Abstract
TANK-binding kinase 1 (TBK1) regulates the interferon regulatory factor (IRF) 3 and IRF7 activation pathways by double strand RNA (dsRNA) via Toll-like receptor (TLR) 3 and by lipopolysaccharide (LPS) via TLR4. Rebamipide is useful for treating inflammatory bowel disease (IBD). Although IBD is associated with nuclear factor-κB (NF-κB), any association with the TBK1-IRF pathway remains unknown. How rebamipide affects the TBK1-IRF pathway is also unclear. We analyzed the relationship between IBD (particularly ulcerative colitis; UC) and the TLR-TBK1-IRF3/7 pathway using human colon tissue, a murine model of colitis and human colonic epithelial cells. Inflamed colonic mucosa over-expressed TKB1, NAP1, IRF3, and IRF7 mRNA compared with normal mucosa. TBK1 was mainly expressed in inflammatory epithelial cells of UC patients. The expression of TBK1, IRF3, IRF7, IFN-α and IFN-β mRNA was suppressed in mice given oral dextran sulfate-sodium (DSS) and daily rectal rebamipide compared with mice given only DSS. Rebamipide reduced the expression of TBK1, IRF3 and IRF7 mRNA induced by LPS/dsRNA, but not of NF-κB mRNA in colonic epithelial cells. Rebamipide might suppress the TLR-TBK1 pathway, resulting in IRF3/7-induction of IFN-α/β and inflammatory factors. TBK1 is important in the induction of inflammation in patients with UC. If rebamipide represses the TLR-TBK1 pathway, then rectal administration should suppress inflammation of the colonic mucosa in patients with UC.
Keywords: TANK-binding kinase 1, toll-like receptor 3/4, interferon regulatory factor 3/7, rebamipide, inflammatory bowel disease
Introduction
The pathogenesis of inflammatory bowel disease (IBD) remains unclear. The immune response to viral or bacterial infection is thought to be one etiology of intestinal inflammation. Toll-like receptors (TLR) induce innate immune responses by recognizing invading microbial pathogens that cause the activation of adaptive immune responses.(1,2) In TLRs, TLR3 and TLR4 impart ligand-specific recognition of double-stranded RNA (dsRNA) of viruses and of bacterial lipopolysaccharide (LPS), respectively.(3,4) The LPS- or polyinosine:polycytidine (poly(I:C))-induced activation of the Toll/IL-1R domain-containing adaptor inducing interferon (IFN)-β (TRIF; TICAM-1), which is an adaptor that functions independently of MyD88, leads to the delayed activation of nuclear factor-κB (NF-κB).(5,6) TRIF also induces activation of the transcriptional regulator, IFN regulatory factor (IRF) 3, and the expression of IFN-β and of IFN-inducible genes through the activation of TANK-binding kinase 1 (TBK1) and inhibitor of kappaB kinase ε (IKKε).(7,8) TLR3 activates primarily the TRIF pathway, whereas TLR4 activates both MyD88- and TRIF-dependent pathways—Both IKKε(9,10) and TBK1(11–13) are key regulators of the IRF3 and IRF7 activation pathways in cells that have been exposed to viruses and/or activated by dsRNA via TLR3.(7,14) NF-κB is induced by poly(I:C)/LPS through TLR3/4,(15) and NF-κB activation is required for the release of proinflammatory cytokines, including interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor (TNF).(16)
The initiation and perpetuation of the inflammatory intestinal responses in IBD might result from an exaggerated host defense reaction of the intestinal epithelium to endogenous luminal bacterial flora and viruses via TLR3/4-signaling. TLR3 is significantly down-regulated in intestinal epithelial cells from patients with active Crohn’s disease (CD) but not in those with ulcerative colitis (UC). In contrast, TLR4 is obviously up-regulated in both UC and CD.(17) Moreover, a relationship has been identified between IBD and NF-κB,(18) but not between IBD and the TBK1-IRF pathway.
Rebamipide is widely used in Japan to treat gastric ulcers(19) and gastric injury.(20) Recently, there was a report of administered rebamipide enemas to a patient with IBD complicated by proctitis.(21) The therapeutic efficacy of rebamipide has been independently confirmed by others using a model of colitis induced by acetic acid(22) or dextran sulfate-sodium (DSS).(23,24) However, the relationship between rebamipide and TBK1 has not been described.
Here, we analyzed the relationship between IBD (in particular, UC) and the TLR-TBK1-IRF3/7 pathway. We then evaluated the effect of rebamipide on the TBK1-IRF3/7-IFN-α/β pathway and on the NF-κB activation pathway using colonic epithelial cells and mice with colitis induced by DSS.
Material and Methods
Human samples and tissue collection
Biopsy specimens of normal and moderately or severely inflammatory mucosa obtained during colonoscopy of 10 patients with UC at Nagoya City University Hospital between 2005 and 2007 were stored at −80°C for mRNA detection.
The Ethics Committee of Nagoya City University Graduate School of Medical Sciences granted approval for this study and written informed consent was obtained from all patients to participate in all procedures associated with the study.
