Abstract
Prostaglandin E2 plays an important role in the maintenance of gastric mucosal integrity. The level of biologically active prostaglandin E2 in the tissue is regulated by the balanced expression of its synthetic enzymes, such as cyclooxygenase, and its catabolic enzyme, 15-hydroxyprostaglandin dehydrogenase. We examined the effect of rebamipide, a mucoprotective drug, on prostaglandin E2 production and metabolism in the gastric tissue and its effect on indomethacin-induced gastric mucosal injury in mice. Rebamipide suppressed indomethacin-induced gastric mucosal injury. Suppressive effect of rebamipide on indomethacin-induced gastric mucosal injury was also observed in cyclooxygenase-2-knockout mice. The mice that were treated with rebamipide showed a 2-fold increase in cyclooxygenase-2 mRNA expression in the gastric tissue, whereas 15-hydroxyprostaglandin dehydrogenase mRNA expression markedly decreased as compared to vehicle-treated control mice. Rebamipide did not affect the expression of cyclooxygenase-1 in the gastric tissue. Rebamipide did not increase prostaglandin E2 production in the gastric tissue; however, it induced a 1.4-fold increase in the concentration of prostaglandin E2 in the gastric tissue as compared to vehicle-treated control mice. These results suggest that the suppressive effect of rebamipide on non-steroidal anti-inflammatory drugs-induced gastric mucosal injury can be attributed to reduced 15-hydroxyprostaglandin dehydrogenase expression, which increases the prostaglandin E2 concentration in the gastric tissue.
Keywords: rebamipide, 15-hydroxyprostaglandin dehydrogenase, prostaglandin E2
Introduction
Prostaglandin (PG) is a bioactive eicosanoid synthesized from arachidonic acid liberated from membrane phospholipids. PG plays important roles in multiple physiological processes, including renal function, vascular homeostasis, bone remodeling, gastrointestinal function, pregnancy, and acute inflammatory responses. PGs, especially PGE2, play an important role in maintenance of gastric mucosal integrity via several mechanisms, including regulation of gastric mucosal blood flow, kinetics of epithelial cells, mucus synthesis, and inhibition of gastric acid secretion.(1) Previous studies of the involvement of PGE2 in gastric mucosal defense have focused on cyclooxygenase (COX), a rate-limiting enzyme for synthesis of PGs. However, the total amount of biologically active PGE2 in gastric tissue is regulated by the balance between PGE2 synthesis and degradation; 15-hydroxyprostaglandin dehydrogenase (15-PGDH), which catalyzes the oxidation of the 15(S)-hydroxyl group of prostaglandins, resulting in the production of 15-keto-prostaglandins, greatly reduces the biological activity of PGE2.(2)
Rebamipide (2-(4-chlorobenzoylamino-3-[2(1H)-quinolinon-4-yl] propionic acid)), a mucosal protective agent, has been used clinically for treating gastritis and peptic ulcers.(3,4) Clinical and experimental data demonstrate that rebamipide accelerates gastric ulcer healing,(5,6) prevents ulcer relapse,(6) and protects gastric mucosa against acute injury caused by various noxious agents.(4,7–10) It is reported that rebamipide actions are mediated by the stimulation of prostaglandin synthesis,(11) inhibition of neutrophil activation,(12,13) and inhibition of generation of, and/or scavenging reactive oxygen species.(14,15) However, the precise mechanism by which rebamipide modulates PGE2 synthesis and metabolism in gastric tissue remains unknown.
In this study, we studied the effect of rebamipide on (1) indomethacin-induced gastric mucosal injury, (2) expression of COX and 15-PGDH in gastric tissue, and (3) PGE2 levels in the gastric tissue.
Materials and Methods
Animals
Seven-week-old C57BL/6 mice were purchased from Charles River Japan, Inc. (Yokohama, Japan). Seven to ten-week-old COX-2-knockout mice (background: C57BL/6) and wild-type littermates were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were housed in polycarbonate cages with paper chip bedding in an air-conditioned, biohazard room with a 12-h light-dark cycle. All animals had free access to food and water. All experimental procedures were approved by the Animal Care Committee of the Osaka City University Graduate School of Medicine.
