Abstract
Liver steatosis is associated with organ dysfunction after hepatic resection and transplantation which may be caused by hepatic ischemia/reperfusion injury. The aim of the current study was to determine the precise mechanism leading to hepatocyte apoptosis after steatotic liver ischemia/reperfusion. Using a murine model of partial hepatic ischemia for 90 min, we examined the levels and pathway of apoptosis, and the peroxynitrite expression, serum alanine aminotransferase levels, and liver histology 1 and 4 h after reperfusion. In the steatotic liver, the peroxynitrite expression increased after ischemia/reperfusion. Significant hepatocyte apoptosis in the steatotic liver was seen after reperfusion, caused by upregulation of cleaved caspases 9 and 3, but not caspase 8. Serum alanine aminotransferase levels were elevated and histological examination revealed severe liver injury in the steatotic liver 4 h after reperfusion. In mice treated with aminoguanidine, ischemia/reperfusion-induced increases in serum alanine aminotransferase levels and apoptosis were significantly reduced in steatotic liver compared with mice treated with phosphate buffered saline. Survival of mice with steatotic livers significantly improved by treatment with aminoguanidine. Our data suggested that the steatotic liver is vulnerable to hepatic ischemia/reperfusion, leading to significant hepatocyte apoptosis by the mitochondrial permeability transition, and thereby resulting in organ dysfunction.
Keywords: steatosis, apoptosis, peroxynitrite, hepatic resection
Introduction
Recently, the number of patients with hepatic steatosis has increased due to alcohol abuse and non-alcoholic fatty liver disease (NAFLD).(1) There are several metabolic disorders involved in the steatotic liver, including oxidative stress, susceptibility to apoptosis, and dysfunction of the mitochondria.(2–4) Steatotis in the liver is associated with postoperative complications after hepatic resection and primary graft nonfunction after liver transplantation.(5–7) Furthermore, the current shortage of organ donors leads to an increase in use of steatotic livers for liver transplantation.(8,9) Steatohepatitis after neoadjuvant chemotherapy for hepatic malignancies is another clinical concern for liver damage and insufficiency of liver regeneration after hepatic resection.(10) For these reasons, further investigation of the cause of liver dysfunction in the steatotic liver after hepatic resection and transplantation is necessary.
One of the major causes of liver dysfunction/damage after hepatic surgery for a steatotic liver is hepatic ischemia/reperfusion injury. In experimental studies, hepatic ischemia/reperfusion injury is caused by the ischemic stress itself, the production of reactive oxygen species, inflammatory responses induced by proinflammatory mediators, neutrophil-mediated proteases, microcirculatory disturbance, and apoptosis of hepatocytes.(11–13)
Nitric oxide (NO) is the metabolic product by NO synthase (NOS). Three isoforms of NOS have been identified; endothelial NOS (eNOS), neural NOS, and inducible NOS (iNOS). NO derived from eNOS has protective effects through maintenance of microcirculation, but NO derived from iNOS is harmful due to production of hazardous reactive oxygen species, such as peroxynitrite.(14) Such hazardous reactive oxygen species may lead to hepatocyte apoptosis which is considered to be an important factor in hepatic ischemia/reperfusion injury.(15) There are two main pathways leading to apoptosis. The type 1 (extrinstic) signaling pathway results from a death signal caused by the expression of tumor necrosis factor-alpha and Fas ligand.(16) Tumor necrosis factor-alpha and Fas associated death domains promote the binding of procaspase 8, and the subsequent proteolytic activation of catalytic caspase 8. Caspase 8 is able to activate procaspase 3, leading to apoptosis.(17) Conversely, the type 2 (intrinstic) signaling pathway is induced by the mitochondrial permeability transition, leading to cytochrome c release.(17,18) The cytochrome c complex activates caspase 9 followed by the cleavage and subsequent activation of caspase 3.(19) Apoptosis requires adenosine triphosphate (ATP), and a switch from apoptosis to necrosis occurs when cells are devoid of ATP.(20) Although much is known regarding hepatic ischemia/reperfusion injury in the normal liver, the cause of steatotic liver ischemia/reperfusion injury has not been thoroughly elucidated. We hypothesized that excessive peroxynitrite production associated with iNOS expression leads to hepatocyte apoptosis, thus resulting in hepatic ischemia/reperfusion injury in the steatotic liver. The aim of the current study was to determine whether hepatocyte apoptosis is augmented in the steatotic liver after ischemia/reperfusion and to examine the production of peroxinitrite and the possible involvement of apoptosis after hepatic ischemia/reperfusion.