Immunohistochemistry of UC patients
Immunohistochemical staining of colon tissues was performed with antibodies against TBK1 (EP611Y, abcam, Tokyo, Japan; dilution 1:50). The procedure was performed with the appropriate positive and negative controls. Briefly, 3-µm-thick sections were deparaffinized and hydrated through a graded series of alcohols. After inhibition of endogenous peroxidase activity by immersion in 3% H2O2/methanol solution, antigen retrieval was achieved by heating the samples in 10 mM citrate buffer (pH 6.0) using a microwave oven for 10 min at 98°C. Then, sections were incubated with the primary antibody. After thorough washing in PBS, the samples were incubated with biotinylated the secondary antibody and then with avidin-biotin horseradish peroxidase complexes (Vectastain Elite ABC kit, Vector Laboratories, Inc., Burlingame, CA). Finally, immune complexes were visualized by incubation with 0.01% H2O2 and 0.05% 3,3'-diaminobenzidine tetrachloride (DAB).
Experimental procedures of DSS mice
Eight-week-old female C57BL/6 mice were housed under conventional conditions in a temperature-controlled room with a 12 h light/dark cycle. Colitis was induced in the mice by orally administering 3% dextran sulfate sodium (DSS; MP Biomedicals Inc., Morgan, CA) in distilled water daily for 5 days. The mice were assigned to groups as follows: Control (no DSS and no rebamipide), DSS (oral DSS alone), DSS + rebamipide (oral DSS plus daily rectal administration of 50 mg/kg/day of rebamipide dissolved in 100 µl of 0.5% carboxymethylcellulose (CMC; Wako Pure Chemical Industries Ltd., Osaka, Japan). All mice were sacrificed 5 days after DSS administration was started. The Animal Care Committee of Nagoya City University approved the study protocol.
Histopathology of DDS mice colon tissues
All specimens were routinely processed and stained with hematoxylin and eosin for histological examination.
Cell culture
Human colonic cancer cells (CaCo2; ATCC number, HTB-37) were seeded in 6-cm dishes at a density of 2 × 106/dish and cultured for 48 h with RPMI1640 (Sigma Chemical Co., St. Louis, MO) supplemented with 10% fetal calf serum, 1% ampicillin and streptomycin in 5% CO2.
Reagents
Lipopolysaccharide (LPS) from Escherichia coli O55:B5 and poly(I:C) were purchased from Sigma Chemical Co. and Amersham Biosciences (Pittsburgh, PA), respectively. Rebamipide was provided by Otsuka Pharmaceutical Co. Ltd. (Tokyo, Japan).
Experimental procedures of human colonic epithelial cells
Sub-confluent CaCo2 cells were incubated with Poly(I:C) or LPS at 37°C for 24 h to examine the TLR3 and TLR4 signaling pathways, respectively, in the presence or absence of rebamipide. Thereafter mRNA and protein expression were examined in the cells as described below.
Real-time RT-PCR
Total RNA was isolated from CaCo2 cells using TRIzol (Invitrogen, Carlsbad, CA). First-strand cDNA was synthesized using Superscript II (Invitrogen) according to the manufacturer’s instructions. Primers for human TLR3, TLR4 and control human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were obtained from Applied Biosystems (CA; TLR3, Hs00152933 m1; TLR4, Hs01061963 m1; GAPDH, 4310884E). The primers for human TBK1, NAP1, IRF3, IRF7, NF-κB and control human acid ribosomal phosphoproteins (ARP) are listed in Table 1 and those for mouse TBK1, IRF3, IRF7, IFN-α, IFN-β and control mouse β-actin are listed in Table 2. Real-time RT-PCR for TLR3 and TLR4 proceeded using an ABI 7500 Fast Real-Time PCR system (Applied Biosystems). Real-time RT-PCR proceeded in a 20-µl volume containing 18 µl of TaqMan Fast Universal PCR Master Mix (Applied Biosystems), 1 µl cDNA and 1 µl primers. Reactions for TBK1, NAP1, IRF3, IRF7, IFN-α, IFN-β, control human ARP and control mouse β-actin proceeded in a 20-µl volume containing 18 µl of Power SYBR Green PCR Master Mix (Applied Biosystems), 1 µl cDNA and 20 µM primers. Uniform amplification of the products was reconfirmed by analyzing the melting curves of the amplified products. All reactions proceeded in triplicate to assess reproducibility.
Table 1.
Human primer sets
| Upper primer | Lower primer | |
|---|---|---|
| IRF3 | tcttccagcagaccatctcc | tgcctcacgtagctcatcac |
| IRF7 | cagatccagtcccaaccaag | gtctctactgcccacccgta |
| TBK1 | agcggcagagttaggtgaaa | ccagtgatccacctggagat |
| NAP1 | tgggaggtggaaaagttgag | ccgtttgtttggctttctgt |
| NF-κB | tctgcttccaggtgacagtg | atcttgagctcggcagtgtt |
| ARP | cgaagccacgctgctgaacatgctcaac | gctgccattgtcgaacacctgctggatg |
Table 2.