Experimental protocol
Sixteen hours after fasting, rebamipide (30 or 100 mg/kg body weight (BW), supplied by Otsuka Pharmaceutical Co., Tokyo, Japan) or vehicle (0.25% carboxymethylcellulose) was given orally to mice. Four hours after administration of rebamipide or vehicle, indomethacin (30 mg/kg BW, Sigma Chemical Company, St. Louis, MO) was given orally to mice and mice stomachs were removed 6 h after administration of indomethacin. Gastric mucosal injury was evaluated by measuring the injured area in the gastric mucosa defined as areas with clots of blood.
Assessing mRNA expression for COX-1, COX-2 and 15-PGDH in the gastric tissue by real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR)
Mice were orally treated with the vehicle or rebamipide (30 or 100 mg/kg BW, respectively) and their stomachs were removed after 4 h. Real-time quantitative RT-PCR analyses were performed as previously described.(16,17) In brief, total RNA was isolated from the gastric tissue using an ISOGEN kit (Nippon Gene, Tokyo, Japan) according to the manufacturer’s protocol. PCR primers and TaqMan probes for COX-1 and -2 were designed using the software program, Primer Express (PE Applied Biosystems, Foster City, CA). The primers and probes for measuring 15-PGDH mRNA in the gastric tissue were chosen using the online Applied Biosystems catalogue. TaqMan probes were labeled with a reporter fluorescent dye (6-carboxyfluorescein) at the 5' end and a fluorescent dye quencher (6-carboxytetramethylrhodamine) at the 3' end. For the mouse COX-1, the sense primer was 5'-CATCAAGGAGTCCCGAGAGAT-3', the antisense primer was 5'-TAAGGCTTCAAGCCAAACCTC-3', and the TaqMan probe was 5'-CGCCTACAGCCCTTCAATGAATACCGA-3'. For mouse COX-2, the sense primer was 5'-AAGCCCTCTACAGTGACA TCGA-3', the antisense primer was 5'-ATGGTCTCCCCAAAGA TAGCAT-3', and the TaqMan probe was 5'-CTGCTGGTGGAA AAACCTCGTCCA-3'. Real-time quantitative RT-PCR analyses were performed using the ABI PRISM 7700 Sequence Detection System and software (PE Applied Biosystems). The reaction mixture was prepared according to the manufacturer’s protocol using the Platinum qRT-PCR ThermoScript One-Step System (Invitrogen, Carlsbad, CA). Thermal cycling conditions were 50°C for 30 min and 95°C for 5 min, followed by 45 cycles of amplification at 95°C for 15 s and 60°C for 1 min. Total RNA was subjected to real-time quantitative RT-PCR for measurement of target genes and GAPDH as an internal standard using TaqMan GAPDH control reagents (PE Applied Biosystems). Expression of mRNA in the gastric tissue was standardized to GAPDH mRNA, and mRNA levels were expressed as ratios to the mean values of GAPDH mRNA in the normal gastric tissue.
Measuring PGE2 synthesis in the gastric tissue
PGE2 production by the gastric tissue was assessed by using a vortex method, developed from that originally described by Whittle et al.(18) Mice were orally treated with the vehicle or rebamipide (30 or 100 mg/kg BW, respectively), their stomachs were removed after 4 h and placed in polypropylene tubes containing 1 ml of Tris-HCl buffer (150 mM, pH 8.2) for 1 min, and then centrifuged at 9,000 rpm for 40 s. Supernatant was discarded and 1.5 ml of fresh Tris-HCl buffer was added. Tubes were then simultaneously mixed in a vortex for 1 min, and 30 µl of 10 mM indomethacin (Sigma Chemical) was added immediately to inhibit excessive COX activity. Tubes were centrifuged at 9,000 rpm for 40 s. Supernatants were collected and stored at −80°C. The amount of PGE2 in the supernatant was determined by enzyme immunoassay (PGE2 EIA kit, Cayman Chemical Co., Ann Arbor, MI). PGE2 production was expressed as nanogram of PGE2 per gram of tissue per minute.