Materials and Methods
Male BKS.Cg-m/+Leprdb mice, genotype Leprdb/Leprdb(db/db mouse, JAX MICE Tsukuba Charles River Laboratories Japan, Inc., Tsukuba, Japan) weighing 37 to 42 g were used as the fatty liver (FL) group and lean littermates weighing 22 to 25 g were used as the wild type (WT) group. The mice were housed in a controlled environment, exposed to a 12-h light/dark cycle, and provided with commercial chow and water ad libitum. This project was approved by the Chiba University Animal Care and Use Committee and was in compliance with National Institutes of Health guidelines.
Hepatic ischemia/reperfusion model and reagents
The model of partial hepatic ischemia and reperfusion employed was prepared as described previously.(11) Briefly, mice were anesthetized with sodium pentobarbital (60 mg/kg administered intraperitoneally). A midline laparotomy was performed and an atraumatic clip was used to interrupt the arterial and the portal venous blood supply to the left lateral and median lobes of the liver for 90 min. Sham control mice underwent the same procedure but without vascular occlusion. The mice were sacrificed prior to surgery (baseline), 1 and 4 h after reperfusion, and liver tissue and blood samples were taken for analysis (n = 6, respectively). In the iNOS inhibition experiment, prior to partial ischemia, mice received either 25 mg/kg of aminoguanidine (AG) in sterile phosphate buffered saline (0.1 ml) or just the phosphate buffered saline (0.1 ml) (Sigma Aldrich, St. Louis, MO). The doses were adopted based on the study by Cottart et al.(21) Survival was examined up to 8 h after reperfusion (n = 6, respectively).
Analysis of apoptosis and histological examination
Liver tissue specimens were obtained, and sections of formalin-fixed paraffin-embedded liver samples were stained with hematoxylin-eosin to assess the degree of liver injury. In order to quantify the apoptotic hepatocytes, we used the terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP) biotin nick end-labeling (TUNEL) method, which labels the DNA double-strand breaks characteristics of apoptosis.(22) The assay was carried out by utilizing an in situ apoptosis detection kit (Wako Pure Chemical, Co. Ltd., Osaka, Japan) according to the manufacture’s instructions. For quantitative evaluation for apoptotic index, percent ratios of TUNEL-positive hepatocyte to the total number of hepatocytes were calculated for each sample in five random fields (400×).
Western blot analysis
Liver tissue specimens were obtained and immediately frozen in liquid nitrogen. Frozen liver tissues were homogenized in lysis buffer containing 10 mM HEPES, pH 7.9, 150 mM NaCl, 1 mM EDTA, 0.6% NP-40, 0.5 mM phenylmethylsulfonyl fluoride, 1 µg/ml leupeptin, 1 µg/ml aprotinin, 10 µg/ml soybean trypsin inhibitor, and 1 µg/ml pepstatin on ice. The homogenates were sonicated and centrifuged at 5,000 rpm to remove cellular debris. The protein concentration was determined using the BCA Protein Assay kit (Pierce Chemical Co. Rockford, IL). Liver lysate protein (20 µg) was subjected to the XV PANTERA system (DRC, Tokyo, Japan). Samples were electrophoresed in a precast 7.5–15% gradient XV PANTERA Gel, and transferred to a polyvinylidene difluoride membrane. Nonspecific binding sites were blocked with TBS (40 mM Tris, pH 7.6, 300 mM NaCl) with 0.1% Tween 20 containing 0.5% nonfat dry milk, for 1 h at room temperature. The membranes were then incubated with antibodies to rabbit polyclonal anti-mouse actin, caspase 9 (Santa Cruz Biotechnology, Santa Cruz, CA), caspase 3, or caspase 8 (CHEMICON International Inc., Temecula, CA) in TBST. After five washes in TBST, membranes were incubated with horseradish peroxidase-conjugated donkey anti-rabbit IgG. Immunoreactive proteins were detected by enhanced chemiluminescence according to the manufacturer’s instructions, and were quantitated with image analysis software (NIH image, Bethesda, MD). The quantification data evaluated by the band-intensity ratio of cleaved caspase 3, caspase 8, and caspase 9 were normalized to that of actin. Results are representative of two independent experiments.