Mouse primer sets
| Upper primer | Lower primer | |
|---|---|---|
| TBK1 | gagagctggaggacgatgag | acggtagccccgtacttctt |
| IRF3 | gatggagaggtccacaagga | gagtgtagcgtggggagtgt |
| IRF7 | cctcttgcttcaggttctgc | gctgcatagggttcctcgta |
| IFN-α | agtgagctgacccagcagat | agacagccttgcaggtcatt |
| IFN-β | ccctatggagatgacggaga | acccagtgctggagaaattg |
| β-Actin | gatctggcaccacaccttct | ggggtgttgaaggtctcaaa |
Western blotting
CaCo2 cells were washed with PBS (−) and subsequently dissolved in 1× lysis buffer (Cell Signaling Technology) containing 20 mmol/l Tris-HCl (pH 7.5), 150 mmol/l NaCl, 1 mmol/l Na2EDTA, 1 mmol/l EGTA, 1% Triton, 2.5 mmol/l sodium pyrophosphate, 1 mmol/l β-glycerophosphate, 1 mmol/l Na3VO4, and 1 µg/ml leupeptin. Cells were disrupted using a Bio-ruptor sonicator (Cosmo Bio, Tokyo, Japan) for 15 s, and then lysates were centrifuged at 15,000 rpm for 10 min at 4°C. All samples were normalized to an equal protein concentration using a protein assay kit (Bio-Rad Laboratories, CA). An equal quantity of 2× SDS-PAGE sample buffer (0.5 mol/l Tris-HCl (pH 7.2), 1% SDS, 100 mmol/l β-mercaptoethanol, and 0.01% bromophenol blue) was added to the samples, and then the mixtures were boiled for 5 min at 100°C. Portions of boiled samples were fractioned on 7.5%, 10% or 12.5% SDS-PAGE gels and then transferred to nitrocellulose membranes (Amersham Pharmacia Biotech, Uppsala, Sweden). Non-specific binding on the membranes was blocked with 5% skimmed milk in PBS (−) for 1 h at room temperature. The membranes were incubated with TBK1 monoclonal antibody (clone: 108A429, Gene Tex, Inc., Irvine, CA) overnight at 4°C followed by three washes with 0.05% Tween 20 in PBS (−) at 5-min intervals. The membranes were incubated with an appropriate secondary antibody for 1 h at room temperature, followed by three washes with 0.05% Tween 20 in PBS (−) at 5-min intervals. Immunoreactive proteins were visualized using the ECL Plus Western blotting Detection system (Amersham Biosciences). Filters were stripped and reprobed using monoclonal β-actin antibody (Abcam Plc., Cambridge, England) as an internal control.
Immunofluorescence microscopy
CaCo2 cells in a subconfluent state were incubated with Poly(I:C) or LPS at 37°C for 24 h respectively, in the presence or absence of rebamipide. Thereafter TBK1 was analyzed by immunofluorescence study. Cells were fixed with ethanol and acetone. Incubation with primary antibody of TBK1 was performed in a solution of PBS containing 0.1% milk at room temperature. Then, sections were incubated with the appropriate secondary antibody and all sections were counterstained with 4',6-diamidino-2-phenylindole (DAPI) (Kirkegaard and Perry Laboratories). Images were obtained with an Eclipse 80i fluorescence microscope (Nikon, Tokyo, Japan).
Results
Inflammatory mucosa of UC over-expressed TBK1-IRF3/7 signaling pathway
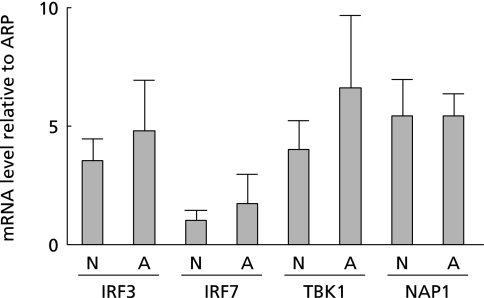
We first performed real-time RT-PCR of TBK1, NAP1, IRF3, and IRF7 to confirm the relationship between the mRNA expression of these genes and UC in 10 patients. The mRNA expression of all these genes was higher in atypical, than in normal mucosa from the patients (Fig. 1). These results indicated that the inflammation associated with UC is related to the TBK1-IRF3/7 signaling pathway.
Fig. 1.
Inflammatory mucosa of UC patients over-expressed TBK1-IRF3/7 signaling pathway in human biopsy tissues. Real-time RT-PCR of TBK1, NAP1, IRF3, and IRF7 confirmed the relationship between mRNA expression of these genes and ulcerative colitis in 10 patients. More TBK1, NAP1, IRF3, and IRF7 mRNA was expressed in atypical, than in the normal mucosa of patients with UC.