Measuring PGE2 levels in the gastric tissue
Mice were orally treated with the vehicle or rebamipide (30 or 100 mg/kg BW, respectively) and their stomachs were removed after 4 h. The gastric tissue was isolated, weighed, and placed in a tube containing 100% ethanol plus 0.1 M indomethacin. The samples were then sheared with scissors, homogenized, and centrifuged at 12,000 rpm for 10 min at 4°C. The supernatant of each sample was used for determination of PGE2 using a PGE2 EIA kit (Cayman Chemical Co.).
Statistical Analysis
Values are the mean ± SE. Kruskal-Wallis H test was used to test for significance of differences among treatment-group means. Results were examined by Mann-Whitney U test with Bonferroni correction. Differences with p values less than 0.05 were considered significant.
Results
The effect of rebamipide on indomethacin-induced gastric mucosal injury
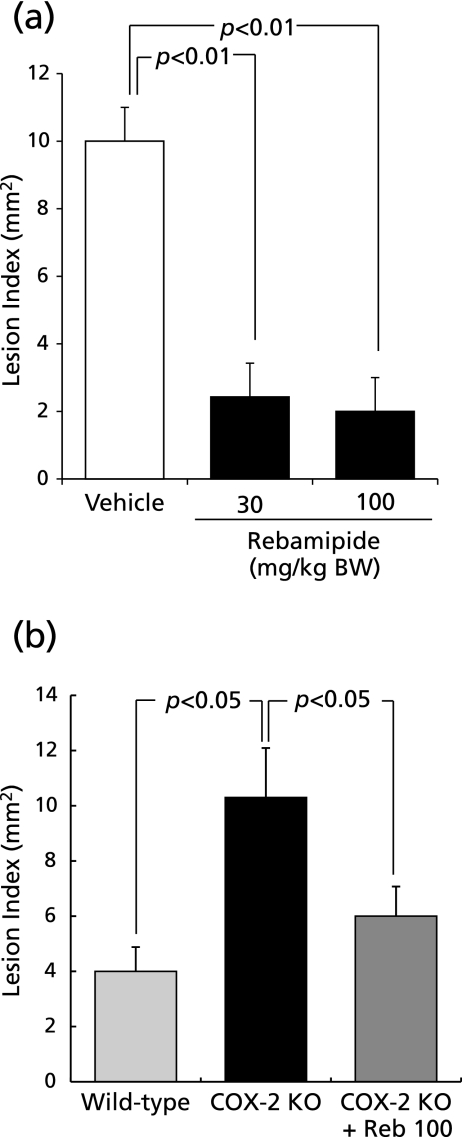
Indomethacin induced hemorrhagic lesions in the stomach. Rebamipide suppressed formation of hemorrhagic lesions induced by indomethacin in a dose-dependent manner (Fig. 1a). Suppressive effect of rebamipide on indomethacin-induced gastric mucosal injury was also observed in COX-2-knockout mice (Fig. 1b).
Fig. 1.
The effect of rebamipide on indomethacin-induced gastric mucosal injury in wild-type or COX-2-knockout mice. (a) Mice were given indomethacin s.c. in a dose of 30 mg/kg and killed 6 h later. Rebamipide (30 or 100 mg/kg) was given p.o. 4 h before indomethacin. (b) COX-2-knockout or wild-type mice were given indomethacin s.c. and killed 6 h later. Rebamipide at 100 mg/kg was given p.o. 4 h before indomethacin. Data are presented as means ± SE for 4–6 mice.