Electrophoretic mobility shift assay (EMSA)
The nuclear extracts of liver tissue were prepared as described previously.(23) The protein concentrations were determined by a bicinchoninic acid assay with trichloroacetic acid precipitation, using BSA as a reference standard. Double-stranded NF-κB consensus oligonucleotide (Promega, Madison, WI) was end-labeled with [γ−32P] ATP (3,000 Ci/mmol, Amersham, Arlington Heights, IL). Binding reactions containing equal amounts of protein (20 µg) and 35 fmols (~50,000 cpm, Cherenkov counting) of oligonucleotide were performed for 30 min in binding buffer (4% glycerol, 1 mM MgCl2, 0.5 mM EDTA, pH 8.0, 0.5 mM dithiothreitol, 50 mM NaCl, 10 mM Tris, pH 7.6, 50 µg/ml poly (dI•dC); Pharmacia, Piscataway, NJ). The reaction volumes were held constant at 15 µl. The reaction products were separated on a 4% polyacrylamide gel and analyzed by autoradiography.
Blood and ELISA quantification of tissue proteins
Blood was obtained by cardiac puncture at the time of sacrifice. Serum samples were analyzed for alanine aminotransferase (ALT) as an index of hepatocellular injury, using a diagnostic kit (Wako Pure Chemical, Japan). Hepatic nitrotyrosine production in the liver was quantitatively assessed by methods described elsewhere.(23) Briefly, specimens were obtained and frozen immediately, and then were homogenized in 10 volumes of homogenization buffer (10 mM ethylenediaminetetraacetic acid, 2 mM phenylmethylsulfonyl fluoride, 0.1 mg/ml soybean trypsin inhibitor, 1.0 mg/ml bovine serum albumin, 0.02% sodium azide, and 0.2 µl/ml protease inhibitor cocktail [1,000 × stock; 1 mg/ml leupeptin, 1 mg/ml aprotinin, and 1 mg/ml pepstatin]). After incubating for 2 h at 4°C, the homogenate was centrifuged at 12,500 g for 10 min. Supernatant was removed and centrifuged again to obtain clear lysate. Total protein concentration of each sample was measured by using a bicinchoninic assay kit, and samples were dispensed for nitrotyrosine EIA kit (OXIS International Inc., Foster City, CA). Protein concentration was calculated per mg total protein.
Statistical analysis
All data were analyzed for statistical significance using the Mann-Whitney test or were analyzed with a one-way analysis of variance and individual group means were then compared with a Student-Newman-Keuls test. All data were expressed as the mean ± SEM. Overall survival was calculated using the Kaplan-Meier method, and comparisons were evaluated using the log rank test. The data were analyzed by using the SigmaStat 3.0 or SPSS 11.5 software program. A value of p<0.05 was considered to be statistically significant.
Results
Liver apoptosis during ischemia/reperfusion
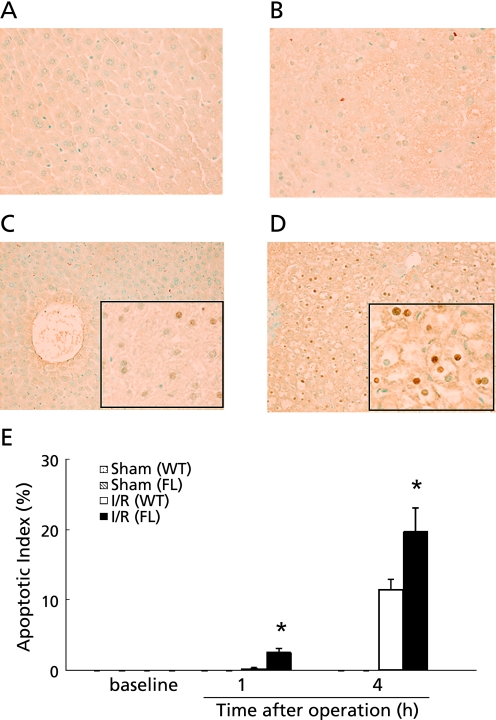
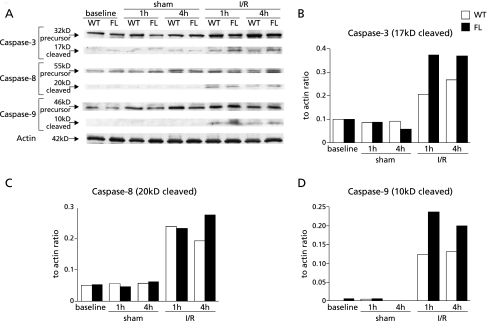
TUNEL staining was performed to investigate whether hepatocyte apoptosis was induced after reperfusion. The number of TUNEL-positive apoptotic hepatocytes quantified by calculating the apoptotic index in FL mice increased significantly compared with that in sham mice 1 h after reperfusion (n = 6, respectively). This increase was not observed in WT mice. A significant increase in TUNEL-positive apoptotic cells in FL mice was seen relative to WT mice 1 and 4 h after reperfusion (Fig. 1). To determine the precise apoptosis pathway activated during ischemia/reperfusion, we performed western blot analysis of precursor and cleaved caspases-3, -8, and -9. In FL mice, cleavage of caspase-3 and -9 was increased more than that in WT mice 1 and 4 h after reperfusion. However, the expression of cleaved caspase-8 did not differ between the FL and WT mice (Fig. 2).