Immunohistochemical analysis of TBK1 in UC
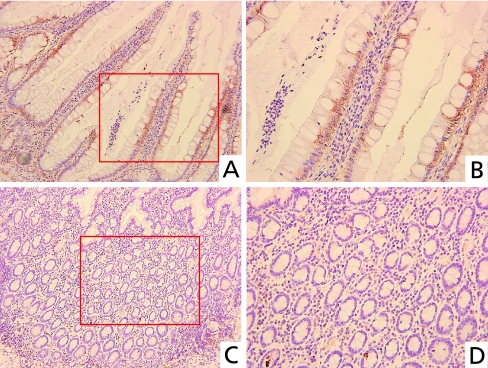
We performed immunohistochemical staining of TBK1 in UC tissues from humans. TBK1 was mainly expressed in obviously inflammatory colon epithelial cells of crypts (Fig. 2 A and B). On the other hand, TBK1 was hardly expressed in colon epithelial cells with weak inflammation (Fig. 2 C and D).
Fig. 2.
Immunohistochemical analysis of TBK1 in UC. TBK1 was mainly expressed in inflammatory colon epithelial cells of crypts (A), but hardly expressed in colon epithelial cells with weak inflammation (C). Higher magnification of A (B). Higher magnification of C (D). (Original magnification: A, C ×200; B, D ×400).
Histological findings in the colons of DDS mice
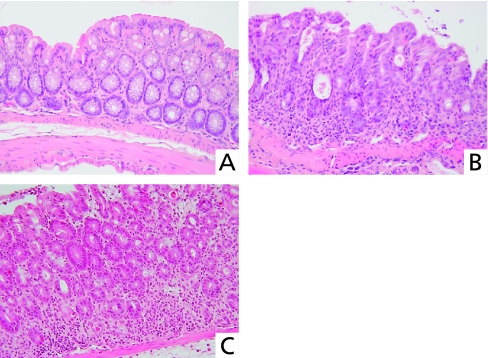
Crypts were diffusely absent and considerable numbers of inflammatory cells had infiltrated colon specimens from mice in the DSS group (Fig. 3B), whereas few crypts had disappeared and inflammatory cell infiltration was minimal in the DSS + rebamipide group (Fig. 3C).
Fig. 3.
Histological findings in the colon of DDS mice. Normal colon mucosa of untreated mouse (A). Colon specimen from DSS mouse shows diffuse crypt disappearance and inflammatory cell infiltration (B) indicating severe colitis. Colon specimen from mouse given oral DSS and daily rectal rebamipide, shows minimal crypt disappearance and inflammatory cell infiltration (C).
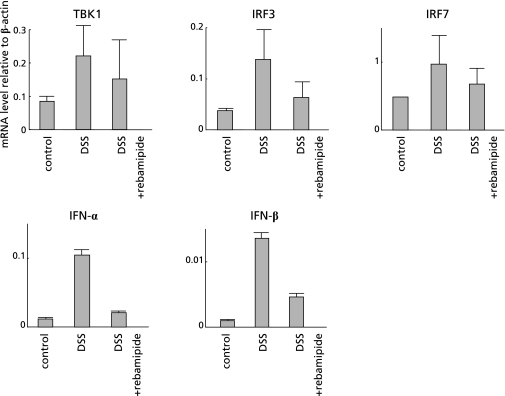
Rebamipide suppressed TBK1-IRF3/7-IFN-α/β signaling pathway in DSS mice
To determine the effect of rebamipide on the TLR-TBK1 signaling pathway in colitis, we performed real-time RT-PCR of TBK1, IRF3, IRF7, IFN-α and IFN-β on colon specimens from DSS and from DSS + rebamipide groups. The mRNA expression of all these genes was increased due to inflammation in the colon of DSS mice, whereas such elevation was suppressed in that of the DSS + rebamipide group (Fig. 4). These results indicated that rebamipide suppresses the TBK1-IRF3/7-IFN-α/β signaling pathway in DDS mice.
Fig. 4.
Rebamipide suppressed TBK1-IRF3/7-IFN-α/β signaling pathway in DSS mice. Effect of rebamipide on TLR-TBK1 signaling pathway in colon specimens from mice given oral DDS examined by real-time RT-PCR of TBK1, IRF3, IRF7, IFN-α and IFN-β. Messenger RNA expression of these genes was increased because of colonic inflammation in DSS mice, but this increase was suppressed in DSS mice given daily rectal rebamipide.