The effect of rebamipide on COX-1, COX-2 and 15-PGDH mRNA expression in the gastric tissue
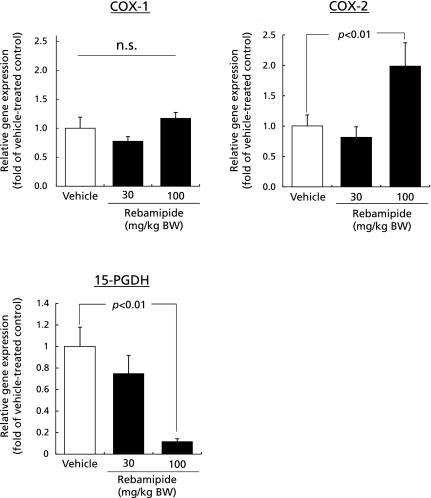
The mice that were treated with 100 mg/kg BW of rebamipide showed a 2-fold increase in COX-2 mRNA expression in the gastric tissue, while 15-PGDH mRNA expression decreased by 26% or 89% in the mice treated with 30 mg/kg BW or 100 mg/kg BW of rebamipide, respectively, compared with vehicle-treated control mice. Rebamipide did not affect COX-1 mRNA expression (Fig. 2).
Fig. 2.
The effect of rebamipide on COX-1, COX-2 and 15-PGDH mRNA expression in the gastric tissue. Mice were given rebamipide (30 or 100 mg/kg) p.o. and killed 4 h later. Data are presented as mean ± SE for 4–6 mice.
Effect of rebamipide on PGE2 synthesis and levels in the gastric tissue
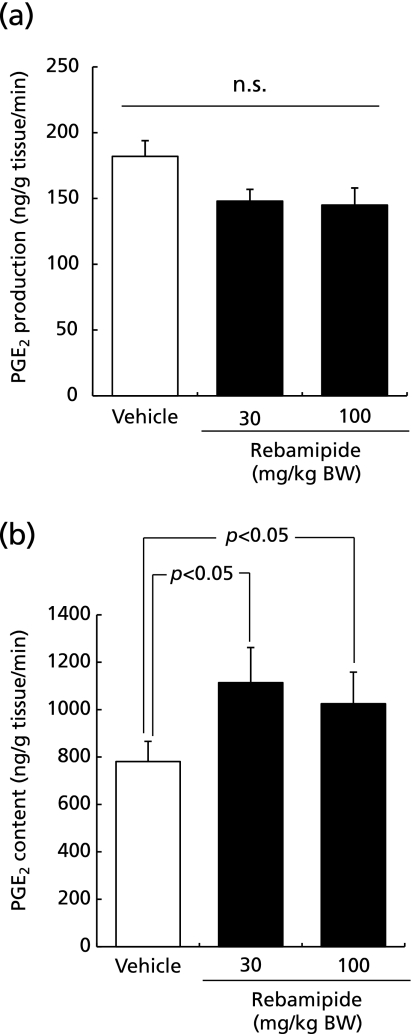
Rebamipide did not increase PGE2 synthesis in the gastric tissue (Fig. 3a); however, it induced a 1.4-fold increase in PGE2 concentration in the gastric tissue compared with vehicle-treated control mice (Fig. 3b).
Fig. 3.
The effect of rebamipide on (a) PGE2 synthesis and (b) PGE2 levels in the gastric tissue. Mice were given rebamipide (30 or 100 mg/kg) p.o. and killed 4 h later. Data are presented as mean ± SE for 4–6 mice.
Discussion
Our present study demonstrated that rebamipide suppresses indomethacin-induced gastric mucosal injury. Notably, even in COX-2-knockout mice, rebamipide exerted preventive effects against indomethacin-induced gastric mucosal injury. Rebamipide up-regulated PGE2 levels accompanied by marked down-regulation of 15-PGDH in the gastric tissue, suggesting that rebamipide suppresses indomethacin-induced gastric mucosal injury via down-regulation of 15-PGDH.
Indomethacin induces gastric mucosal injury by reducing PG levels via inhibition of cyclooxygenase activity. It is reported that prior administration of 16,16-dimethyl PGE2, which is not catabolized by 15-PGDH, prevents injury development in the stomach.(19,20) These results suggest that rescuing physiologically active PGE2 by reducing the activity of its catabolic enzyme, 15-PGDH, could suppress indomethacin-induced gastric mucosal injury. Accumulating evidence suggest that reduction of 15-PGDH levels enhances the physiological effects of PGE2. Recent studies have shown that reduction of 15-PGDH levels occurs in association with an aggressive phenotype and unfavorable prognoses in breast,(21) prostate,(22) lung,(23) colorectal,(24,25) and medullary thyroid cancers.(26) Our recent study suggests that down-regulation of 15-PGDH induces cell proliferation in gastric cancers accompanied by up-regulation of PGE2 level.(27) 15-PGDH in the bladder cancer cell-lines increases motility and anchorage-independent growth.(28) From these findings, it is possible that rebamipide may prevent indomethacin-induced gastric mucosal injury via suppressing PGE2 catabolism by reducing 15-PGDH expression.