Fig. 1.
Time course of hepatocyte apoptosis after reperfusion was assessed using the TUNEL method. Samples were obtained from WT (A) and FL (B) mice prior to the operation, at 1 and 4 h after reperfusion in WT (C) and FL (D) mice. Original magnification ×400. The number of apoptotic cell was quantitated to yield the apoptotic index (E). I/R: ischemia for 90 min and reperfusion. The values represent the mean ± SEM with n = 6 per group. *p<0.05, compared to the WT group.
Fig. 2.
(A) Time course of precursor and cleaved caspase-3, -8, and -9 activation in the liver was assessed by western blotting in WT and FL mice. Samples were obtained from mice undergoing sham operation or ischemia/reperfusion (I/R). To ascertain that there was equal protein loading, the same samples were probed with anti-actin antibody. Each lane represents a sample from an individual animal. The results were quantitated to show the ratio of each of caspase-3 (B), -8 (C), and -9 (D) to actin as determined by an image analysis of an autoradiogram. The results are representative of two independent experiments.
Expression of peroxynitrite during ischemia/reperfusion
Since NOS-mediated NO generates the peroxynitrite oxidant, we examined nitrotyrosine expression by ELISA. Hepatic nitrotyrosine expression was significantly increased in FL mice 1 h after reperfusion, as compared to WT mice (n = 6, respectively; FL, 16.9 ± 9.3, WT 5.2 ± 2.6 pmol/mg, p<0.05).
Activation of NF-κB during ischemia/reperfusion
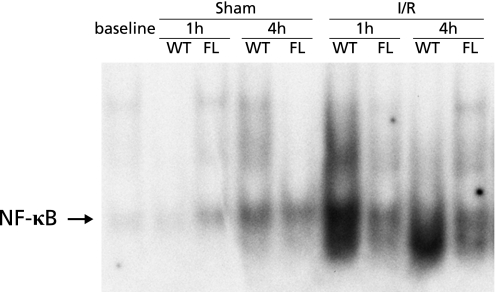
Since NF-κB is known to play a role in both inflammatory responses and anti-apoptosis effects,(11–24) we performed EMSA to determine whether NF-κB activation was induced by hepatic ischemia/reperfusion. After reperfusion, nuclear translocation of NF-κB in WT mice increased 1 and 4 h after reperfusion, but little NF-κB activation was observed in FL mice up to 4 h after reperfusion (Fig. 3).
Fig. 3.
NF-κB activation in whole liver homogenates during hepatic ischemia/reperfusion. Nuclear extracts from liver tissue were subjected to EMSA. The results are representative of two separate time-course experiments. Both WT and FL mice underwent either a sham operation or ischemia for 90 min (I/R).