Rebamipide suppressed TLR-TBK1 signaling pathway in human colonic epithelial cells
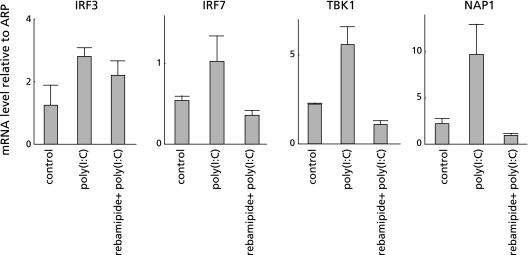
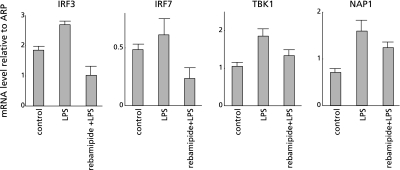
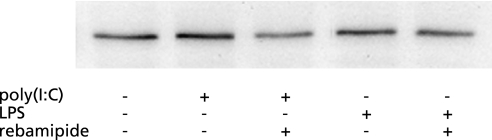
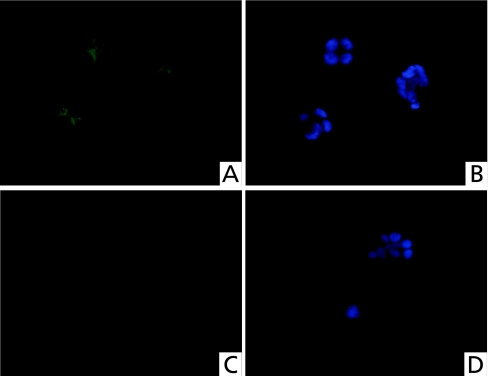
To clarify the mechanism of rebamipide on the TLR-TBK1 signaling pathway in colitis, we performed both real-time RT-PCR of TBK1, NAP1, IRF3 and IRF7, and Western blotting of TBK1 using human colonic epithelial cells. Poly(I:C) (a TLR3 ligand) was added to CaCo2 colonic epithelial cells with or without rebamipide. Poly(I:C) alone increased the mRNA expression of all of these genes in the cells. However, the mRNA expression of these genes was suppressed to control levels when both poly(I:C) and rebamipide were added to the cells (Fig. 5). LPS, which is a ligand for TLR4, increased the mRNA expression of all these genes in CaCo2 cells, whereas rebamipide suppressed these LPS-induced increases to control levels (Fig. 6). Western blotting showed that rebamipide with either poly(I:C) or LPS suppressed TBK1 protein expression (Fig. 7). Immunofluorescence image of TBK1 indicated that rebamipide with LPS suppressed TBK1 protein expression (Fig. 8). Rebamipide also suppressed the expression of TBK-1 with poly(I:C) (data not shown). These results indicated that rebamipide suppresses the TLR3/4-TBK1 signaling pathway in human colonic epithelial and serves as an important factor in anti-inflammation via this pathway.
Fig. 5.
Rebamipide suppressed TLR3-TBK1 signaling pathway in human colonic epithelial cells. Effect of rebamipide on TLR3-TBK1 signaling pathway in human colonic epithelial cells examined by both real-time RT-PCR of TBK1, IRF3 and IRF7 and by Western blotting of TBK1. Poly(I:C) (TLR3 ligand) was added to colonic epithelial cell line, CaCo2 with or without rebamipide. Poly(I:C) alone increased, whereas poly(I:C) plus rebamipide suppressed the mRNA expression of all of these genes to control levels.
Fig. 6.
Rebamipide suppressed TLR4-TBK1 signaling pathway in human colonic epithelial cells. Lipopolysaccharide (TLR4 ligand) was added to CaCo2 colonic epithelial cells with or without rebamipide. LPS alone increased, whereas LPS plus rebamipide suppressed mRNA expression of all of these genes to control levels in CaCo2 cells.
Fig. 7.
Effect of rebamipide on protein expression level of TBK1. Western blots show that rebamipide suppressed TBK1 protein expression in human colonic epithelial cells induced by either poly(I:C) or LPS. These results indicate that rebamipide suppresses TLR3/4-TBK1 signaling in these cells.
Fig. 8.
Immunofluorescent image of TBK1. TBK1 expression (green) were visualized by immunofluorescent microscopy (A, C). Nuclei were stained blue by DAPI (B, D). TBK1 was clearly expressed in the cytoplasm of CaCo2 cells treated with LPS (A). On the other hand, TBK1 expression was reduced in the cytoplasm of CaCo2 cells treated with LPS and rebamipide (C).
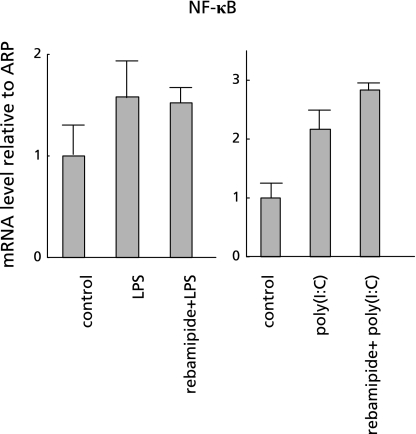
To determine the effect of rebamipide on the NF-κB signaling pathway, we performed real-time RT-PCR of NF-κB in CaCo2 cells using the same procedure as described above. We found that LPS increased the expression of NF-κB mRNA in CaCo2 cells (Fig. 9), whereas LPS/poly(I:C) plus rebamipide did not suppress the expression and the level was similar to that in the presence of LPS/poly(I:C) (Fig. 9). These results indicated that rebamipide does not exert anti-inflammatory effects via the NF-κB signaling pathway.
Fig. 9.
Effect of rebamipide on NF-κB signaling pathway. Real-time RT-PCR of NF-κB in human colonic epithelial cells shows that LPS and poly(I:C) each increased NF-κB gene mRNA expression in colonic epithelial cells, and that adding rebamipide did not suppress either of these increases.