The mechanisms whereby rebamipide reduces 15-PGDH expression in the gastric tissue remain unknown. Activation of epidermal growth factor-receptor (EGFR) signaling pathway has been proposed as a possible mechanism whereby 15-PGDH expression may be reduced.(29) A recent study suggests that rebamipide activates EGFR and mitogen-activated protein kinase (MAPK) in conjunctival goblet cells.(30) From these findings, we speculate that rebamipide may down-regulate 15-PGDH expression via activation of EGFR and MAPK in gastric epithelial cells.
Previous reports demonstrate that rebamipide induces COX-2 expression, increases PGE2 levels, and enhances gastric mucosal defense in a COX-2-dependent manner in rat gastric mucosa(31) and rat gastric epithelial cells.(32) In addition of induction of COX-2, it is also possible that reduction of 15-PGDH by rebamipide may also contribute to increase PGE2 levels in the gastric tissue. We demonstrate that rebamipide promotes healing of gastric ulcers through both COX-2-dependent and COX-2-independent mechanisms.(12) On the contrary, Kleine A. et al.(11) concluded that the increase in prostaglandin formation results from stimulation of biosynthesis and not inhibition of degradation because release of 15-keto-PGE2, a metabolites of PGE2, in gastric mucosal tissue of rats was not impaired by rebamipide. This discrepancy might be due to differences among the studies in the animal model,the study design, the period of treatment with rebamipide and the methods of assessment used.
In conclusion, rebamipide inhibits indomethacin-induced gastric mucosal injury. Rescuing physiologically active PGE2 by reducing 15-PGDH expression may be a potential mechanism for this effect.
Abbreviations
- PG
prostaglandin
- COX
cyclooxygenase
- 15-PGDH
15-hydroxyprostaglandin dehydrogenase
- EGFR
epidermal growth factor-receptor
- MAPK
mitogen-activated protein kinase
Conflicts of Interest
The authors disclose the following: T. Arakawa has received a research support and honorarium for consultancies from Otsuka Pharmatheutical Co. Ltd.. K. Takeuchi has received a research support from Otsuka Pharmatheutical Co. Ltd.. The remaining authors disclose no financial conflicts of interest.
References
- 1.Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself ? Physiol Rev. 2008;88:1547–1565. doi: 10.1152/physrev.00004.2008. [DOI] [PubMed] [Google Scholar]
- 2.Ensor CM, Tai HH. 15-Hydroxyprostaglandin dehydrogenase. J Lipid Mediat Cell Signal. 1995;12:313–319. doi: 10.1016/0929-7855(95)00040-w. [DOI] [PubMed] [Google Scholar]
- 3.Arakawa T, Higuchi K, Fujiwara Y, et al. 15th anniversary of rebamipide: looking ahead to the new mechanisms and new applications. Dig Dis Sci. 2005;50(Suppl 1):S3–S11. doi: 10.1007/s10620-005-2800-9. [DOI] [PubMed] [Google Scholar]
- 4.Arakawa T, Kobayashi K, Yoshikawa T, Tarnawski A. Rebamipide: overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing. Dig Dis Sci. 1998;43(Suppl):5S–13S. [PubMed] [Google Scholar]
- 5.Watanabe T, Higuchi K, Taira K, et al. Rebamipide reduces delay in gastric ulcer healing in cyclooxygenase-2-deficient mice. Dig Dis Sci. 2005;50(Suppl 1):S63–S69. doi: 10.1007/s10620-005-2808-1. [DOI] [PubMed] [Google Scholar]
- 6.Arakawa T, Watanabe T, Fukuda T, Yamasaki K, Kobayashi K. Rebamipide, novel prostaglandin-inducer accelerates healing and reduces relapse of acetic acid-induced rat gastric ulcer. Comparison with cimetidine. Dig Dis Sci. 1995;40:2469–2472. doi: 10.1007/BF02063257. [DOI] [PubMed] [Google Scholar]