Hepatocellular injury after hepatic ischemia/reperfusion
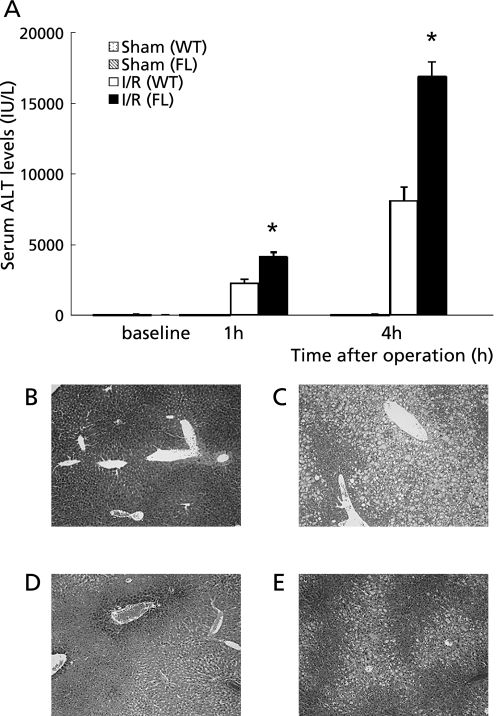
To assess the extent of hepatic injury during ischemia/reperfusion, serum levels of ALT were examined (n = 6, respectively). The serum ALT levels in the FL mice were significantly increased relative to the WT mice 1 and 4 h after reperfusion (Fig. 4A). The histological findings showed that focal hepatic necrosis was observed in the FL mice 4 h after reperfusion (Fig. 4B–E).
Fig. 4.
(A) Hepatocellular injury as indicated by serum levels of alanine aminotransferase (ALT). Samples were obtained from mice undergoing the sham operation or ischemia for 90 min and reperfusion (I/R). Values represent the mean ± SEM with n = 6 per group. *p<0.05, compared to the WT group. Histological findings of the liver assessed by hematoxylin and eosin staining. (B) Sham operated WT group. (C) Sham operated FL group. (D) WT group 4 h after reperfusion. (E) FL group 4 h after reperfusion. Original magnification ×100.
Role of iNOS in apoptosis and liver damage during ischemia/reperfusion
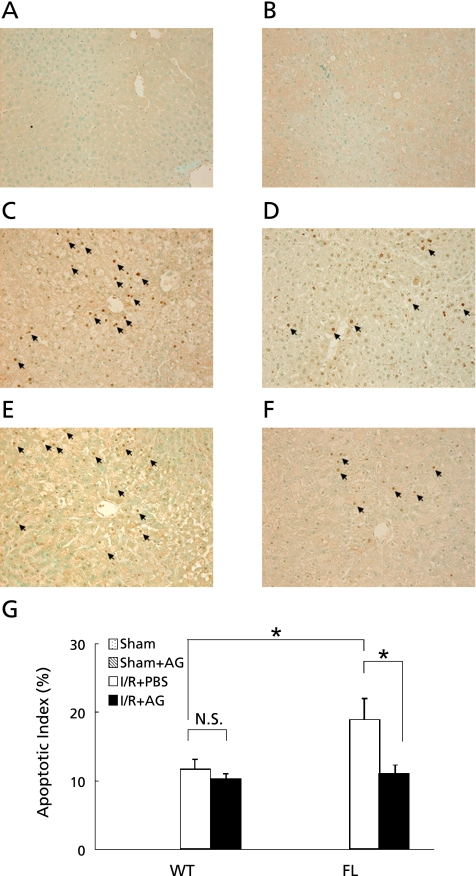
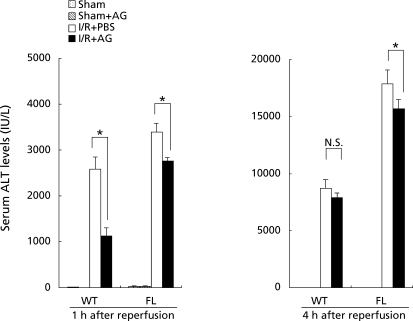
To determine whether iNOS was involved in liver apoptosis during hepatic ischemia/reperfusion, we performed TUNEL staining. The number of TUNEL positive cells, quantified by calculating the apoptotic index, was significantly upregulated 4 h after reperfusion in WT and FL mice relative to sham-operated controls (n = 6, respectively; Fig. 5). There was significant difference in apoptotic index of WT mice administered AG as an iNOS specific inhibitor. Treatment with AG significantly reduced the number of apoptotic cells in FL mice relative to treatment with PBS (Fig. 5). To determine whether iNOS was involved in hepatic ischemia/reperfusion injury, we examined the serum alanine aminotransferase (ALT) levels following the administration of AG. In sham-operated controls, there were no differences in serum ALT levels among WT and FL mice treated with PBS or AG. One hour after reperfusion, serum ALT levels were significantly decreased by administration of AG (56% decrease, p = 0.002) in the WT mice. In the FL mice, administration of AG also attenuated serum ALT levels (20% decrease, p = 0.025) compared with the FL mice treated with PBS 1 and 4 h after reperfusion (Fig. 6).