Discussion
The TLRs are the best-characterized family of innate immune receptors and they recognize conserved microbial motifs including dsRNA and LPS. Several intestinal cell types including epithelial cells, dendritic cells, monocyte/macrophages, granulocytes and lymphocytes express TLRs.(25–27) Studies using mice deficient in the MyD88 gene, which is the major signaling adaptor of the TLR family, suggest that the predominant role of TLR/MyD88 signal transduction is to prevent intestinal inflammation.(28,29) However, TLR/MyD88 signaling also promotes intestinal inflammation,(30–32) and the inhibition of NF-κB, a major target of this pathway, can ameliorate murine colitis.(33) On the other hand, few studies have investigated TRIF signaling in IBD.(34) Here, we found that the over-expression of TBK1, NAP1 and IRF3/7 activated TRIF signaling in a murine model of UC induced by DSS. These findings suggest that TRIF signaling plays an important role in the etiology of colitis.
Rebamipide is widely used to treat gastric ulcers in Japan(19) and the first successful case study of rebamipide administered rectally to a patient with IBD complicated by proctitis has recently been published.(21) The encouraging outcome of that study indicated a need for a prospective study to assess the safety and efficacy of rebamipide enemas in a cohort of patients with active UC. Rebamipide is a mucosal protective and ulcer-healing agent that is used to treat patients with acute and chronic gastritis and to heal gastric ulcers in Asian countries including Japan.(35–37) Clinical and experimental data indicate that rebamipide has anti-inflammatory properties; it scavenges free radicals,(38) and suppresses the production of pro-inflammatory mediators(39,40) as well as the migration and adherence of inflammatory cells.(41,42) Rebamipide also has mucosal protective and healing actions through promoting prostaglandin biosynthesis,(35,43,44) mucus production and release,(45) mucosal cell turnover,(46) and cell proliferation.(47) Because rebamipide is a radical scavenger, it has been investigated as an alternative treatment for chronic inflammatory diseases including colitis.(22–24,48) Anal administration of rebamipide has protective anti-colitis effects in the colitis model induced by tri-nitrobenzene sulfonic acid, in which colonic epithelial cells produce low levels of antioxidants.(48) The established DSS-induced mouse model of colitis has often been used to detect inflammatory factors including cytokines in the colon.(49) The therapeutic value of rebamipide has been independently confirmed by others using models of colitis induced by acetic acid(22) or DSS.(23,24) To our knowledge, an association between rebamipide and TBK1-IRF3/7 signaling in colitis has not been reported. Here, we demonstrated that rebamipide inhibited the TBK1-IRF3/7 pathway and IFN-α and IFN-β in mice with colitis induced by DSS. We also investigated how rebamipide causes such inhibition in colonic epithelial cells. Our data demonstrated that rebamipide inhibited the TLRs-TBK1-IRF3/7 pathway in these cells independently of NF-κB activation, indicating that rebamipide directly suppresses TBK1 activation.
The type I interferons, IFN-α and IFN-β, have been evaluated as therapy for active UC in pilot clinical trials.(50,51) IFN-β has induced the remission of ulcerative colitis in a Japanese patient with type C chronic hepatitis.(52) Our findings seem to contradict these reports. Whether IFN-β had a positive or a negative effect upon the mucosa was unclear. However, a systematic review concluded that type I IFNs cannot be recommended as treatment for active UC.(53) The contradictory effect of IFN-β might be dose-related.
In conclusion, the TLR-TBK1-IRF3/7 pathway might be one etiology of IBD and rebamipide directly thwarts this pathway. However, further studies are required to clarify the mechanism of action.
References
- 1.Medzhitov R, Preston-Hurlburt P, Janeway CA,, Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 2.O’Neill LA. TLRs: Professor Mechnikov, sit on your hat. Trends Immunol. 2004;25:687–693. doi: 10.1016/j.it.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Rhee SH, Hwang D. Murine TOLL-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NF kappa B and expression of the inducible cyclooxygenase. J Biol Chem. 2000;275:34035–34040. doi: 10.1074/jbc.M007386200. [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 5.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto M, Sato S, Mori K, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald KA, McWhirter SM, Faia KL, et al. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 8.Toshchakov V, Jones BW, Perera PY, et al. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat Immunol. 2002;3:392–398. doi: 10.1038/ni774. [DOI] [PubMed] [Google Scholar]