- 7.Ogino K, Hobara T, Ishiyama H, et al. Antiulcer mechanism of action of rebamipide, a novel antiulcer compound, on diethyldithiocarbamate-induced antral gastric ulcers in rats. Eur J Pharmacol. 1992;212:9–13. doi: 10.1016/0014-2999(92)90065-c. [DOI] [PubMed] [Google Scholar]
- 8.Tarnawski A, Arakawa T, Kobayashi K. Rebamipide treatment activates epidermal growth factor and its receptor expression in normal and ulcerated gastric mucosa in rats: one mechanism for its ulcer healing action? Dig Dis Sci. 1998;43(Suppl):90S–98S. [PubMed] [Google Scholar]
- 9.Naito Y, Kuroda M, Mizushima K, et al. Transcriptome analysis for cytoprotective actions of rebamipide against indomethacin-induced gastric mucosal injury in rats. J Clin Biochem Nutr. 2007;41:202–210. doi: 10.3164/jcbn.2007029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naito Y, Iinuma S, Yagi N, et al. Prevention of indomethacin-induced gastric mucosal injury in Helicobacter pylori-negative healthy volunteers: a comparison study rebamipide vs famotidine. J Clin Biochem Nutr. 2008;43:34–40. doi: 10.3164/jcbn.2008041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleine A, Kluge S, Peskar BM. Stimulation of prostaglandin biosynthesis mediates gastroprotective effect of rebamipide in rats. Dig Dis Sci. 1993;38:1441–1449. doi: 10.1007/BF01308601. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T, Higuchi K, Hamaguchi M, et al. Rebamipide prevents delay of acetic acid-induced gastric ulcer healing caused by Helicobacter pylori infection in Mongolian gerbils. Dig Dis Sci. 2002;47:1582–1589. doi: 10.1023/a:1015879421739. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida N, Yoshikawa T, Iinuma S, et al. Rebamipide protects against activation of neutrophils by Helicobacter pylori. Dig Dis Sci. 1996;41:1139–1144. doi: 10.1007/BF02088229. [DOI] [PubMed] [Google Scholar]
- 14.Naito Y, Yoshikawa T, Tanigawa T, et al. Hydroxyl radical scavenging by rebamipide and related compounds: electron paramagnetic resonance study. Free Radic Biol Med. 1995;18:117–123. doi: 10.1016/0891-5849(94)00110-6. [DOI] [PubMed] [Google Scholar]
- 15.Kim CD, Hong KW. Preventive effect of rebamipide on gastric lesions induced by ischemia-reperfusion in the rat. J Pharmacol Exp Ther. 1995;275:340–344. [PubMed] [Google Scholar]
- 16.Xu Y, Watanabe T, Tanigawa T, et al. Bile acids induce cdx2 expression through the farnesoid x receptor in gastric epithelial cells. J Clin Biochem Nutr. 2010;46:81–86. doi: 10.3164/jcbn.09-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanigawa T, Watanabe T, Higuchi K, et al. Lansoprazole, a proton pump inhibitor, suppresses production of tumor necrosis factor-alpha and interleukin-1beta induced by lipopolysaccharide and Helicobacter pylori bacterial components in human monocytic cells via inhibition of activation of nuclear factor-kappaB and extracellular signal-regulated kinase. J Clin Biochem Nutr. 2009;45:86–92. doi: 10.3164/jcbn.08-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whittle BJ, Higgs GA, Eakins KE, Moncada S, Vane JR. Selective inhibition of prostaglandin production in inflammatory exudates and gastric mucosa. Nature. 1980;284:271–273. doi: 10.1038/284271a0. [DOI] [PubMed] [Google Scholar]
- 19.Arakawa T, Nakamura H, Chono S, et al. Absence of effect of 16,16-dimethyl prostaglandin E2 on reduction of gastric mucosal blood flow caused by indomethacin in rats. Dig Dis Sci. 1989;34:1369–1373. doi: 10.1007/BF01538071. [DOI] [PubMed] [Google Scholar]