Fig. 5.
Hepatocyte apoptosis induced by ischemia/reperfusion assessed by the TUNEL method. To assess the effect of the iNOS inhibitor on hepatocyte apoptosis, samples from mice treated with PBS or aminoguanidine (AG) prior to ischemia for 90 min were examined. Samples were obtained from (A) sham operated WT, (B) sham operated FL, and (C) FL mice undergoing 90 min ischemia and 4 h reperfusion (I/R) with PBS treatment, (D) WT mice undergoing I/R with PBS treatment, (E) FL mice undergoing I/R with AG treatment. (F) WT mice undergoing I/R with AG treatment. Original magnification ×200. Arrows indicate hepatocyte apoptosis. The apoptotic index was also calculated in mice 4 h after reperfusion (G). The data represent the mean ± SEM with n = 6 per group. *p<0.05 compared to mice treated with PBS.
Fig. 6.
To assess the effects of the iNOS inhibitor on hepatocellular injury induced by ischemia/reperfusion, samples from mice treated with PBS or aminoguanidine (AG) were examined. Samples were obtained from the sham operated group (A), at 1 h (B), and at 4 h (C) after reperfusion. Hepatocellular injury was assessed by serum ALT levels. Values represent the mean ± SEM with n = 6 per group. *p<0.05, compared to mice treated with PBS.
Survival after ischemia/reperfusion
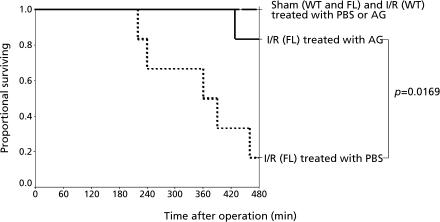
To determine whether apoptosis and subsequent hepatic ischemia/reperfusion injury may be related to death, we examined the survival of mice after administration of AG. In sham-operated controls, neither WT nor FL mice died after treatment with PBS or AG (Fig. 7). None of WT mice undergoing ischemia for 90 min died during 8 h after reperfusion. On the other hand, the survival rate in FL mice undergoing ischemia for 90 min after reperfusion was significantly worse. However, treatment with AG significantly ameliorated survival after reperfusion in the FL mice (Fig. 7).
Fig. 7.
Survival after hepatic ischemia for 90 min up to 8 h after reperfusion (I/R). WT and FL mice were treated with either PBS or AG intravenously. For all groups, n = 6.
Discussion
Liver surgery for steatotic liver poses risks of post-operative morbidity, mortality, and primary graft nonfunction.(7,8) One of the possible causes of this event is hepatic ischemia/reperfusion injury. We herein provide evidence that the steatotic liver is vulnerable to hepatic ischemia/reperfusion.
We have also demonstrated that the production of reactive oxygen species is related to this type of injury. In fact, NO derived from iNOS is harmful because of production of hazardous reactive oxygen species, such as peroxynitrite.(14) Peroxynitrite concentrations were significantly upregulated in FL mice compared with WT mice in the current srudy, suggesting that upregulation of reactive oxygen species produced by iNOS may initiate hepatocyte apoptosis in the steatotic liver after ischemia/reperfusion. This was confirmed by the fact that AG administration reduced the hepatocyte apoptosis, suggesting that the induction of apoptosis is, at least in part, attributable to upregulation of iNOS.
The activation of cleaved caspase 8 did not differ between the WT and FL mice, suggesting that death signaling is unlikely to play a role in augmenting apoptosis in the steatotic liver. However, the activation of cleaved caspase 9 was prominent in the initial phase of reperfusion, and cleaved caspase 3 was activated in the steatotic liver, thus suggesting that the mitochondrial permeability transition plays an important role in apoptosis associated with steatotic liver ischemia/reperfusion injury. This pathway has been known to occur in hepatocytes, and to induce apoptosis more rapidly than type 1 pathway.(19) Immunohistochemistry revealed iNOS expression in hepatocytes, especially in the periportal area (personal observation). It has also been demonstrated that periportal hepatocytes are vulnerable to hypoxia-reoxygenation and that Kupffer cells in periportal areas engulf apoptotic cells, thus resulting in the release of reactive oxygen species.(25,26) Taken together, the early induction of hepatocyte apoptosis was significantly observed after reperfusion in the steatotic liver.