- 9.Peters RT, Liao SM, Maniatis T. IKKepsilon is part of a novel PMA-inducible IkappaB kinase complex. Mol Cell. 2000;5:513–522. doi: 10.1016/s1097-2765(00)80445-1. [DOI] [PubMed] [Google Scholar]
- 10.Shimada T, Kawai T, Takeda K, et al. IKK-i, a novel lipopolysaccharide-inducible kinase that is related to IkappaB kinases. Int Immunol. 1999;11:1357–1362. doi: 10.1093/intimm/11.8.1357. [DOI] [PubMed] [Google Scholar]
- 11.Pomerantz JL, Baltimore D. NF-kappaB activation by a signaling complex containing TRAF2, TANK and TBK1, a novel IKK-related kinase. EMBO J. 1999;18:6694–6704. doi: 10.1093/emboj/18.23.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnard M, Mirtsos C, Suzuki S, et al. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription. EMBO J. 2000;19:4976–4985. doi: 10.1093/emboj/19.18.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tojima Y, Fujimoto A, Delhase M, et al. NAK is an IkappaB kinase-activating kinase. Nature. 2000;404:778–782. doi: 10.1038/35008109. [DOI] [PubMed] [Google Scholar]
- 14.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 15.Faure E, Equils O, Sieling PA, et al. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 16.Baeuerle PA. IkappaB-NF-kappaB structures: at the interface of inflammation control. Cell. 1998;95:729–731. doi: 10.1016/s0092-8674(00)81694-3. [DOI] [PubMed] [Google Scholar]
- 17.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizushima T, Sasaki M, Ando T, et al. Blockage of angiotensin II type 1 receptor regulates TNF-alpha-induced MAdCAM-1 expression via inhibition of NF-kappaB translocation to the nucleus and ameliorates colitis. Am J Physiol Gastrointest Liver Physiol. 2010;298:G255–G266. doi: 10.1152/ajpgi.00264.2009. [DOI] [PubMed] [Google Scholar]
- 19.Arakawa T, Kobayashi K, Yoshikawa T, Tarnawski A.Rebamipide: overview of its mechanisms of action and efficacy in mucosal protection and ulcer healing Dig Dis Sci 1998; 435S–13S. [PubMed] [Google Scholar]
- 20.Ono S, Kato M, Imai A, et al. Preliminary trial of rebamipide for prevention of low-dose aspirin-induced gastric injury in healthy subjects: a randomized, double-blind, placebo-controlled, cross-over study. J Clin Biochem Nutr. 2009;45:248–253. doi: 10.3164/jcbn.09-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makiyama K, Takeshima F, Kawasaki H, Zea-Iriarte WL. Anti-inflammatory effect of rebamipide enema on proctitis type ulcerative colitis: a novel therapeutic alternative. Am J Gastroenterol. 2000;95:1838–1839. doi: 10.1111/j.1572-0241.2000.02154.x. [DOI] [PubMed] [Google Scholar]
- 22.Sakurai K, Osaka T, Yamasaki K. Protection by rebamipide against acetic acid-induced colitis in rats: relationship with its antioxidative activity. Dig Dis Sci. 1998;43(Suppl):125S–133S. [PubMed] [Google Scholar]
- 23.Iwai A, Iwashita E. Changes in colonic inflammation induced by dextran sulfate sodium (DSS) during short- and long-term administration of rebamipide. Dig Dis Sci. 1998;43(Suppl):143S–147S. [PubMed] [Google Scholar]
- 24.Kishimoto S, Haruma K, Tari A, Sakurai K, Nakano M, Nakagawa Y. Rebamipide, an antiulcer drug, prevents DSS-induced colitis formation in rats. Dig Dis Sci. 2000;45:1608–1616. doi: 10.1023/a:1005525313856. [DOI] [PubMed] [Google Scholar]
- 25.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19:59–69. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Himmel ME, Hardenberg G, Piccirillo CA, Steiner TS, Levings MK. The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease. Immunology. 2008;125:145–153. doi: 10.1111/j.1365-2567.2008.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michelsen KS, Arditi M. Toll-like receptors and innate immunity in gut homeostasis and pathology. Curr Opin Hematol. 2007;14:48–54. doi: 10.1097/00062752-200701000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Gibson DL, Ma C, Bergstrom KS, Huang JT, Man C, Vallance BA. MyD88 signalling plays a critical role in host defence by controlling pathogen burden and promoting epithelial cell homeostasis during Citrobacter rodentium-induced colitis. Cell Microbiol. 2008;10:618–631. doi: 10.1111/j.1462-5822.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 30.Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe T, Asano N, Murray PJ, et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118:545–559. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karrasch T, Kim JS, Muhlbauer M, Magness ST, Jobin C. Gnotobiotic IL-10-/-;NF-kappa B(EGFP) mice reveal the critical role of TLR/NF-kappa B signaling in commensal bacteria-induced colitis. J Immunol. 2007;178:6522–6532. doi: 10.4049/jimmunol.178.10.6522. [DOI] [PubMed] [Google Scholar]