- 20.Kunikata T, Araki H, Takeeda M, Kato S, Takeuchi K. Prostaglandin E prevents indomethacin-induced gastric and intestinal injury through different EP receptor subtypes. J Physiol Paris. 2001;95:157–163. doi: 10.1016/s0928-4257(01)00021-3. [DOI] [PubMed] [Google Scholar]
- 21.Wolf I, O’Kelly J, Rubinek T, et al. 15-hydroxyprostaglandin dehydrogenase is a tumor suppressor of human breast cancer. Cancer Res. 2006;66:7818–7823. doi: 10.1158/0008-5472.CAN-05-4368. [DOI] [PubMed] [Google Scholar]
- 22.Swami S, Krishnan AV, Moreno J, Bhattacharyya RB, Peehl DM, Feldman D. Calcitriol and genistein actions to inhibit the prostaglandin pathway: potential combination therapy to treat prostate cancer. J Nutr. 2007;137:205S–210S. doi: 10.1093/jn/137.1.205S. [DOI] [PubMed] [Google Scholar]
- 23.Ding Y, Tong M, Liu S, Moscow JA, Tai HH. NAD+-linked 15-hydroxyprostaglandin dehydrogenase (15-PGDH) behaves as a tumor suppressor in lung cancer. Carcinogenesis. 2005;26:65–72. doi: 10.1093/carcin/bgh277. [DOI] [PubMed] [Google Scholar]
- 24.Backlund MG, Mann JR, Holla VR, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem. 2005;280:3217–3223. doi: 10.1074/jbc.M411221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myung SJ, Rerko RM, Yan M, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci USA. 2006;103:12098–12102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quidville V, Segond N, Lausson S, Frenkian M, Cohen R, Jullienne A. 15-Hydroxyprostaglandin-dehydrogenase is involved in anti-proliferative effect of non-steroidal anti-inflammatory drugs COX-1 inhibitors on a human medullary thyroid carcinoma cell line. Prostaglandins Other Lipid Mediat. 2006;81:14–30. doi: 10.1016/j.prostaglandins.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Tatsuwaki H, Tanigawa T, Watanabe T, et al. Reduction of 15-hydroxyprostaglandin dehydrogenase expression is an independent predictor of poor survival associated with enhanced cell proliferation in gastric adenocarcinoma. Cancer Sci. 2010;101:550–558. doi: 10.1111/j.1349-7006.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng-Rogenski S, Gee J, Ignatoski KW, et al. Loss of 15-hydroxyprostaglandin dehydrogenase expression contributes to bladder cancer progression. Am J Pathol. 2010;176:1462–1468. doi: 10.2353/ajpath.2010.090875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L, Amann JM, Kikuchi T, et al. Inhibition of epidermal growth factor receptor signaling elevates 15-hydroxyprostaglandin dehydrogenase in non-small-cell lung cancer. Cancer Res. 2007;67:5587–5593. doi: 10.1158/0008-5472.CAN-06-2287. [DOI] [PubMed] [Google Scholar]
- 30.Rios JD, Shatos MA, Urashima H, Dartt DA. Effect of OPC-12759 on EGF receptor activation, p44/p42 MAPK activity, and secretion in conjunctival goblet cells. Exp Eye Res. 2008;86:629–636. doi: 10.1016/j.exer.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Sun WH, Tsuji S, Tsujii M, et al. Induction of cyclooxygenase-2 in rat gastric mucosa by rebamipide, a mucoprotective agent. J Pharmacol Exp Ther. 2000;295:447–452. [PubMed] [Google Scholar]
- 32.Murata H, Yabe Y, Tsuji S, et al. Gastro-protective agent rebamipide induces cyclooxygenease-2 (COX-2) in gastric epithelial cells. Dig Dis Sci. 2005;50(Suppl 1):S70–S75. doi: 10.1007/s10620-005-2809-0. [DOI] [PubMed] [Google Scholar]