In general, the role of NF-κB during hepatic ischemia/reperfusion injury has been considered proinflammatory. Previous studies have shown that the therapeutic modalities which reduce inflammatory injury after ischemia/reperfusion, also reduce NF-κB activation.(11) On the other hand, data from other models of liver injury have suggested that hepatocyte activation of NF-κB is associated with its anti-apoptotic effects.(24,27) In the current study, NF-κB activation in whole livers (which is largely representative of hepatocytes) was decreased in steatotic livers after hepatic ischemia/reperfusion, as demonstrated by EMSA. The steatotic liver after reperfusion was vulnerable to hepatocyte apoptosis, which may be associated with decreased NF-κB-related anti-apoptotic properties.
The steatotic liver is known to have low ATP levels after hepatic ischemia/reperfusion.(28,29) Our previous studies have demonstrated that cellular apoptosis consumes a large amount of nicotinamide adenine dinucleotide (NAD+), and to resynthesize NAD+, resulting in a decrease in ATP levels.(30) Combination with hepatocyte apoptosis and ATP depletion changes the cellular response to secondary oncotic necrosis.(31) These findings suggest that hepatocyte apoptosis in conjunction with a decrease in ATP levels causes secondary oncotic necrosis, leading to significant liver injury. This is consistent with our observation that the serum ALT levels were greatest in the steatotic liver, which resulted in severe liver damage and lower survival in the late phase of reperfusion in the steatotic liver. Treatment with AG improved the survival of FL mice after reperfusion, suggesting that iNOS-related hepatocyte apoptosis seems to initiate hepatic ischemia/reperfusion injury in the steatotic liver. However, the current study demonstrated that the reduction in liver injury as defined by serum ALT levels under the blockade of iNOS is modest, thus suggesting that other mechanism might be related to this type of injury.
In conclusion, our data suggest that the steatotic liver is vulnerable to ischemia/reperfusion injury due to hepatocyte apoptosis associated with the early upregulation of iNOS-related peroxinitrite, which thus leads to subsequent oncotic necrosis. The present data are considered to expand our knowledge of steatosis and therefore clinically applicable for patients with ischemia/reperfusion injury during liver surgery for steatotic liver.
Acknowledgment
This work was supported in part by grants from the Japan Society for the Promotion of Science (#20591601, 22591500).
Abbreviations
- FL
fatty liver
- NF-κB
nuclear factor-kappa B
- NAFLD
non-alcoholic fatty liver disease
- ALT
alanine aminotransferase
- TUNEL
terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate biotin nick end-labeling
- EMSA
electrophoretic mobility shift assay
- ELISA
enzyme-linked immunosorbent assay
- NOS
nitric oxide synthase
- AG
aminoguanidine
References
- 1.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 2.Roskams T, Yang SQ, Koteish A, et al. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163:1301–1311. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 4.Vendemiale G, Grattagliano I, Caraceni P, et al. Mitochondrial oxidative injury and energy metabolism alteration in rat fatty liver: effect of the nutritional status. Hepatology. 2001;33:808–815. doi: 10.1053/jhep.2001.23060. [DOI] [PubMed] [Google Scholar]
- 5.Todo S, Demetris AJ, Makowka L, et al. Primary nonfunction of hepatic allografts with preexisting fatty infiltration. Transplantation. 1989;47:903–905. doi: 10.1097/00007890-198905000-00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20:829–838. doi: 10.1002/hep.1840200410. [DOI] [PubMed] [Google Scholar]
- 7.Kooby DA, Fong Y, Suriawinata A, et al. Impact of Steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–113. doi: 10.1055/s-2001-12933. [DOI] [PubMed] [Google Scholar]
- 9.Marsman WA, Wiesner RH, Rodriguez L, et al. Use of fatty donor liver is associated with diminished early patient and graft survival. Transplantation. 1996;62:1246–1251. doi: 10.1097/00007890-199611150-00011. [DOI] [PubMed] [Google Scholar]