- 34.Rahman FZ, Smith AM, Hayee B, Marks DJ, Bloom SL, Segal AW. Delayed resolution of acute inflammation in ulcerative colitis is associated with elevated cytokine release downstream of TLR4. PLoS One. 2010;5:e9891. doi: 10.1371/journal.pone.0009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamasaki K, Kanbe T, Chijiwa T, Ishiyama H, Morita S. Gastric mucosal protection by OPC-12759, a novel antiulcer compound, in the rat. Eur J Pharmacol. 1987;142:23–29. doi: 10.1016/0014-2999(87)90649-2. [DOI] [PubMed] [Google Scholar]
- 36.Uchida M, Tabusa F, Komatsu M, Morita S, Kanbe T, Nakagawa K. Studies on 2(1H)-quinolinone derivatives as gastric antiulcer active agents. Synthesis and antiulcer activities of optically active alpha-amino acid derivatives of 2(1H)-quinolinone and oxindole. Chem Pharm Bull (Tokyo) 1987;35:853–856. doi: 10.1248/cpb.35.853. [DOI] [PubMed] [Google Scholar]
- 37.Higuchi K, Arakawa T, Nebiki H, et al. Rebamipide prevents recurrence of gastric ulcers without affecting Helicobacter pylori status. Dig Dis Sci. 1998;43(Suppl):99S–106S. [PubMed] [Google Scholar]
- 38.Yoshikawa T, Naito Y, Tanigawa T, Kondo M. Free radical scavenging activity of the novel anti-ulcer agent rebamipide studied by electron spin resonance. Arzneimittelforschung. 1993;43:363–366. [PubMed] [Google Scholar]
- 39.Nagano C, Wakebe H, Azuma A, Imagawa K, Kikuchi M. IFN-gamma-induced iNOS mRNA expression is inhibited by rebamipide in murine macrophage RAW264.7 cells. Dig Dis Sci. 1998;43(Suppl):118S–124S. [PubMed] [Google Scholar]
- 40.Aihara M, Imagawa K, Funakoshi Y, Ohmoto Y, Kikuchi M. Effects of rebamipide on production of several cytokines by human peripheral blood mononuclear cells. Dig Dis Sci. 1998;43(Suppl):160S–166S. [PubMed] [Google Scholar]
- 41.Yoshida N, Yoshikawa T, Iinuma S, et al. Rebamipide protects against activation of neutrophils by Helicobacter pylori. Dig Dis Sci. 1996;41(Suppl):1139–1144. doi: 10.1007/BF02088229. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida M, Wakabayashi G, Ishikawa H, et al. Rebamipide attenuates gastric microcirculatory disturbances in the early period after thermal injury in rats. Dig Dis Sci. 1998;43(Suppl):148S–153S. [PubMed] [Google Scholar]
- 43.Kleine A, Kluge S, Peskar BM. Stimulation of prostaglandin biosynthesis mediates gastroprotective effect of rebamipide in rats. Dig Dis Sci. 1993;38:1441–1449. doi: 10.1007/BF01308601. [DOI] [PubMed] [Google Scholar]
- 44.Sun WH, Tsuji S, Tsujii M, et al. Induction of cyclooxygenase-2 in rat gastric mucosa by rebamipide, a mucoprotective agent. J Pharmacol Exp Ther. 2000;295:447–452. [PubMed] [Google Scholar]
- 45.Suetsugu H, Ishihara S, Moriyama N, et al. Effect of rebamipide on prostaglandin EP4 receptor gene expression in rat gastric mucosa. J Lab Clin Med. 2000;136:50–57. doi: 10.1067/mlc.2000.107303. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi M, Takada H, Takagi K, Kataoka S, Soma R, Kuwayama H. Gastric restitution is inhibited by dexamethasone, which is reversed by hepatocyte growth factor and rebamipide. Aliment Pharmacol Ther. 2003;18 (Suppl 1):126–132. doi: 10.1046/j.1365-2036.18.s1.19.x. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe T, Higuchi K, Hamaguchi M, et al. Rebamipide prevents delay of acetic acid-induced gastric ulcer healing caused by Helicobacter pylori infection in Mongolian gerbils. Dig Dis Sci. 2002;47:1582–1589. doi: 10.1023/a:1015879421739. [DOI] [PubMed] [Google Scholar]
- 48.Zea-Iriarte WL, Makiyama K, Goto S, et al. Impairment of antioxidants in colonic epithelial cells isolated from trinitrobenzene sulphonic acid-induced colitis rats. Protective effect of rebamipide. Scand J Gastroenterol. 1996;31:985–992. doi: 10.3109/00365529609003118. [DOI] [PubMed] [Google Scholar]
- 49.Nishimura T, Andoh A, Hashimoto T, Kobori A, Tsujikawa T, Fujiyama Y. Cellobiose prevents the development of dextran sulfate sodium (DSS)-induced experimental colitis. J Clin Biochem Nutr. 2010;46:105–110. doi: 10.3164/jcbn.09-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madsen SM, Schlichting P, Davidsen B, et al. An open-labeled, randomized study comparing systemic interferon-alpha-2A and prednisolone enemas in the treatment of left-sided ulcerative colitis. Am J Gastroenterol. 2001;96:1807–1815. doi: 10.1111/j.1572-0241.2001.03875.x. [DOI] [PubMed] [Google Scholar]
- 51.Nikolaus S, Rutgeerts P, Fedorak R, et al. Interferon beta-1a in ulcerative colitis: a placebo controlled, randomised, dose escalating study. Gut. 2003;52:1286–1290. doi: 10.1136/gut.52.9.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miike T, Tahara Y, Yamaguchi Y, et al. A case study: interferon-beta-induced remission of ulcerative colitis in a patient with type C chronic hepatitis. Nippon Shokakibyo Gakkai Zasshi. 2008;105:1362–1366. [PubMed] [Google Scholar]
- 53.Seow CH, Benchimol EI, Griffiths AM, Steinhart AH. Type I interferons for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2008:CD006790. doi: 10.1002/14651858.CD006790.pub2. [DOI] [PubMed] [Google Scholar]