmunol.174.9.5224. [DOI] [PubMed] [Google Scholar]
- 44.Haskó G, Szabó C, Németh ZH, Deitch EA. Dopamine suppresses IL-12 p40 production by lipopolysaccharide-stimulated macrophages via a beta-adrenoceptor-mediated mechanism. J Neuroimmunol. 2002;122:34–39. doi: 10.1016/s0165-5728(01)00459-3. [DOI] [PubMed] [Google Scholar]
- 45.Agarwal SK, Marshall GD., Jr Beta-adrenergic modulation of human type-1/type-2 cytokine balance. J Allergy Clin Immunol. 2000;105:91–98. doi: 10.1016/s0091-6749(00)90183-0. [DOI] [PubMed] [Google Scholar]
- 46.Speidl WS, Toller WG, Kaun C, et al. Catecholamines potentiate LPS-induced expression of MMP-1 and MMP-9 in human monocytes and in the human monocytic cell line U937: possible implications for peri-operative plaque instability. FASEB J. 2004;18:603–605. doi: 10.1096/fj.03-0454fje. [DOI] [PubMed] [Google Scholar]