- 10.Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 11.Yoshidome H, Kato A, Edwards MJ, Lentsch AB. Interleukin-10 suppresses hepatic ischemia/reperfusion injury in mice: implications of a central role for nuclear factor KappaB. Hepatology. 1999;30:203–208. doi: 10.1002/hep.510300120. [DOI] [PubMed] [Google Scholar]
- 12.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 13.Rüdiger HA, Clavien PA. Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology. 2002;122:202–210. doi: 10.1053/gast.2002.30304. [DOI] [PubMed] [Google Scholar]
- 14.Laroux FS, Pavlick KP, Hines IN, et al. Role of nitric oxide in inflammation. Acta Physiol Scand. 2001;173:113–118. doi: 10.1046/j.1365-201X.2001.00891.x. [DOI] [PubMed] [Google Scholar]
- 15.Song SW, Tolba RH, Yonezawa K, Manekeller S, Minor T. Exogenous superoxide dismutase prevents peroxynitrite-induced apoptosis in non-heart-beating donor livers. Eur Surg Res. 2008;41:353–361. doi: 10.1159/000162294. [DOI] [PubMed] [Google Scholar]
- 16.Peter ME, Krammer PH. Mechanisms of CD95 (APO-1/Fas)-mediated apoptosis. Curr Opin Immunol. 1998;10:545–551. doi: 10.1016/s0952-7915(98)80222-7. [DOI] [PubMed] [Google Scholar]
- 17.Soeda J, Miyagawa S, Sano K, Masumoto J, Taniguchi S, Kawasaki S. Cytochrome c release into cytosol with subsequent caspase activation during warm ischemia in rat liver. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1115–G1123. doi: 10.1152/ajpgi.2001.281.4.G1115. [DOI] [PubMed] [Google Scholar]
- 18.Morin D, Pires F, Plin C, Tillement JP. Role of the permeability transition pore in cytochrome c release from mitochondria during ischemia-reperfusion in rat liver. Biochem Pharmacol. 2004;68:2065–2073. doi: 10.1016/j.bcp.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 20.Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–1257. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- 21.Cottart CH, Do L, Blanc MC, et al. Hepatoprotective effect of endogenous nitric oxide during ischemia-reperfusion in the rat. Hepatology. 1999;29:809–813. doi: 10.1002/hep.510290317. [DOI] [PubMed] [Google Scholar]
- 22.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeuchi D, Yoshidome H, Kato A, et al. Interleukin 18 causes hepatic ischemia/reperfusion injury by suppressing anti-inflammatory cytokine expression in mice. Hepatology. 2004;39:699–710. doi: 10.1002/hep.20117. [DOI] [PubMed] [Google Scholar]
- 24.Iimuro Y, Nishiura T, Hellerbrand C, et al. NFκB prevents apoptosis and liver dysfunction during liver regeneration. J Clin Invest. 1998;101:802–811. doi: 10.1172/JCI483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taniai H, Hines IN, Bharwani S, et al. Susceptibility of murine periportal hepatocytes to hypoxia-reoxygenation: role for NO and Kupffer cell-derived oxidants. Hepatology. 2004;39:1544–1552. doi: 10.1002/hep.20217. [DOI] [PubMed] [Google Scholar]
- 26.Cursio R, Gugenheim J, Ricci JE, et al. A caspase inhibitor fully protects rats against lethal normothermic liver ischemia by inhibition of liver apoptosis. FASEB J. 1999;13:253–261. doi: 10.1096/fasebj.13.2.253. [DOI] [PubMed] [Google Scholar]
- 27.Iida A, Yoshidome H, Shida T, et al. Hepatocyte nuclear factor-kappa beta (NF-kappaB) activation is protective but is decreased in the cholestatic liver with endotoxemia. Surgery. 2010;148:477–489. doi: 10.1016/j.surg.2010.01.014. DOI: 10.1016/j.surg.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Selzner N, Selzner M, Jochum W, Clavien PA. Ischemic preconditioning protects the steatotic mouse liver against reperfusion injury: an ATP dependent mechanism. J Hepatol. 2003;39:55–61. doi: 10.1016/s0168-8278(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 29.Sakuragawa T, Hishiki T, Ueno Y, et al. Hypotaurine is an energy-saving hepatoprotective compound against ischemia-reperfusion injury of the rat liver. J Clin Biochem Nutr. 2010;46:126–134. doi: 10.3164/jcbn.09-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iida A, Yoshidome H, Shida T, et al. Does prolonged biliary obstructive jaundice sensitize the liver to endotoxemia? Shock. 2009;31:397–403. doi: 10.1097/SHK.0b013e31818349ea. [DOI] [PubMed] [Google Scholar]
- 31.Jaeschke H, Gujral JS, Bajt ML. Apoptosis and necrosis in liver disease. Liver Int. 2004;24:85–89. doi: 10.1111/j.1478-3231.2004.0906.x. [DOI] [PubMed] [Google Scholar]